Ecological trade-offs in development and defence in a specialist moth when feeding on four congeneric host plants
Abstract
Herbivore diet breadth has been hypothesised to be driven by plant quality (physiological efficiency hypothesis) or natural enemies (enemy-free space hypothesis). These hypotheses on their own are now known to be insufficient explanations for the range of herbivore diet breadths and more integrative approaches consider how trade-offs and ecological contingencies shape host plant use by herbivores. I carried out laboratory experiments to test whether physiological efficiency and defence against natural enemies trade off when larvae of the ornate bella moth, Utetheisa ornatrix (L.) (Lepidoptera: Erebidae), a genus-specialist pod-boring herbivore, feeds on leaves and pods of four Crotalaria species (Fabaceae). Different components of U. ornatrix physiological efficiency traded off amongst each other and with defence against adult predation by the spider Lycosa erythrognatha Lucas (Araneae: Lycosidae). Crotalaria micans Link provided the highest survival and fast development but low pupal weight and defence. Crotalaria ochroleuca G. Don provided poor survival, slow development, and low defence, but high pupal weight. Crotalaria pallida Aiton provided slow development and intermediate survival and pupal weight, but the best defence. Crotalaria vitellina Ker Gawl. provided high survival, fast development, and efficient defence, but low pupal weight. Compared to leaves, feeding on green seeds provided greater defence against predation, and developmental benefits, independent of host plant species, but there was a large cost in boring into the pod to reach the seeds. I therefore provide evidence that the trade-offs among physiological efficiency components and between physiological efficiency and defence could maintain multiple host plant use in specialist herbivores. I also show that feeding on green seeds incurs a cost through pod-boring but gives the moth benefits in adult defence. Therefore, the evolution of host plant use and pod-boring in insect herbivores could be driven by defence as much as by physiological efficiency.
INTRODUCTION
Understanding herbivore diet breadth is fundamental for understanding the evolution of biodiversity, particularly in the tropics where the diversity of plant-herbivore interactions is believed to be responsible for a large part of global biodiversity (Novotny et al., 2006; Dyer et al., 2007). The study of host plant use by herbivorous insects was initially driven by the comparison between polyphagous and strictly monophagous feeding habits. However, recent studies focussing on intermediate strategies of host plant use have revealed important information about the selective pressures and constraints in the evolution of diet breadth (Singer, 2008; Kelly & Bowers, 2016). Even for herbivores that are highly specialised on a single genus it is important to ask which ecological and evolutionary pressures maintain the use of multiple host plants within the genus. Plant species within a genus are unlikely to be equal as resources or in natural enemy presence and activity, so studying these herbivores will give us insights into whether they suffer similar ecological and evolutionary pressures as occurs for polyphagous herbivores, but on a more restricted taxonomic scale. Therefore, understanding these variations in diet breadth is essential for understanding when and how strict monophagy evolves.
Among the functional hypotheses that have been proposed to explain the evolution of diet breadth in herbivorous insects, the physiological efficiency hypothesis, the enemy-free space hypothesis, and the slow-growth/high-mortality hypothesis have received the most attention, but mixed empirical support (Moran & Hamilton, 1980; Clancy & Price, 1987; Bernays & Graham, 1988; Scriber, 2005; Singer, 2008; Forister et al., 2012). The physiological efficiency hypothesis posits that host plant use is primarily driven by selection for plants that lead to increased fitness through fastest development, largest accumulation of biomass, and highest resulting reproductive investment (Dethier, 1954; Scriber, 1983). The enemy-free space hypothesis on the other hand, predicts that specialization is driven by the use of host plants on which the herbivore suffers lower mortality to natural enemies due to, among other mechanisms, physical refuges or plant-provided defence compounds (Bernays & Graham, 1988; Denno et al., 1990; Stamp, 2001). Finally, the slow-growth/high-mortality hypothesis states that low-quality plants will necessarily subject the herbivore to greater predation through increased development time and host plant specialisation will result from the mutually reinforcing effects of plant quality and natural enemy pressure (Moran & Hamilton, 1980; Clancy & Price, 1987).
