Parasitoid venom alters the lipid composition and development of microorganisms on the wax moth cuticle
Abstract
Ectoparasitoids may change host metabolism, making the environment more favorable for the development of their larvae. These alterations may also be suitable for growth and proliferation of commensal microorganisms and pathogens. This is especially important in relation to cuticular microbiota, with which ectoparasitoids interact directly. However, biochemical and microbiological changes occurring on insect cuticles in response to parasitoid venoms are insufficiently understood. We assayed the biochemical and microbiological changes in cuticles of larvae of the wax moth, Galleria mellonella (L.) (Lepidoptera: Pyralidae), after envenomation by Habrobracon brevicornis (Wesmael) (Hymenoptera: Braconidae). In particular, lipid composition in the epicuticle, total nitrogen (N) and carbon (C) amounts in the cuticle, bacterial colony-forming unit (CFU) counts, and bacterial community structure (16S rRNA gene-based metagenomics) on the insect surface were analyzed. The susceptibility of wax moth larvae to the entomopathogenic fungus (EPF) Metarhizium robertsii J.F. Bisch., S.A. Rehner & Humber (Hypocreales: Clavicipitaceae) was also assessed. Envenomation led to an increase in the hydrocarbon amount, as well as alterations in the fatty acid composition of the epicuticle; in particular, a large decrease in the amount of ω-1 hydroxy fatty acids. The total N and C amounts in the cuticle also slightly increased after envenomation. These changes were correlated with a decrease in bacterial diversity and an increase in Enterococcus abundance on the surface of envenomated larvae. Envenomation also led to a substantial increase in larval susceptibility to M. robertsii infection; differences between the LC50 values of envenomated and control larvae were 505 000-fold. We suggest that the hyper-lipidation of the cuticle is related to processes occurring in the host’s hemocoel after envenomation by the parasitoid. The effects of the changes in the lipid composition on the proliferation and development of microorganisms are discussed.
INTRODUCTION
Parasitoids may dramatically change the behavior, metabolic processes, and immune reactions of host insects. In particular, venoms of parasitoid wasps may lead to developmental arrest and alterations in lipid, protein, and carbohydrate metabolism, as well as humoral and cellular immunity, which may be beneficial for the development of parasitoid larvae (reviewed by Pennacchio & Strand, 2006; Mrinalini & Werren, 2016). At the same time, these alterations may affect the development of commensal and pathogenic microorganisms localized in the gut or on the surface of insects (Richards & Dani, 2010; Richards et al., 2011; Kryukov et al., 2013; Polenogova et al., 2019). The active proliferation of opportunistic and pathogenic fungi and bacteria causes competition between microorganisms and parasitoid larvae and, in some cases, may lead to the death of both host and parasitoid (Roy & Pell, 2000). Changes in microbial communities and the behaviors of certain microorganisms in insect hosts in response to ectoparasitoid infections are insufficiently understood. These could be especially important for cuticular microorganisms, with which ectoparasitoids interact directly.
Alterations in insect metabolism have been well studied for several host-ectoparasitoid systems. For example, the venom of Nasonia vitripennis (Walker) increases the amount of lipids, carbohydrates, and amino acids in Sarcophaga bullata (Parker) (Rivers & Denlinger, 1994, 1995; Martinson et al., 2014). Nakamatsu & Tanaka (2004) showed that the venom of Euplectrus separatae Kamijo disrupts the host’s fat body, which leads to the release of lipid particles. Similar effects were established for the Bracon nigricans (Szépligeti)–Spodoptera littoralis (Boisduval) system (Becchminazi et al., 2017). Increases in lipid amounts may be useful for the development of parasitoid larvae, as many parasitic insect species exhibit reduced lipogenesis, i.e., they consume host lipids instead of synthesizing them de novo (Visser & Ellers, 2008). In a previous study (Kryukova et al., 2020), we established that wax moth larvae, Galleria mellonella (L.) (Lepidoptera: Pyralidae), envenomated by Habrobracon brevicornis (Wesmael) [syn. Habrobracon hebetor (Say)] (Hymenoptera: Braconidae) were also characterized by fat body disruption and increased levels of total lipids and trehalose in the hemolymph. In addition, the envenomated larvae exhibited dramatically increased susceptibility to the entomopathogenic fungi (EPF) Beauveria, Metarhizium, and Cordyceps (Kryukov et al., 2013, 2018a,b). We recorded more active germination of EPF on the cuticle of envenomated larvae (Kryukov et al., 2018a), although the reasons for these changes remained unclear. In addition, spontaneous bacterioses were frequently observed in wax moth larvae envenomated by Habrobracon (Polenogova et al., 2019), probably due to the penetration of bacteria through the gut or cuticular injuries. However, possible changes in bacterial load on the insect surface, or biochemical changes in the epicuticle, were not addressed.
Opportunistic and pathogenic microorganisms are sensitive to the chemical composition of insect cuticles. In particular, the susceptibility of insects to EPF is determined by the amount and composition of the main epicuticular lipids – saturated and unsaturated hydrocarbons and fatty acids (Pedrini et al., 2007, 2013; Ortiz-Urquiza & Keyhani, 2013). The lipid layer determines the hydrophobicity of the epicuticle and the adhesion force of fungal propagules to the insect surface (e.g., Sosa-Gomez et al., 1997). In addition, EPF grow on hydrocarbons and fatty acids. Metarhizium and Beauveria spp. utilize linear and methyl-branched hydrocarbons (Napolitano & Juárez, 1997; Crespo et al., 2000) that positively affect the differentiation of infection structures (germ tubes and appressoria) and change fungal metabolism and virulence (Crespo et al., 2002; Jarrold et al., 2007; Lin et al., 2011; Huarte-Bonnet et al., 2018). Saturated and unsaturated fatty acids may also be assimilated by fungi (Zhang et al., 2012). Fatty acids are present in the cuticle in free and bound forms (Juárez & Fernandez, 2007; Sutton et al., 2013). Fungi and bacteria liberate free fatty acids from triglycerides (Keyhani, 2018; Fischer, 2020). Many fatty acids may inhibit fungal growth, especially at high concentrations, by increasing the fluidity of fungal cell membranes and disintegrating fungal cells (reviewed by Pohl et al., 2011). Fatty acids may inhibit germination and mycelial growth or change the enzymatic activities of EPF, as observed in studies of Conidiobolus coronatus (Costantin) A. Batko (Bogus et al., 2010, Wrońska et al., 2018), Beauveria bassiana sensu lato (Bals.-Criv.) Vuill., Cordyceps fumosorosea (Wize) Kepler, B. Shrestha & Spatafora (Saito & Aoki, 1983; Szafranek et al., 2001), Metarhizium brunneum Petch (Ment et al., 2013), and Metarhizium robertsii J.F. Bisch., S.A. Rehner & Humber (Hypocreales: Clavicipitaceae) (Kryukov et al., 2018c). However, some fatty acids (C20:0) that are present at low concentrations may stimulate appressoria formation, as shown in M. brunneum (Ment et al., 2013). Others (saturated and unsaturated C14-C20) may stimulate germination and mycelial growth, as shown in B. bassiana and C. fumosorosea (Saito & Aoki, 1983; Peng et al., 2020). Incubation on media supplemented with C16:1 and C18:1 increases the virulence of B. bassiana (Ortiz-Urquiza et al., 2016) and C. coronatus (Bogus et al., 2010). Thus, the interactions between fungi and fatty acids are not straightforward and depend on the concentrations of certain fatty acids and the species of microorganisms.
Many bacteria use hydrocarbons and fatty acids as carbon sources (Campbell et al., 2003; Brzeszcz & Kaszycki, 2018). However, fatty acids exhibit antimicrobial activity against many gram-negative and -positive bacteria (reviewed by Desbois & Smith, 2010; Fischer et al., 2020; Borrelli et al., 2021). This activity is caused by interference with the electron transport chain, disruption of oxidative phosphorylation, inhibition of bacterial fatty acid biosynthesis, alterations in membrane fluidity and permeability, and a decrease in the expression of virulence factors (Desbois & Smith, 2010). The level of bactericidal and bacteriostatic action depends on the fatty acid structure, the target bacterium, and environmental factors.