Stemming from Price et al.’s (1980) seminal ideas on how the multitrophic environment moulds plant-herbivore interactions, a more integrative approach for understanding herbivore diet breadth is being constructed. This approach considers that plant quality and natural enemy pressure interactively determine herbivore fitness (Singer et al., 2004b). Use of different host plants by herbivores may therefore lead to specific costs and benefits that can often trade off (Singer et al., 2004b; Singer, 2008). These trade-offs are explicitly incorporated into more recent hypotheses such as the tritrophic hypothesis, which predicts that herbivore fitness on different host plants is determined by the interaction between physiological efficiency and natural enemy pressure (Mooney et al., 2012). Thus, multiple host plant use can be maintained if feeding on plants that improve development causes higher mortality to predators, whereas feeding on plants that provide enemy-free space result in developmental costs (Ballabeni et al., 2001; Mira & Bernays, 2002; Singer et al., 2004a,b; Müller & Arand, 2007; Rodrigues et al., 2010; Rodrigues & Freitas, 2013; Murphy & Loewy, 2015; Stead et al., 2021).
Though ecological trade-offs are being incorporated into our understanding of host-plant use, most work has focussed on trade-offs within the same developmental stage. Larval development usually correlates well with adult physiological components, such as the well-established relationship among larval feeding, weight gain, and adult potential fecundity (LaMunyon, 1997; Awmack & Leather, 2002). However, the natural enemy species that attack larvae are often different to those that attack adults and therefore host plants could provide different levels of defence for each life stage. These trade-offs within and among herbivore life stages are likely to cause contingency and context dependence in the benefits and ecological effects of host plant use as mortality of different life stages may vary with changes in natural enemy communities (Martins et al., 2015; Katsanis et al., 2016).
The ornate bella moth, Utetheisa ornatrix (L.) (Lepidoptera: Erebidae), is a lepidopteran herbivore specialised on host plants in the genus Crotalaria (Fabaceae). Utetheisa ornatrix bores into green pods to feed on the developing seeds, which are the best nutritional resource and provide the highest amounts of pyrrolizidine alkaloids (PAs), which the larva sequesters for larval and adult defence and for male pheromone production as an adult (Conner et al., 1981; Rossini et al., 2003; Martins et al., 2015). Large costs of pod-boring have been detected, and the smaller larvae also feed extensively on leaves (Ferro et al., 2006; Magalhães et al., 2017). Furthermore, this moth feeds on many species within the genus Crotalaria, which differ in physical characteristics such as pod size and pod hardness (M Pareja, unpubl.) as well as PA profiles, which may provide different levels of defence (Silva & Trigo, 2002; Flores et al., 2009; Martins et al., 2015). Among the insect herbivores, pod-borers are an understudied group and studying the selective pressures in insects that feed on both leaves and pods will help understand the evolution of endophagy, especially if combined with information on host plant use (Tooker & Giron, 2020).
In this study I tested (1) whether feeding on different Crotalaria host plants leads to ecological trade-offs for U. ornatrix between physiological efficiency of host plant use and adult defence against spider predation. If there were trade-offs between the functional explanations, I expected that the Crotalaria species that provided U. ornatrix with the fastest development, heaviest pupae, and highest survival would also result in the highest susceptibility to spider predation. This would indicate that multiple Crotalaria host plant use by U. ornatrix is maintained by diverging costs and benefits when feeding on each species. Furthermore, as we know that the seeds themselves are a superior resource (Ferro et al., 2006), I tested (2) whether boring through the pod shell to reach the green seeds of the various species incurs a cost to U. ornatrix. If there were a cost to boring into the pods, I expected development would be enhanced when the seeds were presented in open pods that did not require boring. If these costs differ among host plant species, I expected the magnitude of difference between open vs. intact pods to depend on host plant species (i.e., a significant interaction between pod treatment and host plant species).
MATERIALS AND METHODS
Study system
Utetheisa ornatrix is native to the American continent and is specialised on the genus Crotalaria. There are 702 described species of Crotalaria, of which 64 are found in South America (le Roux et al., 2013). Though there is no systematic information on how many species of Crotalaria U. ornatrix can feed on, literature reports indicate that it has been able to feed on all 14 species so far presented in the laboratory, even species introduced from the Paleotropics, but there are no comprehensive studies of host plant use in the field (Sourakov, 2015; Trigo et al., 2017). Females lay their eggs on the first few leaves below the inflorescence, and first instars feed mainly on leaf tissue. From the second larval stadium onwards, the larvae migrate to the inflorescences where they feed on flowers and mainly on green seeds, after boring into the pods (Sourakov, 2015). Larvae are mobile, but the extent to which individual larvae feed on several plant individuals of the same or different species during their development is unclear and likely depends on the amount of foliage and the number of pods available on an individual plant, which varies among species (Sourakov, 2015), as well as on the occurrence of mixed-species stands. Pyrrolizidine alkaloid concentration in larval food has been shown to alter defence levels and male pheromone titres, so these secondary metabolites are associated with components of both larval and adult fitness (Conner et al., 1981; González et al., 1999). Crotalaria species are known to vary as a resource for U. ornatrix, both in nutritional compounds and PA concentration (Martins et al., 2015; Trigo et al., 2017; Verçosa et al., 2019).
Utetheisa ornatrix adults were continuously collected in the field in rural Campinas, São Paulo State, Brazil (22°44′55.3″S, 47°03′19.9″W) in 2009 and 2010. Groups of adults were placed in paper cylinders to oviposit. The larvae that emerged from these eggs were then used directly in experiments.
Four Crotalaria species were used as host plants for U. ornatrix. Crotalaria micans Link and Crotalaria pallida Aiton were chosen because they are two of the most common species in the region, though they occupy slightly different habitats: C. micans is a native shrub that grows to around 2 m in height and thrives in semi-disturbed open habitats (Lorenzi, 2008). Crotalaria pallida is an exotic (Paleotropical) shrub that grows to 0.5–1 m in height and thrives in highly disturbed open habitats such as pastures and roadsides (Lorenzi, 2008). Crotalaria vitellina Ker Gawl. is also a small native shrub (0.5–1 m tall) that occupies more permanent habitats in sandy soils and is therefore more common in humid areas near water (Flores & Tozzi, 2008). Crotalaria ochroleuca G. Don is an introduced Indotropical species, often planted for nematode control and nitrogen fixation as a part of intercrops and crop rotations (Debiasi et al., 2016; de Souza et al., 2019). Crotalaria micans and C. pallida seeds were collected in the region of Campinas. Crotalaria vitellina seeds were collected in the Picinguaba region of the Parque Estadual da Serra do Mar, Ubatuba, São Paulo State (23°21′36.8″S, 44°50′52.7″W). Crotalaria ochroleuca seeds were available from the stock collected by Flores (2004) and kept at the Chemical Ecology Laboratory, UNICAMP (Campinas, SP, Brazil). Seeds were germinated in the laboratory and plants were grown in pots filled with natural (local) unfertilised soil under open conditions at the Universidade Estadual de Campinas. Once these plants set seed, seeds were regularly collected to replenish the seed stock.
To test U. ornatrix defence against predation I used the wolf spider Lycosa erythrognatha Lucas (Araneae: Lycosidae), which is an abundant predator in pastures and meadows of southeastern Brazil. It is not yet known how important predation by wolf spiders is, but U. ornatrix adults spend most of their resting time in the grass (personal observation) so they are likely to come into frequent contact with L. erythrognatha. Furthermore, wolf spiders have been shown to be an excellent model system to test defences against spider predation (Eisner & Eisner, 1991; González et al., 1999). Lycosa erythrognatha individuals were collected at the UNICAMP campus (Campinas, SP, Brazil) during the night using headlamps and placed individually in 5-l glass jars with a 1-cm layer of sand at the bottom. These jars then served as the bioassay arenas for the experiments described below. Spiders were fed twice a week with larvae of the mealworm Tenebrio molitor L. (Coleoptera: Tenebrionidae), reared in our laboratory on a diet of bread and apple, until use in bioassays. Each spider was used in bioassays only once.
General statistical methods
All analysis were carried out in the R programming environment, using R v.4.0.5 (R Core Team, 2021). Linear mixed effects models were fitted using the lmer function of the lme4 package (Bates et al., 2015). Generalised linear mixed effects models were fitted using the glmer function of the same package. Generalised linear models were fitted using the glm function of core R. Model diagnostics were checked using the DHARMa package (Hartig, 2017) and variance inflation was checked using the car and performance packages (Fox & Weisberg, 2019; Lüdecke et al., 2021). Type II ANOVA tables were generated using the lmerTest package (Kuznetsova et al., 2017). Pairwise comparisons and effect size were calculated using the emmeans, pairs, and eff_size functions of the emmeans package (Lenth, 2021).