In addition to lipids, various water-soluble compounds are present in the cuticle. Among them, carbohydrates, proteins, and amino acids may stimulate the development of microorganisms, as observed for fungi (Jarrold et al., 2007; Ment et al., 2013). Notably, the carbon-to-nitrogen (C:N) ratio in various growth substrates may also affect the growth, enzymatic activity, and virulence of fungal pathogens. For example, Shah et al. (2005) showed that a change in the C:N ratio from 35:1 to 10:1 in media led to a decrease in Metarhizium biomass and an increase in germination rate, spore-bound proteinase (Pr1) levels, and virulence. In addition, the pH of the cuticle represents a physiological signal that triggers the gene expression of fungal chitinases and proteinases (St Leger & Roberts, 1998).
Importantly, various groups of microorganisms may interfere with each other on insect surfaces. For example, certain bacteria exhibit antagonistic properties toward fungi, acting as one of the host’s defense systems, although some bacteria can promote fungal infection (reviewed by Boucias et al., 2018). Therefore, alterations in the bacterial community and density on the insect surface may influence fungal growth and the susceptibility of insects to mycopathogens. These interactions have not yet been specifically considered for insects envenomated by parasitoids.
We hypothesized that the envenomation of wax moth larvae by the ectoparasitoid H. brevicornis leads to changes in both the chemical composition of the epicuticle and the bacterial load (and/or structure) on the insect surface, which will affect fungal behavior and insect susceptibility to infections. In the present study, we analyzed the epicuticular lipid composition of envenomated and non-envenomated larvae using gas chromatography-mass spectrometry (GC-MS). In addition, we assessed the C and N amounts in the cuticles, the bacterial load and composition on the insect surface, and insect susceptibility to the EPF M. robertsii.
MATERIALS AND METHODS
Insects
In the study, we used laboratory populations of the wax moth G. mellonella, from the Institute of Systematics and Ecology of Animals of the Siberian Branch of the Russian Academy of Sciences (SB RAS) (Novosibirsk, Russia), and the parasitoid H. brevicornis, of Uzbekistan origin (Kittel & Maeto, 2019). Wax moth larvae were reared at 28 °C in the dark on artificial medium containing 22.5% cornmeal, 12.5% honey, 12.5% glycerol, 12.5% beeswax, 10% wheat flour, 12.5% milk solids, 5% yeast, and 12.5% water. Habrobracon brevicornis was maintained on fifth and sixth instars of G. mellonella. Adult parasitic wasps were reared on a diet of 12% liquid honey at 28 °C and L14:D10 photoperiod.
Envenomation procedure
Sixth instar wax moths and recently emerged H. brevicornis adults were used. Parasitoids and wax moth larvae (3:10, respectively) were placed together in 9-cm-diameter Petri dishes for 8 h. All larvae were envenomated by parasitoids during this time. Control larvae were incubated without parasitoids. After envenomation, parasitoids were removed from the Petri dishes, and their eggs were also removed from the cuticle of wax moth larvae using wet cotton wool. Control larvae were also treated with this cotton wool. Envenomated and control larvae were incubated in the same dishes with 1 g of artificial medium at 27 °C and 90% r.h. in the dark for 48 h. Then, the larvae were used for sample preparations.
Cuticle thickness measurements
Control (n = 15) and envenomated (n = 20) larvae were fixed with 70% ethanol and stored at 4 °C until sample preparation (sample sizes are given in Table S1). The processing of the larvae consisted of washing with ethanol in phosphate-buffered saline (PBS), incubating in a PBS-saccharose solution (30%) overnight for cryoprotection, and freezing in liquid nitrogen. Three sections of eight sternites were prepared for each larva using a Microm HM 520 cryotome (Thermo Fisher Scientific, Walldorf, Germany) at -25 °C. The thickness of the slices was 30 μm. Five measurements were conducted in each of the three sections, and then the average thickness for each larva was calculated. Measurements were performed with an Axio Imager M1 microscope (Carl Zeiss, Munich, Germany) and analyzed using AXIOVISION v.4.6.3 software (Carl Zeiss).
Epicuticular lipid extraction
A modified version of the technique described by Ment et al. (2013) was used to extract total epicuticular lipids. Control and envenomated larvae (n = 5, each replicate consisting of 45 insects; Table S1) were weighed and then exposed to a stream of CO2 for 5 min to prevent defecation and regurgitation in solvents. Forty-five larvae were placed in 45 ml of a 1:1 (vol:vol) mixture of HPLC grade n-hexane and methyl tertbutyl ether (PanReac AppliChem, Barcelona, Spain) in a glass flask. After 5 min of incubation at 150 r.p.m. and 27 °C, the solvents were placed in a new flask, evaporated in a vacuum concentrator, and stored at -20 °C until GC-MS analysis. The weight of the lipid extracts was assessed as well.
Derivatization and gas chromatography-mass spectrometry analysis
The amounts of hydrocarbons, sterols, and fatty acids in the extracts were analyzed. We estimated the total amount of fatty acids (free + bound) because both forms may exhibit antimicrobial properties (Szafranek et al., 2001). Total epicuticular lipids were derivatized with 1% H2SO4 in MeOH at 80 °C for 3 h (Koukos et al., 2015; He et al., 2019). The obtained compounds were dissolved in a mixture of acetone-hexane and analyzed using GC-MS.
We used a 6890N gas chromatograph and a 5975N mass-selective detector (Agilent Technologies, Santa Clara, CA, USA). CHEMSTATION software (Agilent Technologies) was used for the identification and quantification of hydrocarbons and fatty acid methyl esters (FAMEs). Separation was achieved with an HP-5ms capillary column (30 m × 0.25 mm inner diameter, 0.25 μm film thickness; Agilent Technologies). The carrier gas was helium with a flow rate of 1.0 ml per min. The temperature of the injector and interface was 280 °C. The column oven temperature cycle began at 50 °C for 2 min, and then the temperature was increased to 280 °C (10 °C per min). The final temperature was maintained for 40 min. The mass spectrum was obtained under electron impact ionization at 70 eV. The following temperature model was used to calculate linear retention indices: the column oven temperature cycle began at 50 °C for 2 min, then the temperature was increased to 310 °C (2 °C per min), and the final temperature was maintained for 13 min. The relative quantity of compounds was determined by calculating the mean area of the chromatographic peak among two replicate injections. The quantification of hydrocarbons and fatty acids was carried out in a solution with an extract concentration of 1.0 mg ml-1 using n-triacontane and methyl stearate as standards. Supelco 37-Component FAME Mix (47885-U; Supelco, Bellefonte, PA, USA) was used for the identification of FAMEs. In addition, the NIST 14 MS spectral library (LIPID MAPS Lipidomics Gateway, https://www.lipidmaps.org/) was used to identify individual FAMEs. The GC qualitative standard AG5080-8716 (Agilent Technologies) was used to calculate linear retention indices (LRIs) (Mühlen & Marriott, 2011; He et al., 2013).
The results are presented in two units: relative amount (μg per mg extract), which characterizes a portion of certain lipids in composition, and absolute amount (μg per larva).
Elemental analysis
Larvae were dissected in cold saline and intestines, fatty bodies, and muscle tissue were removed. The cleaned cuticle was washed twice with saline and homogenized in liquid nitrogen. The C and N amounts in the cuticles (n = 3, each replicate consisting of 10 insects; Table S1) were determined using a CHN analyzer (EURO EA 3000 Elemental Analyzer; EuroVector, Pavia, Italy). Samples were burned in a vertical reactor (oxidation tube) dynamically at 1050 °C under a helium flow with the addition of oxygen (10 ml) at the instant of sample introduction. Portions of the sample were placed in the automated sampler in tin capsules, from which they were transferred to the oxidation tube at regular intervals. After pyrolysis, the resulting products were oxidized in the lower part of the reactor filled with a catalytic oxide, and then passed through the reduction zone.
pH of the cuticular water extracts
Cuticles of envenomated and non-envenomated larvae (n = 3, each replicate consisting of 80 insects; Table S1) were dissected from other tissues and ground with a mortar and pestle in liquid nitrogen. Ground cuticles were added to deionized distilled water (weight ratio 1:2) and sonicated in an ultrasonic bath (Sonorex RK 100 H; Bandelin Electronic, Berlin, Germany) for 30 min at 25 °C. Then, the samples were centrifuged at 10000 g, and the supernatants were collected and lyophilized. Lyophilisate from each sample (15 mg) was diluted in 500 μl of deionized distilled water, and the pH was determined using a pH Meter Multitest IPL-301 (Semiko, Novosibirsk, Russia).
Bacterial colony-forming unit counts on the cuticle
As it was previously shown (Allonsius et al., 2019) that the wax moth microbiota is predominantly determined by enterococci, and less frequently by other bacilli and enterobacteria, we used the following media for colony-forming unit (CFU) counts: (1) Luria-Bertani agar (LB), universal medium for determination of the total load of cultivable bacteria; (2) bile esculin azide agar, selective media for enterococci; and (3) endo agar, selective media for enterobacteria (all obtained from HiMedia, Mumbai, India).
Larvae were exposed to a stream of CO2 for 5 min to prevent subsequent regurgitation and defecation. In each sample, five larvae were placed in a 15-ml tube with 5 ml of a cooled 0.1 M PBS–Tween-80 (0.05%) solution (pH 7.2). Tubes were shaken horizontally at 180 r.p.m. for 10 min at 4 °C, and then insects were removed. Suspensions were diluted to 1:100, 1:500, and 1:1000, and 20 μl was plated on 9-cm-diameter Petri dishes with the LB (n = 8), bile esculin azide (n = 10), and endo agar media (n = 6; for all experiments, each replicate consisting of five insects; Table S1). The Petri dishes were incubated for 72 h at 28 °C, and then the bacterial colonies were counted.
To confirm the growth of certain taxa of bacteria on these media, representative colonies were collected for identification (n = 3). Cultures were purified by three passages on LB agar and identified as described previously (Polenogova et al., 2019). A bacterial colony was placed in 20 ml water and heated at 95 °C for 5 min, after which the cell debris was precipitated by centrifugation at 10000 g for 5 min. The supernatant containing bacterial DNA was used to amplify the 16S rRNA gene. Amplification was performed using the primers 16S-8-f-b 5’-AGR GTT GAT CCC GGC TCA-3’ and 16S-1350-r-B 5’-ACG GCG GGT GTG TAC AAN G-3’ to obtain 1342-bp DNA fragments. Sequencing was performed using the same primers and a Terminator v.3.1 BigDye kit (Applied Biosystems, Waltham, MA, USA). The nucleotide sequences were verified using SEQUENCHER v.4.0.5. BLAST v.2.12.0+ with GenBank and the type material was used to determine the putative species. Eighteen sequences were deposited in the GenBank database (MW672446–MW672463).
16S rRNA gene metagenomic analysis of cuticular bacteria
In each sample, five larvae, immobilized by CO2, were placed in a 15-ml tube with 5 ml of a sterile cooled 0.1 M PBS-Tween 80 (0.05%) solution. The tubes were shaken horizontally at 180 r.p.m. for 10 min at 4 °C, and then insects were removed. Then, the suspensions were frozen in liquid nitrogen, lyophilized, and stored at -80 °C until analysis. Six replicates of envenomated and non-envenomated larvae (each replicate consisting of five insects) were used (Table S1). In addition, two replicates of the larva-free PBS-Tween 80 solution were used as a negative control (blank).
DNA was extracted using a DNA-sorb-B kit (Central Research Institute of Epidemiology, Moscow, Russia) according to the manufacturer’s instructions. The DNA was amplified with a pair of primers targeting the V3-V4 regions of the 16S rRNA gene. The primers contained Illumina-specific adapters attached at the 5’-end: forward 5’-TCG GCA GCG TCA GAT GTG TAT AAG AGA CAG CCT ACG GGN GGC WGC AG-3’ and reverse 5’-GTC TCG TGG GCT CGG AGA TGT GTA TAA GAG ACA GGA CTA CHV GGG TAT CTA ATC C-3’ (Klindworth et al., 2013). The library was prepared and sequenced by Evrogen (Moscow, Russia) using the Illumina MiSeq platform with v3 (600 cycles as 2 × 300-bp paired-end reads) chemistry (Illumina, San Diego, CA, USA).
Microbiota analysis was performed using QIIME 2 2021.11 (Bolyen et al. 2019). The FastaQ files without barcode information and primer sequences were demultiplexed using the q2-demux plugin. The quality of the sequencing was inspected by visualizing the forward and reverse read quality profiles (visualization paired-end-demux.qzv). Paired-end sequences were trimmed, denoised, joined, and dereplicated using the DADA2 plugin (via q2-dada2) (Callahan et al., 2016) with the following options: --p-trim-left-f 20, --p-trim-left-r 20, --p-trunc-len-f 250, and --p-trunc-len-r 230. Default values were applied for other parameters. At the last stage of the dada2 plugin, chimeric sequences were identified and removed. A taxonomic sequence classification of the resulting amplicon sequence variants (ASVs) was assigned with the QIIME 2 q2-feature-classifier plugin. It was trained using the V3 and V4 regions of the 16S rRNA gene sequences retrieved from the Silva version 138 database (Quast et al., 2013), clustered at 99% sequence similarity. Predominant ASVs were also compared against GenBank-available sequences from type material (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
All rarefaction curves reached a saturation plateau (Figure S1). In the final dataset, ASVs unassigned at the kingdom taxa level as well as ASVs belonging to mitochondria and chloroplasts were excluded. In addition, ASVs that were represented in the negative control (mainly Actinobacteria and Proteobacteria) were also excluded (see dataset). The final dataset included 201 170 reads (16765 ± 2429 per replicate) belonging to 140 ASVs (see dataset). Alpha diversity metrics richness index (Chao 1) and evenness index (Shannon H) were calculated for the ASV level using PAST 3 (Hammer et al., 2001) after rarefying.
Fungal virulence
Metarhizium robertsii strain P-72 from the collection of SB RAS (Novosibirsk) was used. Conidia were grown on Sabouraud agar (HiMedia) and suspended in a water-Tween 80 solution (0.03%). Concentrations of conidia were determined using a Neubauer hemocytometer. At 48 h after envenomation or the control treatment, larvae (n = 3 per concentration, each replicate consisting of 10 insects; Table S1) were dipped for 10 s in suspensions containing concentrations ranging from 101 to 109 conidia ml-1. The control consisted of dipping in a conidia-free water–Tween-80 solution. After dipping, the larvae were dried for 10 s on filter paper and placed in 9-cm-diameter Petri dishes lined with moistened filter paper. The Petri dishes were incubated at 26 °C in the dark, and mortality was assessed for 8 days. Cadavers were placed in Petri dishes with filter paper moistened with sterile water to observe mycelial growth and to confirm death due to M. robertsii.
Statistical analysis
Normality of data distribution was assessed using the Shapiro-Wilk W test. Normally distributed data were analyzed using a two-tailed Student’s t-test, and not normally distributed data were analyzed using the Mann-Whitney U test. Half-lethal concentrations (LC50) of M. robertsii conidia were calculated using the Spearman-Karber test. Principal component analysis (PCA) of the lipid composition was performed using the variance-covariance matrix. Hydrocarbon and fatty acid amounts were considered in one total matrix. We extracted only the first and second components which explained 82% of the total variance for relative units (μg mg-1 extract) and 95% of total variance for absolute units (μg per larva). The data were visualized using scatterplots and biplots. Analyses were performed with STATISTICA v.8 (StatSoft, Tulsa, OK, USA) and PAST v.3 software (Hammer et al., 2001).
RESULTS
General parameters
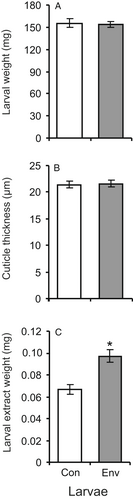
At 48 h after envenomation by H. brevicornis, the treated wax moth larvae had a similar weight as the control larvae (t = 0.25, d.f. = 8, P = 0.80; Figure 1A). Also the cuticle thickness did not change (t = 0.21, d.f. = 30, P = 0.84; Figure 1B). However, the weight of epicuticular extracts from envenomated larvae was 1.5-fold greater than that of control larvae (t = 4.2, d.f. = 8, P = 0.003; Figure 1C).