Development on leaves
To test how larval feeding on leaves of different Crotalaria species affects U. ornatrix performance caterpillars were reared on the four plant species and development time and pupal weight were measured as proxies for performance. Neonate larvae (<24 h old) emerging from eggs from field-collected adults were randomly assigned to a diet treatment: (1) C. micans, (2) C. ochroleuca, (3) C. pallida, or (4) C. vitellina. Each larva was individually placed in a plastic pot (5 cm diameter, 6 cm deep) in a controlled temperature chamber (EletroLab, São Paulo, Brazil) at 25 ± 2 °C and fed with young leaves (defined as the five fully expanded leaves closest to the meristem of a branch) of the corresponding diet treatment. Every other day the leaves were changed, and the pot was cleaned, noting whether the larva was alive. From day 15 onwards the pots were checked daily to determine whether the larvae had pupated. Twenty-four h after entering the pupal stage, the pupa was weighed, as this is known to correlate with adult fecundity and longevity in this species (LaMunyon, 1997; Long & Sourakov, 2017). When the adult emerged, it was sexed.
The experiment was set up in a randomized block design, with four separate blocks, each with a varying number of replicates (the smallest block had eight replicates per treatment and the largest 24).
Statistical analysis
To understand how host plant diet affected U. ornatrix performance I used days to pupation and pupal weight as response variables. For each response variable I fitted a linear mixed effects model to test the main effects of diet treatment and sex, as well as their two-way interaction. The blocking factor was fitted as a random effect. Significance of the fixed effects was tested using an F-test on the ratio of the variance of the fixed effects and the residual variance. Post-hoc pairwise comparisons were carried out using paired t-tests with a Tukey P-value correction. Standardised effect sizes (Cohen’s d) were calculated for comparisons between treatments and for each treatment relative to the overall mean.
For testing how diet treatment affected survival to pupation, I fitted a mixed effects generalised linear model (GLMM) with binomial errors using survival as a binary response variable (survived or died). The model was fitted with the main effect of diet treatment as an explanatory variable and block as a random effect. Significance was tested by removing the treatment term and testing the change in deviance against a χ2 distribution (Crawley, 2013). Odds ratios were calculated to compare the effect sizes of rearing on different plant species. The standardised effect size for each treatment relative to the overall mean was calculated by transforming the odds ratio to Cohen’s d using d = log(OR) × √3/π (Borenstein et al., 2009). For graphical presentation the estimated probability of survival on each plant was calculated from the GLMM. The risk ratio was also calculated (Table S5).
Development on pods
A second experiment was set up to test whether rearing on pods of the four Crotalaria species affects development and survival, as well as to test whether there is a cost to perforating pods to reach the green seeds, which has been shown to be U. ornatrix’s preferred resource (Ferro et al., 2006). The experiment was designed with two factors in a factorial design in two randomised blocks. The first factor was host plant diet and had four levels: (1) C. micans, (2) C. ochroleuca, (3) C. pallida, and (4) C. vitellina. The second factor was pod treatment: pods were presented either (1) intact (closed) and required the larvae to bore into the pod to reach the seeds, or (2) open – pods were opened by pressing the pod to split open the dorsal suture and thus expose the seeds. The open pod shells were presented containing the seeds still attached to the funiculus. This eliminated the larva’s need to perforate the pods, even though the pods were presented along with the seeds. As the seeds were presented still attached to the pod, the seeds did not dry out over 2 days. Thus, the experiment consisted of eight treatments, which were each replicated 4× in each block, giving a total of eight replicates per treatment. The pods used were always green, and always contained green seeds that were close to full size.
First instars (see above) were randomly assigned to a treatment and placed individually in a plastic pot (5 cm diameter, 6 cm deep) in a controlled temperature chamber at 25 ± 2 °C (EletroLab). The larvae were fed on leaves of the corresponding plant diet treatment, grown as described above, through the first and second instars (as occurs in the field). From the third instar onwards, larvae were fed exclusively on pods from the corresponding plant diet treatment. Every other day the leaves/pods were changed, and the pot was cleaned, noting whether the larva was alive. From day 15 onwards the pots were checked daily to determine whether the larvae had pupated. Twenty-four h after entering the pupal stage the pupa was weighed, as this has been shown to correlate with adult fecundity (LaMunyon, 1997). When the adult emerged, it was sexed.
Statistical analysis
To test how host plant diet and pod treatment affected U. ornatrix, I used days to pupation and pupal weight as response variables. For each response variable I fitted a linear mixed effects model to test the main effects of plant diet treatment, pod treatment, and sex, as well as all the two-way interactions among those three factors. The test of the interaction term between plant diet treatment and pod treatment is a test of the hypothesis that the cost of pod boring is species-dependent. Block was fitted as a random effect. The three-way interaction term could not be tested because some three-way combinations of factor levels had single observations. Significance of the fixed effects was tested using an F-test on the ratio of the variance of the fixed effects and the residual variance. Post-hoc pairwise comparisons were carried out using paired t-tests with a Tukey P-value correction. Standardised effect sizes (Cohen’s d) were calculated for between treatment comparisons and for each treatment relative to the overall mean.