Changes in hydrocarbon and fatty acid compositions
Linear long-chain saturated and unsaturated cuticular hydrocarbons C16-C33 were detected in epicuticular extracts, and C27:0, C29:0, C31:0, C31:1, and C33:1 were the most abundant (Table 1). Hydrocarbons over C33 were not detected when analyzing samples at both 280 and 310 °C. Among FAMEs, saturated and unsaturated chains C12-C34 were detected, with the highest amounts of C16:0, C16:1, C18:0, C18:1, and C18:2. In addition, cholesterol, ω-1 hydroxy-, β-hydroxy-, and oxo-FAMEs C14-18 were detected in the extracts.
| Compound | LRI | Relative amount (μg per mg extract) (mean ± SE) | t (d.f. = 8) | Z (n = 5) | P | Absolute amount (μg per larva) (mean ± SE) | t (d.f. = 8) | Z (n = 5) | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Envenomated | Control | Envenomated | |||||||||||
| Saturated hydrocarbons | 1 | Hexadecane | 1600 | C16:0 | 0.27 ± 0.03 | 0.40 ± 0.09 | 1.33 | 0.22 | 0.02 ± 0.00 | 0.04 ± 0.01 | 2.69 | 0.028 | ||
| 2 | Heptadecane | 1700 | C17:0 | 0.58 ± 0.15 | 0.48 ± 0.14 | 0.48 | 0.64 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.47 | 0.65 | |||
| 3 | Pentacosane | 2500 | C25:0 | 1.89 ± 0.22 | 2.59 ± 0.72 | 0.93 | 0.38 | 0.13 ± 0.02 | 0.24 ± 0.05 | 2.05 | 0.075 | |||
| 4 | Hexacosane | 2600 | C26:0 | 0.67 ± 0.09 | 1.05 ± 0.15 | 2.20 | 0.059 | 0.04 ± 0.01 | 0.10 ± 0.01 | 3.68 | 0.0062 | |||
| 5 | Heptacosane | 2700 | C27:0 | 13.95 ± 1.08 | 9.63 ± 0.60 | 3.50 | 0.0081 | 0.93 ± 0.08 | 0.92 ± 0.04 | 0.02 | 0.98 | |||
| 6 | Nonacosane | 2900 | C29:0 | 12.17 ± 1.33 | 12.92 ± 2.10 | 0.30 | 0.77 | 0.81 ± 0.09 | 1.21 ± 0.12 | 2.75 | 0.025 | |||
| 7 | Hentriacontane | 3100 | C31:0 | 9.07 ± 1.40 | 23.61 ± 4.60 | 3.02 | 0.017 | 0.60 ± 0.08 | 2.19 ± 0.29 | 5.24 | 0.0008 | |||
| 8 | Tritriacontane | 3300 | C33:0 | 1.37 ± 0.17 | 5.01 ± 1.09 | 3.31 | 0.011 | 0.09 ± 0.01 | 0.47 ± 0.08 | 4.41 | 0.0023 | |||
| Total | 39.97 ± 3.74 | 55.70 ± 8.75 | 1.65 | 0.14 | 2.65 ± 0.25 | 5.22 ± 0.48 | 4.76 | 0.0014 | ||||||
| Unsaturated hydrocarbons | 9 | Heptadecene | 1693 | C17:1 | 1.03 ± 0.22 | 0.96 ± 0.16 | 0.27 | 0.79 | 0.07 ± 0.01 | 0.09 ± 0.02 | 1.23 | 0.25 | ||
| 10 | Hentriacontene | 3073, 3080 | C31:1 | 11.67 ± 1.74 | 14.72 ± 3.98 | 0.70 | 0.50 | 0.78 ± 0.13 | 1.34 ± 0.27 | 1.87 | 0.098 | |||
| 11 | Tritriacontene | 3277 | C33:1 | 36.62 ± 2.66 | 60.22 ± 13.62 | 1.70 | 0.13 | 2.43 ± 0.23 | 5.54 ± 0.87 | 3.46 | 0.0086 | |||
| 12 | Tritriacontadiene | 3253 | C33:2 | 2.12 ± 0.17 | 2.43 ± 0.48 | 0.61 | 0.56 | 0.14 ± 0.02 | 0.23 ± 0.03 | 2.40 | 0.043 | |||
| Total | 51.43 ± 4.54 | 78.32 ± 17.99 | 1.45 | 0.19 | 3.42 ± 0.37 | 7.20 ± 1.15 | 3.12 | 0.014 | ||||||
| Total hydrocarbons | 91.40 ± 5.07 | 134.02 ± 26.54 | 1.58 | 0.15 | 6.07 ± 0.48 | 12.42 ± 1.57 | 3.83 | 0.0050 | ||||||
| Sterols | 13 | Cholesterol | 3088 | 2.24 ± 0.45 | 0.57 ± 0.24 | 3.24 | 0.020 | 0.15 ± 0.03 | 0.06 ± 0.03 | 2.34 | 0.047 | |||
| Saturated fatty acid methyl esters (FAMEs) | 14 | Dodecanoic | 1526 | C12:0 | 1.03 ± 0.18 | 0.51 ± 0.05 | 2.76 | 0.025 | 0.07 ± 0.02 | 0.05 ± 0.00 | 1.50 | 0.17 | ||
| 15 | Tridecanoic | 1626 | C13:0 | 0.14 ± 0.04 | 0.06 ± 0.01 | 0.75 | 0.45 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.23 | 0.82 | |||
| 16 | Tetradecanoic | 1730 | C14:0 | 8.35 ± 1.52 | 4.54 ± 0.66 | 2.30 | 0.050 | 0.57 ± 0.13 | 0.43 ± 0.05 | 1.03 | 0.34 | |||
| 17 | Pentadecanoic | 1831 | C15:0 | 3.06 ± 0.76 | 2.47 ± 0.20 | 0.76 | 0.47 | 0.21 ± 0.06 | 0.24 ± 0.02 | 0.44 | 0.67 | |||
| 18 | Hexadecanoic | 1933 | C16:0 | 148.18 ± 12.0 | 162.35 ± 8.97 | 0.95 | 0.37 | 10.03 ± 1.43 | 15.74 ± 1.29 | 2.96 | 0.018 | |||
| 19 | Heptadecanoic | 2028 | C17:0 | 2.58 ± 0.41 | 2.74 ± 0.61 | 0.21 | 0.84 | 0.18 ± 0.04 | 0.26 ± 0.06 | 1.19 | 0.27 | |||
| 20 | Octadecanoic | 2131 | C18:0 | 33.88 ± 3.50 | 42.21 ± 5.26 | 1.67 | 0.095 | 2.30 ± 0.38 | 3.99 ± 0.25 | 3.74 | 0.0057 | |||
| 21 | Eicosanoic | 2330 | C20:0 | 3.40 ± 0.56 | 4.80 ± 0.52 | 1.84 | 0.10 | 0.23 ± 0.05 | 0.47 ± 0.06 | 2.94 | 0.019 | |||
| 22 | Docosanoic | 2545 | C22:0 | 3.15 ± 0.21 | 2.87 ± 0.53 | 0.49 | 0.64 | 0.21 ± 0.01 | 0.28 ± 0.06 | 1.20 | 0.26 | |||
| 23 | Tetracosanoic | 2731 | C24:0 | 10.32 ± 0.91 | 4.36 ± 0.60 | 2.51 | 0.012 | 0.70 ± 0.10 | 0.41 ± 0.03 | 2.20 | 0.028 | |||
| 24 | Hexacosanoic | 2933 | C26:0 | 5.63 ± 0.48 | 3.07 ± 0.60 | 3.33 | 0.010 | 0.38 ± 0.05 | 0.29 ± 0.03 | 1.52 | 0.17 | |||
| 25 | Octacosanoic | 3133 | C28:0 | 3.60 ± 0.65 | 1.38 ± 0.08 | 3.38 | 0.0096 | 0.24 ± 0.05 | 0.13 ± 0.01 | 2.09 | 0.070 | |||
| 26 | Triacontanoic | 3337 | C30:0 | 4.78 ± 0.81 | 3.78 ± 0.48 | 1.07 | 0.31 | 0.33 ± 0.07 | 0.36 ± 0.05 | 0.42 | 0.68 | |||
| 27 | Dotriacontanoic | 3541 | C32:0 | 3.27 ± 0.61 | 4.24 ± 0.85 | 0.63 | 0.53 | 0.22 ± 0.05 | 0.39 ± 0.06 | 2.22 | 0.057 | |||
| 28 | Tetratriacontanoic | 3746 | C34:0 | 3.19 ± 0.39 | 2.90 ± 0.27 | 0.63 | 0.55 | 0.21 ± 0.03 | 0.28 ± 0.02 | 1.80 | 0.11 | |||
| Total | 234.57 ± 18.80 | 242.28 ± 13.59 | 0.33 | 0.75 | 15.90 ± 2.30 | 23.32 ± 1.25 | 2.83 | 0.022 | ||||||
| Unsaturated FAMEs | 29 | Tetradecenoic | 1703 | C14:1 | 0.96 ± 0.37 | 0.36 ± 0.05 | 1.60 | 0.15 | 0.06 ± 0.03 | 0.03 ± 0.00 | 0.21 | 0.83 | ||
| 30 | 9-Tetradecenoic (n5) | 1713 | C14:1 | 0.40 ± 0.06 | 0.15 ± 0.03 | 4.05 | 0.0037 | 0.03 ± 0.00 | 0.01 ± 0.00 | 2.02 | 0.043 | |||
| 31 | Pentadecenoic | 1807 | C15:1 | 0.92 ± 0.34 | 0.39 ± 0.07 | 1.51 | 0.17 | 0.06 ± 0.02 | 0.04 ± 0.00 | 0.93 | 0.38 | |||
| 32 | Hexadecenoic | 1903 | C16:1 | 11.18 ± 3.33 | 5.80 ± 0.24 | 1.61 | 0.15 | 0.75 ± 0.24 | 0.56 ± 0.04 | 0.79 | 0.45 | |||
| 33 | 9-Hexadecenoic (n7) | 1909 | C16:1 | 22.64 ± 2.97 | 17.04 ± 3.08 | 1.67 | 0.095 | 1.53 ± 0.28 | 1.69 ± 0.37 | 0.34 | 0.75 | |||
| 34 | Heptadecenoic | 2024 | C17:1 | 9.26 ± 0.85 | 10.65 ± 0.96 | 0.63 | 0.53 | 0.63 ± 0.10 | 1.04 ± 0.13 | 2.56 | 0.034 | |||
| 35 | 9-Octadecenoic (n9) | 2102 | C18:1 | 239.77 ± 16.38 | 227.33 ± 12.72 | 0.21 | 0.83 | 16.14 ± 2.07 | 22.14 ± 2.05 | 1.88 | 0.060 | |||
| 36 | Octadecadienoic | 2106 | C18:2 | 3.37 ± 0.27 | 2.11 ± 0.09 | 4.52 | 0.0020 | 0.23 ± 0.03 | 0.20 ± 0.01 | 0.21 | 0.83 | |||
| 37 | 9,12-Octadecadienoic (n6) | 2095 | C18:2 | 23.38 ± 2.31 | 18.82 ± 1.44 | 1.68 | 0.13 | 1.57 ± 0.21 | 1.84 ± 0.22 | 0.89 | 0.40 | |||
| 38 | 9-Eicosenoic (n11) | 2300 | C20:1 | 4.97 ± 1.38 | 7.64 ± 1.92 | 1.13 | 0.29 | 0.35 ± 0.11 | 0.72 ± 0.17 | 1.76 | 0.12 | |||
| Total | 316.87 ± 21.75 | 290.29 ± 17.80 | 0.94 | 0.37 | 21.36 ± 2.76 | 28.29 ± 2.78 | 1.77 | 0.11 | ||||||
| Hydroxy- and oxo-FAMEs | 39 | 13-Hydroxytetradecanoic1 | 1936 | C14:0 13-OH | 26.94 ± 3.74 | 1.30 ± 0.22 | 2.51 | 0.012 | 1.79±0.29 | 0.13±0.03 | 5.74 | 0.0004 | ||
| 40 | 15-Hydroxyhexadecanoic | 2136 | C16:0 15-OH | 20.93 ± 2.59 | 2.40 ± 0.28 | 7.1 | 0.0001 | 1.41 ± 0.23 | 0.24±0.04 | 4.96 | 0.0011 | |||
| 41 | 3-Hydroxyoctadecanoic2 | 2281 | C18:0 3-OH | 20.84 ± 4.39 | 16.84 ± 4.82 | 0.84 | 0.40 | 1.46 ± 0.41 | 1.69 ± 0.53 | 0.34 | 0.75 | |||
| 42 | 15-Oxohexadecanoic | 2134 | C16:0 15-O | 16.31 ± 2.45 | 0.90 ± 0.13 | 6.28 | 0.0002 | 1.10 ± 0.22 | 0.09 ± 0.01 | 4.63 | 0.0017 | |||
| Total | 85.00 ± 10.11 | 21.42 ± 4.91 | 5.66 | 0.0005 | 5.76 ± 1.03 | 2.14 ± 0.55 | 2.51 | 0.012 | ||||||
| Total fatty acids | 636.47 ± 46.50 | 554.00 ± 27.98 | 1.04 | 0.30 | 43.02 ± 5.94 | 53.74 ± 4.25 | 1.46 | 0.14 | ||||||
- 1 Mass spectra of FAMEs C14:0 13-OH and C16:0 15-OH from peaks in the GC-MS chromatogram are presented in Figure S4. Trace amounts of ω-1 hydroxy-derivatives of FAMEs C12:0, C18:0, and C20:0 have also been identified. 2Trace amounts of β-hydroxy-derivatives of FAMEs C16:0, C20:0, and C22:0 acids have also been identified (Figure S5). LRI = linear retention index. Each replicate includes 45 larvae.
Parasitoid venom decreased the FAME:hydrocarbon ratio in the wax moth epicuticle from 7:1 (control) to 4:1 (envenomated larvae), at a level of marginal significance (t = 2.1, d.f. = 8, P = 0.07). The total hydrocarbon amount in envenomated compared to control larvae increased 1.5-fold when calculated as relative units (μg per mg extract) and 2.1-fold when calculated as the absolute amount (μg per larva). Trends of an increase in the amounts of long-chain hydrocarbons were visible on chromatograms (Figures S2–S3). In terms of relative units (μg per mg extract), significant increases were registered for C31:0 and C33:0. In the case of absolute amounts (μg per larva), significant increases were observed for C16:0, C26:0, C29:0, C31:0, C33:0, C33:1, and C33:2 (Table 1). The ratio of unsaturated:saturated hydrocarbons (1.34–1.36) was not changed after envenomation (t = 0.07, d.f. = 8, P = 0.94).
The total FAME amount was not significantly altered by parasitoid envenomation; however, alterations in the FAME composition were detected (Table 1). In particular, trends or significant decreases were observed for the relatively short-chain FAMEs C12:0, C14:0, and C14:1 and for the very long-chain FAMEs C24:0, C26:0, and C28:0. In contrast, the amounts of long-chain FAMEs C16-C20 (C16:0, C17:1, C18:0, and C20:0) were significantly increased or tended to increase after envenomation. A notably significant increase was registered for the total amounts of saturated, but not unsaturated fatty acids. The ratio of unsaturated:saturated FAMEs (excluding hydroxyl- and oxo-FAMEs) was 1.4 in control larvae and 1.2 in envenomated larvae; however, the differences were not significant (t = 2.00, d.f. = 8, P = 0.09).
Importantly, the amounts of ω-1 hydroxy- and oxo-FAMEs were substantially decreased after envenomation (Table 1). In particular, the relative amount of ω-1 C14:0 13-OH decreased 21-fold (μg per mg extract), and the absolute amount decreased 14-fold (μg per larva). The relative amounts of hydroxy-FAME ω-1 C16:0 15-OH decreased 9-fold, the absolute amount decreased 6-fold, and the relative and absolute amounts of oxo-FAME ω-1 C16:0 15-O decreased 18- and 13-fold, respectively. The amount of β-hydroxy-FAME C18:0 3-OH did not change significantly.