For testing how diet treatment and pod penetration affected survival to pupation, I fitted a mixed effects generalised linear model (GLMM) with binomial errors using survival as a binary response variable (survived or died). The model was fitted with the main effects of diet treatment and pod treatment as explanatory variables, as well as block as a random effect. The interaction was left out of the model because, when fitted, the model exhibited severe collinearity and inflation of standard errors. Significance was tested by removing the terms for the explanatory variables and testing the change in deviance against a χ2 distribution (Crawley, 2013). Odds ratios were calculated to compare the effect sizes of rearing on different plant species. For graphical presentation the estimated probability of survival on each plant was calculated from the GLMM. The risk ratio was also calculated (Table S5).
Defence against spider predation
Utetheisa ornatrix is well known for sequestering plant chemical compounds for its own defence (Rossini et al., 2003; Martins et al., 2015). I tested whether feeding on the four host plants affected the defence obtained by U. ornatrix against the wolf spider L. erythrognatha. Furthermore, I reared the larvae on leaves and pods containing green seeds to test whether pods, which have greater concentrations of PAs, confer greater protection.
First instars obtained as described above were randomly assigned to a host plant treatment and a tissue treatment (leaves or pods) and placed individually in a plastic pot (5 cm diameter, 6 cm deep) in a controlled temperature chamber at 25 ± 2 °C (EletroLab). The experiment consisted of a factorial design with two factors, in two randomised blocks. The first factor was host plant diet with four levels: (1) C. micans, (2) C. ochroleuca, (3) C. pallida, and (4) C. vitellina. The second factor was tissue treatment: leaves or pods. For the pod-fed treatment, the larvae were fed on leaves of the corresponding plant diet treatment through the first and second instars (as occurs in the field). From the third instar onwards, larvae were fed exclusively on intact pods from the corresponding plant diet treatment. Every other day the leaves/pods were changed, and the pot was cleaned. Once in the pupal stage, the pupa was placed in a glass vial for adult emergence. Within 24 h of adult emergence adults were sexed and used in bioassays. Due to availability of material from the four plant species and larval mortality, the host plant diet treatment was replicated 24× for C. micans, 16× for C. ochroleuca, 13× for C. pallida, and 20× for C. vitellina. The tissue treatment was replicated 38× for leaves and 35× for pods.
For each bioassay, the adult moth was tossed into the jar containing one L. erythrognatha individual, which was also sexed. Once the moth was tossed into the jar, I measured whether the spider preyed on or rejected the moth. Spiders were starved for 48 h prior to the bioassay.
Statistical analysis
Using the binary variable of the spider preying or not on the moth, I fitted a generalised linear model (GLM) with binomial errors. The model was fitted with the main effects of plant diet treatment and tissue treatment (leaves or pods) as explanatory variables, as well as sex of the moth and of the spider. As moths fed on C. pallida were all released, this factor level for plant diet treatment was excluded from the dataset before fitting the model, as it had no variability. Interactions between the fixed effects were left out of the model due to severe collinearity and variance inflation. I tested significance by removing the terms for the explanatory variables and testing the change in deviance against a χ2 distribution (Crawley, 2013). Odds ratios were calculated to compare the effect sizes of rearing on different plant species. The standardised effect size for each treatment relative to the overall mean was calculated by transforming the odds ratio to Cohen’s d using d = log(OR)× √3/π (Borenstein et al., 2009). For graphical presentation the estimated probability of predation on each plant was calculated from the GLMM. The risk ratio was also calculated (Table S5).
RESULTS
Development on leaves
On average it took U. ornatrix 19.6 days to reach pupation, but this time was affected by diet treatment (F3,172 = 22.52, P<0.001), whereas an effect of sex was not significant (F1,172 = 1.31, P = 0.26; Table S1). It took the larvae 17.1 days (95% CI: 14.5–19.8) to pupate when fed on C. vitellina, 17.9 days (15.2–20.6) on C. micans, 20.9 days (18.3–23.6) on C. ochroleuca, and 22.3 days (19.6–25.0) on C. pallida. Development time was significantly slower on C. ochroleuca and C. pallida than on C. micans and C. vitellina (Figure 1A; Table S2).