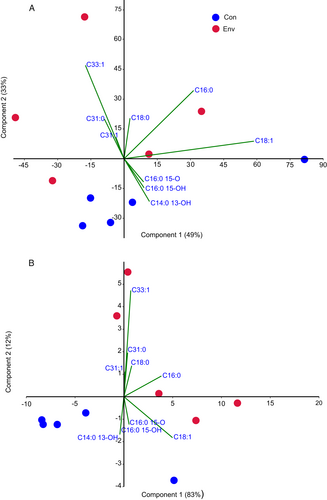
Principal component analysis showed clear clusters of lipid compositions obtained from control and envenomated larvae (Figure 2), which were observed for both the relative amount (μg per mg of extract; Figure 2A) and the absolute amount (μg per larva; Figure 2B). The main factors contributing to the discrimination between the lipid compositions were hydrocarbons C31.0, C31.1, and C33.1, and saturated fatty acids C16.0 and C18.0, which were elevated in extracts of envenomated larvae, as well as all ω-1 hydroxy- and oxo-FAMEs, which were decreased in extracts of envenomated larvae.
Total carbon and nitrogen amounts in the cuticle
Envenomation of wax moth larvae by H. brevicornis led to slight but significant increases in the C and N amounts in the cuticle after 48 h (Table 2). The C amount increased 1.05-fold and the N amount increased 1.06-fold compared to the control. The C:N ratio was slightly (1.01-fold) and significantly decreased in the cuticle of envenomated larvae compared to the control.
| Control | Envenomated | t1 (d.f. = 4) | P | |
|---|---|---|---|---|
| C (%) | 43.90 ± 0.20 | 45.99 ± 0.46 | 4.2 | 0.01 |
| N (%) | 10.63 ± 0.05 | 11.26 ± 0.13 | 4.7 | 0.01 |
| C:N ratio | 4.13 ± 0.01 | 4.08 ± 0.01 | 4.6 | 0.01 |
- 1 Student’s t-test.
pH of cuticular water extract
The pH of the cuticular water extract in control larvae was close to neutral (6.8). In envenomated larvae, the pH of the extract increased slightly (6.9) (t = 3.9, d.f. = 4, P = 0.018).
Bacterial colony-forming unit counts on the insect surface
Plating wax moth cuticular rinses on culture media revealed a substantial increase in the CFU count from envenomated larvae relative to the control (Figure 3): on LB agar it was increased 5-fold and 23-fold on bile esculin azide agar was increased 23-fold (Mann-Whitney U test: Z = 2.7, n = 8, P = 0.01 and Z = 3.1, n = 10, P = 0.006 correspondently). The CFU count on endo agar was relatively low, 2×103 per control larva, and no colonies from envenomated larvae were detected. The whole experiment was conducted independently 2×, and the results were consistent.

16S rRNA gene sequencing of selected isolates showed that the predominant species that grew on LB agar from control and envenomated larvae were bacilli Enterococcus faecalis (Andrewes & Horder) Schleifer & Kilpper-Bälz and Staphylococcus spp. (Table S2). One isolate of Bacillus thuringiensis Berliner was also detected in the cuticular rinses of control larvae. Bacteria isolated from bile esculin azide agar included E. faecalis (in control larvae), and E. faecalis and Enterococcus casseliflavus (Vaughan et al.) Collins et al. (in envenomated larvae). From endo agar, we isolated only Enterobacter spp., which were closest to Enterobacter cancerogenus (Urosevic) Dickey & Zumoff and Enterobacter bugandensis Doijad et al. All isolates of enterobacteria were obtained only from control larvae (Table S2). Thus, an increase in the CFU count of Gram-positive bacteria (Enterococcus spp.) was observed on the surface of wax moth larvae envenomated by the parasitoid.
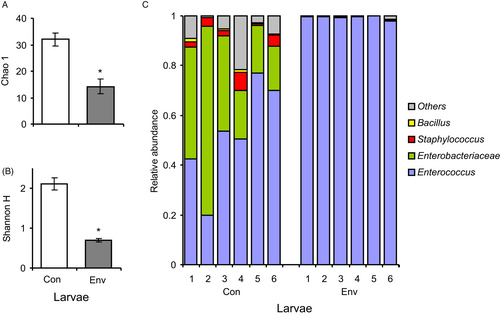
Metagenomic analysis of bacterial communities
The results of the metagenomic analysis of surface bacteria correlated well with the data obtained from culture methods. We registered 140 ASVs, and the more abundant ASVs belonged to Enterococcus, unclassified Enterobacteriaceae, and Staphylococcus. Notably, the two most abundant ASVs belonging to Enterococcus showed 99.8-100% similarity only with E. faecalis. Changes in the bacterial community were observed in envenomated larvae (Figure 4), as indicated by a substantial decrease in the diversity indices due to an increase in the abundance of Enterococcus (Figure 4C). The Chao and Shannon indices decreased 2–3-fold in envenomated larvae compared to the control (Mann-Whitney U test: Z>2.8, P<0.005; Figure 4A,B). The relative abundance of Enterococcus increased 2-fold after envenomation (Z = 2.8, P = 0.005), reaching approximately 100% of the relative abundance. Enterobacteria almost completely disappeared in envenomated insects (a 270-fold decrease compared to the control; Z = 2.8, P = 0.005). In addition, the envenomated larvae showed a decrease in the abundance of Staphylococcus (Z>2.8, P<0.005) and other taxa with low abundance. Thus, the data confirm a substantial increase in the proliferation of Enterococcus on the wax moth cuticle after envenomation.
Changes in fungal virulence and development
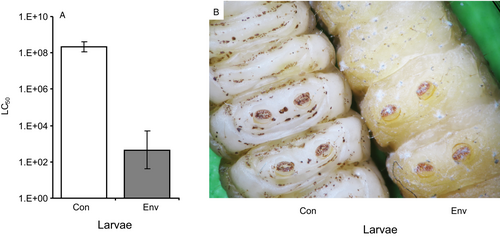
Envenomated larvae exhibited a greater susceptibility to M. robertsii than non-envenomated larvae. In particular, the LC50 value for non-envenomated larvae was 2×108 conidia ml-1, whereas it was 400 conidia ml-1 for envenomated larvae; i.e., the susceptibility to the fungus increased 505 000-fold after envenomation (Figure 5A). We also observed a change in the behavior of the fungus on envenomated larvae after infection with high concentrations (>107 conidia ml-1): in addition to growing inside the cuticle (as in the control insects), the fungus simultaneously formed aerial mycelium and conidia on the cuticle (Figure 5B). This phenomenon was observed starting on day 2 or 3 after inoculation. Colonization of the hemocoel by hyphal bodies and formation of sclerotia (dense entanglements of hyphae within the cadavers) were observed in both non-envenomated and envenomated larvae.
DISCUSSION
The envenomation of wax moth larvae by H. brevicornis causes lysis of fat body cells by controlled necrotic death (necroptosis) and the release of lipids, resulting in the hyperlipidemia of the hemolymph (Kryukova et al., 2020). In addition, larval hemolymph becomes richer in amino acids and carbohydrates after envenomation by Habrobracon spp. (Becchimanzi et al., 2017; Kryukova et al., 2020). Moreover, envenomation of the host by the parasitoid leads to changes in gut microbiota and the development of spontaneous infection (Polenogova et al., 2019). In the present study, we showed that parasitoid envenomation leads to changes in the lipid content and microbiota structure, not only in the host’s internal tissues, but also in the cuticle. We established that envenomation of wax moth larvae by H. brevicornis causes epicuticular hyper-lipidation, increases hydrocarbon amount, and changes the fatty acid composition. In addition, the cuticle became more hospitable to bacteria and fungi. An increase in the bacterial load on the insect surface, and a substantial increase in the susceptibility of the larvae to the fungus M. robertsii and modifications in the development of this pathogen were observed. We discuss the possible physiological mechanisms of the changes in the cuticle and their impact on the development of microbiota.
After envenomation, the weight of the lipid epicuticular extract of G. mellonella was increased 1.5-fold. It is likely that high lipid amounts along with insect immobilization can promote the adhesion of microorganisms, particularly EPF. Removal of cuticular lipids by solvents leads to a decrease in the adhesion of Metarhizium anisopliae sensu lato (Metschn.) Sorokīn conidia to the cuticle of the bug Nezara viridula L. (Sosa-Gomez et al., 1997). A positive correlation between hydrocarbon amount and the level of conidial adhesion was observed in the M. robertsii–Colorado potato beetle (Leptinotarsa decemlineata Say) system (Tomilova et al., 2019). An increase in lipid amounts could also exert a positive effect on bacterial adhesion (see below).
An increase in hydrocarbon amount was observed after envenomation, and the greatest increases were detected for C31:0 and C31:1. As reviewed by Blomquist (2010), the synthesis of hydrocarbons occurs in oenocytes associated with the epidermal layer of integuments and fat body periphery. Hydrocarbons are transported through the hemolymph by lipophorin to epidermal cells; however, the mechanisms by which they cross through the integument are insufficiently understood (Bagnères & Blomquist, 2010). Habrobracon venom disrupts fat body cells and different types of hemocytes (Kryukova et al., 2015, 2020), which may lead to the release of hydrocarbons to the hemolymph. We speculate that elevated hydrocarbon amounts in the epicuticle of envenomated larvae were due to the incomplete cessation of lipid transport. Hydrocarbons may promote the differentiation of fungal infection structures. For example, straight-chain hydrocarbons С24–С36 strongly stimulated the germination of Metarhizium acridum (Driver & Milner) J.F. Bisch., S.A. Rehner & Humber conidia in vitro, and treatment of the host locust cuticle with pentane (to remove non-polar cuticular compounds) decreased the level of germination (Jarrold et al., 2007). Extracts of Colorado potato beetle epicuticle containing mainly alkanes C25–C38 promoted germination of M. robertsii, M. brunneum, and Metarhizium pemphigi (Driver & Milner) Kepler, Humber & Rehner (Tyurin et al., 2016; Tomilova et al., 2019). A wax moth line susceptible to M. brunneum was characterized by elevated epicuticular alkane and alkene amounts and a high rate of conidial germination on the cuticle compared to a resistant line (Grizanova et al., 2019). In addition, an n-hexane epicuticular extract of wax moth larvae enhanced germination of B. bassiana, and the fungus displayed increased germination on the extract from envenomated compared to non-envenomated larvae (Kryukov et al., 2018a). Thus, an increase in hydrocarbon amounts in envenomated larvae might explain their increased susceptibility to fungi.
The observed composition of wax moth fatty acids was consistent with previous studies (Gołebiowski et al., 2008, 2021; Guil-Guerrero et al., 2018; Wrońska et al., 2018; Kazek et al., 2019). In particular, the prevalence of С16:0, С18:1, and С18:0 was reported previously. Some minor differences were related to fatty acids present in low amounts, which may be caused by different methodologies and GC-MS apparatuses, population variations, or different diets (Kazek et al., 2019). However, we detected ω-1 hydroxy-, oxo-, and β-hydroxy-fatty acids in this study, which has not been reported by other authors. We showed that the total amount of fatty acids in the wax moth epicuticle did not change after envenomation; however, the ratios between various groups of fatty acids were altered. The amounts of fatty acids with relatively short chains (С12-С14) and very long chains (С24-С28) were decreased, whereas the С16-С20 amounts tended to increase. Saturated and unsaturated fatty acids С12–С14 may exhibit stronger antifungal properties than С16–С20, as shown for the human pathogen Candida sp. (Clement et al., 2007) and the insect pathogen С. fumosorosea (Saito & Aoki, 1983). In particular, short-chain fatty acids (from C6:0 to C14:0) inhibited conidial germination to a greater extent than C16–C24; in addition, long-chain saturated and unsaturated fatty acids (C14–C24) increased the dry biomass of С. fumosorosea fungus in vitro (Saito & Aoki, 1983). Elevated amounts of the saturated and unsaturated fatty acids С16–С18 in wax moth epicuticles correlated positively with susceptibility to the fungus C. coronatus (Gołebiowski et al., 2008). Kazek et al. (2019) showed that a wax moth line fed a semi-natural diet, which increased susceptibility to C. coronatus, was characterized by elevated amounts of 16:0 but decreased amounts of very-long-chain fatty acids (С21–С26) compared to the same line fed a natural diet, which increased resistance. This is consistent with the effects observed here: an increase in 16:0 and a decrease in very-long-chain fatty acid amounts in larvae susceptible to fungi (i.e., envenomated larvae). We also observed a slight decreasing trend in the ratio of unsaturated:saturated fatty acids in envenomated larvae. This change might also affect the fungistatic properties of the epicuticle, because unsaturated fatty acids exhibit stronger antifungal activities than saturated fatty acids (Pohl et al., 2011).
We also detected a substantial decrease in the amounts of ω-1 hydroxy- and oxo-fatty acids after envenomation. The oxidation of fatty acids to their ω-hydroxy forms is catalyzed by the cytochrome P450 enzyme system (Miura, 2013). We hypothesize that a shift toward anaerobic metabolism and disturbance in the P450 system in envenomated wax moth larvae might lead to a decrease in ω-1 hydroxy-acid amounts. Deregulation of P450 gene expression and a decrease in lipid metabolism were reported previously in Sarcophaga spp. in response to N. vitripennis venom (Danneels et al., 2013; Mrinalini et al., 2014). The switch toward anaerobic metabolism in G. mellonella after envenomation by H. brevicornis is shown by the inhibition of the Krebs cycle through metabolomic analysis (N Kryukova & Yu Tsentalovich, unpubl.). Hydroxy- and oxo-fatty acids are chemically unstable compounds with a high viscosity and reactivity compared to other types of fatty acids (Pohl et al., 2011). Their effects on EPF have not been studied; however, pronounced fungicidal properties of these compounds against a wide range of human and plant mycopathogens are reported (Pohl et al., 2011). Sjögren et al. (2004) proposed that hydroxy-fatty acids increase the permeability of fungal membranes and release intracellular electrolytes and proteins. The activity of hydroxy-fatty acids against Gram-positive bacteria was also reported (Shin et al., 2004). Theoretically, decreases in hydroxy- and oxo-fatty acid amounts in envenomated larvae might increase susceptibility to EPF and increase bacterial proliferation. The localization of hydroxy- and oxo-fatty acids in wax moth larvae and their effects on EPF require further study.
We observed slight but significant increases in the total N and C amounts in the wax moth cuticle after envenomation. It is likely that increased N amounts are associated with increases in protein and free amino acid amounts in the internal tissues of the larvae. Parasitoid venoms increase protein and free amino acid amounts in the whole body or hemolymph of insects, as shown in the host-parasitoid systems N. vitripennis–S. bullata (Rivers & Denlinger, 1994) and B. nigricans–S. littoralis (Becchimanzi et al., 2017). This increase is proposed to occur due to a blockade of the tricarboxylic acid cycle, leading to disrupted protein synthesis and the destruction of host cells. It is possible that increased levels of N-containing compounds in the wax moth cuticle exerted a positive effect on both the development of the fungus and the proliferation of bacteria.
We showed that the bacterial community of the wax moth surface was composed of enterococci, enterobacteria, and staphylococci, which is consistent with previous assays (Allonsius et al., 2019). There was a substantial decrease in bacterial diversity and an increase in the abundance of aerobic Gram-positive bacteria – enterococci (mostly E. faecalis) – on the surfaces of envenomated larvae. It is possible that an increase in bacterial proliferation was caused by hyperlipidemia of the epicuticle and an increase in the N amount. Enterococcus and Staphylococcus bacilli attach to hydrophobic substrates, form biofilms, and degrade hydrocarbons (Toledo-Arana et al., 2001; Solyanikova & Golovleva, 2019). We also hypothesize that decreases in the ratios of fatty acids/hydrocarbons and unsaturated/saturated fatty acids and in hydroxyl- and oxo-fatty acid amounts after envenomation might exert a positive effect on E. faecalis proliferation. Carson & Daneo-Moore (1980) showed that saturated fatty acids (C16:0 and C18:0) had little or no effect on the growth of whole cells or protoplasts of E. faecalis; however, unsaturated fatty acids (C18:1 and C18:2) induced the lysis of whole cells and protoplasts. The authors suggested that unsaturated fatty acids are more effective membrane destabilizers of E. faecalis than saturated fatty acids. In contrast, Saito et al. (2017) showed that unsaturated fatty acids were less toxic to E. faecalis than saturated fatty acids. It is likely that the sensitivity depends on the strain or environment. However, overall, unsaturated fatty acids tend to have greater efficacy against various bacteria than saturated fatty acids (Desbois & Smith, 2010). A slight change in the pH of the wax moth cuticle after envenomation (from 6.8 to 6.9) could not lead to a significant impact on the proliferation of bacteria because E. faecalis and Enterobacter spp. are capable of growing in a wide range of pH values, from approximately 4 to 10 (e.g., Nakajo et al., 2006; Azis et al., 2019; Chantarasiri, 2020). Notably, conclusions about the effect of pH as well as C and N amounts on the development of microorganisms may have limitations due to the low sample size (three replicates) and the analysis of the whole cuticle but not its surface layer.
Our understanding of why active proliferation on the epicuticle was observed specifically for Enterococcus but not for other aerobic bacteria is insufficient. The proliferation of Enterococcus is most likely related to native wax moth microbiota, and the impact of contamination by Habrobracon attack and oviposition seems to be negligible. The bacterial community of the studied Habrobracon laboratory population is dominated by Wolbachia, whose relative abundance is >90% (N Kryukova, MR Kabilov & VY Kryukov, unpubl.). Notably, in envenomated larvae, we detected a few reads of Wolbachia (see dataset). The phenomenon of increased enterococci proliferation probably occurred due to the initially higher density of these bacteria on the wax moth surface. Interestingly, the opposite situation was observed in the midgut of envenomated wax moth larvae; in particular, enterococci were replaced by facultative anaerobic enterobacteria that may be caused by the cessation of peristalsis (Polenogova et al., 2019). Thus, envenomation leads to increased proliferation of different groups of bacteria in various tissues, which correlates with the upregulation of antimicrobial peptide genes in hosts observed in different host-parasitoid systems (Martinson et al., 2014; Polenogova et al., 2019).
The substantial increase in the susceptibility of envenomated larvae to fungal infection was consistent with our previous findings (Kryukov et al., 2013, 2018a,b). Larger differences in susceptibility between envenomated and control larvae were found in the current study after infection with M. robertsii than in the previous study after infection with B. bassiana (500 000- vs. 5000-fold, respectively), which were most likely caused by the relatively low virulence of M. robertsii (strain P-72) toward the studied wax moth line (Dubovskiy et al., 2013), i.e., larger differences were observed due to the higher LC50 of M. robertsii conidia for control larvae. Interestingly, M. robertsii was able to form aerial mycelium on envenomated larvae while simultaneously colonizing hemocoel. Previously, we documented a similar phenomenon for the development of phytopathogenic, saprotrophic, and several entomopathogenic fungi on wax moth larvae envenomated by Habrobracon (Kryukov et al., 2017; Figure S6). In particular, Fusarium oxysporum sensu Smith & Swingle and Scopulariopsis brevicaulis Bainier, which were avirulent for non-envenomated larvae, formed aerial mycelium on the cuticle of envenomated larvae, although the colonization of hemocoel by these fungi was not observed. Topical application of envenomated larvae with Lecanicillium muscarium (Petch) Zare & Gams conidia and Cordyceps militaris (L.) Link ascospores also led to aerial mycelium formation at the site of inoculation, simultaneously with the colonization of internal tissues (Figure S6). This alteration in fungal development might be caused by the biochemical modifications of the cuticle and the absence of mechanical action on fungi due to immobilization of larvae. However, the change in fungal development was unlikely to be solely determined by the lipid composition and N amount. Supplementation of nutrient media with lipids usually does not lead to a substantial increase in mycelia and conidia biomasses and can even lead to a decrease in these parameters (Napolitano & Juárez, 1997; Szafranek et al., 2001; Bogus et al., 2010). An increase in the bacterial load might only inhibit and not stimulate fungal growth. As shown in our previous study, E. faecalis inhibits the mycelial growth of the investigated M. robertsii strain (Kryukov et al., 2020). It is possible that changes in fungal development are caused by the increased levels of carbohydrates in the cuticle. A substantial increase in the amounts of carbohydrates in the hemolymph of G. mellonella and S. littoralis after envenomation by Habrobracon has been reported (Becchimanzi et al., 2017; Kryukova et al., 2020). It is well known that carbohydrates stimulate the germination and mycelial growth of entomopathogenic, saprotrophic, and phytopathogenic fungi. However, a quantitative evaluation of carbohydrates in wax moth cuticles requires the development of specific techniques. Notably, in the case of EPF, stopping the circulation of the hemolymph in response to venom could be the cause of simultaneous internal and external growth of hyphae after penetration of the cuticle, but additional histological investigation is required.
The proliferation of bacteria and changes in differentiation of fungal structures might also be mediated by changes in immunity after envenomation, but this topic was outside the scope of the present study. We previously reported inhibitions in a large set of immune reactions in response to Habrobracon venom, including decreases in the activity of phenoloxidases and antioxidant enzymes, the production of reactive oxygen species (ROS), the viability and spreading of hemocytes, and the rate of encapsulation (Kryukova et al., 2011, 2015, 2020; Kryukov et al., 2018a). Inhibition of these systems should lead to a strong increase in susceptibility to EPF (Lu & St Leger, 2016). However, the abovementioned parameters were estimated in the hemolymph, fat body, and hemocytes of envenomated larvae and do not explain the increased bacterial proliferation and fungal growth on the cuticle. It is unknown how immune parameters (e.g., ROS, proteinase inhibitors, and antimicrobial peptides) change in the cuticle after envenomation and whether these changes can lead to an increase in bacterial proliferation and alterations in fungal development. Therefore, the next step in the study of the Habrobracon–wax moth–fungi–bacteria system should be focused on changes of immune parameters in integuments after envenomation by the parasitoid.
CONCLUSIONS
This study analyzed alterations in cuticular lipids and microbial compositions on the insect host surface in response to parasitoid venom. Еnvenomation of wax moth larvae by H. brevicornis causes: (1) epicuticular hyperlipidemia, mainly due to the increases in hydrocarbon and saturated fatty acid amounts; (2) substantial decreases in ω-1 hydroxy- and oxo-fatty acid amounts in the epicuticle; and (3) slight increases in total C and N amounts in the cuticle. These alterations were correlated with changes in the community and development of surface microorganisms, in particular: (1) restructuring of the bacterial microbiota on the insect surface, due to the substantial proliferation of Enterococcus; and (2) modification of M. robertsii development and a substantial increase in larval susceptibility to the fungus. Further investigations may be focused on the immune reactions in the cuticle of envenomated and non-envenomated larvae as well as on the expression of genes related to virulence, lipid metabolism, differentiation of infection structures, and formation of biofilms in microorganisms developing on wax moth larvae with an altered lipid composition.
AUTHOR CONTRIBUTIONS
Vadim Kryukov: Conceptualization (equal); formal analysis (lead); methodology (equal); visualization (lead); writing – original draft (lead); writing – review and editing (lead). Elena I Chernyak: Formal analysis (supporting); investigation (lead); methodology (supporting); visualization (equal); writing – original draft (supporting); writing – review and editing (supporting). Natalia Kryukova: Investigation (equal); methodology (supporting); resources (equal); writing – review and editing (supporting). Maksim Tyurin: Investigation (equal). Anton Krivopalov: Formal analysis (lead); methodology (equal); visualization (supporting); writing – original draft (supporting); writing – review and editing (equal). Olga Yaroslavtseva: Funding acquisition (lead); investigation (supporting); project administration (equal); resources (lead); writing – review and editing (supporting). Igor V. Senderskiy: Investigation (equal); writing – review and editing (equal). Olga Polenogova: Investigation (equal); writing – review and editing (supporting). Elena Zhirakovskaia: Data curation (equal); formal analysis (supporting); investigation (equal); resources (supporting); writing – review and editing (supporting). Viktor V Glupov: Funding acquisition (equal); project administration (equal); resources (equal); writing – review and editing (supporting). Sergey V Morozov: Formal analysis (equal); investigation (supporting); methodology (equal); resources (lead); writing – review and editing (equal).
Acknowledgements
We express our special thanks to Tatyana Marchenko and Evgenia Buntova for their technical assistance. We also thank Yulia M. Deryabina for help with the elemental analysis and Alexander A. Alekseev for consultation in pH measurement.
Funding
The pathogenesis model was developed within the project of the Russian Foundation for Basic Research (project No. 18-04-00335). Analyses of lipid and microbiota compositions were conducted within a project of the Russian Science Foundation (No. 20-74-10043). Maintenance of insect laboratory lines was supported by Federal Fundamental Scientific Research Program (no. 122011800141-7).
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Open Research
Data availability STATEMENT
The sequences of the 16S rRNA regions were deposited in the GenBank database under accession numbers MW672446–MW672463. Experimental row data are presented in the dataset.