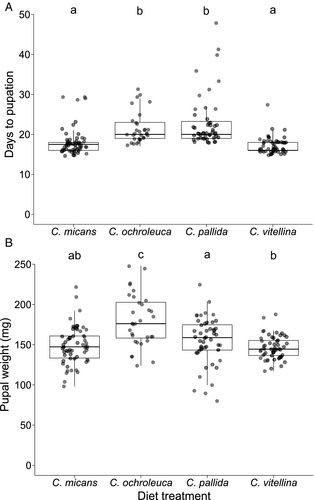
Utetheisa ornatrix pupae reared on leaves weighed, on average, 158 mg, but this was affected by both diet treatment (F3,172 = 19.16, P<0.001) and moth sex (F1,172 = 10.09, P = 0.002). Males were heavier than females: 164 mg (95% CI: 155–173) vs. 153 mg (144–162). The plant diet that produced the heaviest pupae was C. ochroleuca (181 mg; 171–192), followed by C. pallida (158 mg; 149–167), C. micans (148 mg; 139–158), and C. vitellina (146 mg; 137–155) (Figure 1B). Pupae from larvae reared on C. ochroleuca were significantly heavier than those reared on every other host plant, and pupae from larvae reared on C. pallida were heavier than those from larvae reared on C. vitellina (Figure 1B; Tables S3 and S4).
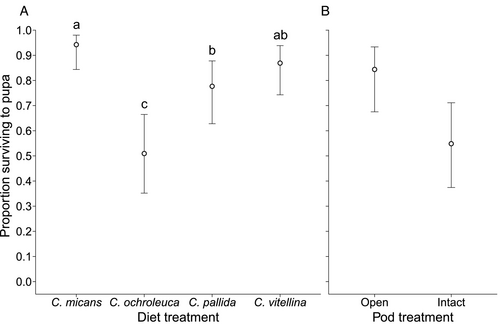
Survival to pupal stage was analysed by means of GLMM simplification. Plant diet was significant upon model simplification and comparison (χ2 = 38.45, d.f. = 3, P<0.001) and was retained in the model. The plant diet on which U. ornatrix was most successful in reaching the pupal stage was C. micans (probability of 0.94; 95% CI: 0.84–0.98). This was followed by C. vitellina (probability of 0.87; 0.74–0.94), C. pallida (probability of 0.78; 0.63–0.88), and the lowest probability was observed on C. ochroleuca (0.51; 0.35–0.67) (Figure 2A). The effect sizes of pairwise comparisons were estimated using the odds ratio of survival on each pair of plants. Survival on C. ochroleuca was significantly lower than on all other species. Survival on C. pallida was lower than survival on C. micans (Table S5).
Development on pods
When feeding on intact or open pods of the four plant species, time to pupation was only affected by pod treatment (F1,23 = 12.94, P = 0.002). When pods were opened and U. ornatrix had access to the seeds without having to bore into the pods, time to pupation was 2.2 days shorter (mean of 16.0 days; 95% CI: 14.7–17.3) than when they had to bore into the pods (18.1 days; 16.9–19.5) (Figure 3A). None of the other main effects or two-way interactions were significant (Table S6).

When the larvae had to bore into closed pods, pupae suffered 25% reduction in pupal weight: mean pupal weight was 191 mg (95% CI: 175–208) for the open pod treatment vs. 143 mg (127–160) for the closed pod treatment (F1,23 = 33.45, P<0.001). For diet treatments (F1,23 = 11.59, P<0.001; Table S7), the highest mean weight was observed when U. ornatrix was fed on pods of C. ochroleuca (191 mg; 168–213), followed by C. pallida (179 mg; 161–196), C. micans (163 mg; 147–180), and C. vitellina (137 mg; 116–157) (Figure 3B). Weight was significantly lower when reared on C. vitellina than when reared on C. ochroleuca and C. pallida (Table S8). This represents a standardised effect size of 2.52 (Table S8), and 28% decrease in pupal weight between feeding on C. ochroleuca and C. vitellina. Males (169 mg; 145–194) were again slightly heavier than females (165 mg; 144–187), but this effect was marginal (F1,23 = 4.18, P = 0.052).
GLMM simplification revealed that only pod treatment affected survival to pupa (pod: χ2 = 7.20, d.f. = 1, P = 0.007; diet: χ2 = 4.61, d.f. = 3, P = 0.20). The odds ratio of survival on closed vs. open pods was 0.23 (95% CI: 0.07–0.74), representing a 77% decrease in the survival odds when pods are closed. This translates to a survival probability of 0.55 (0.37–0.71) when feeding on closed pods and 0.84 (0.68–0.93) when feeding on open pods (Figure 2B).
Defence against spider predation
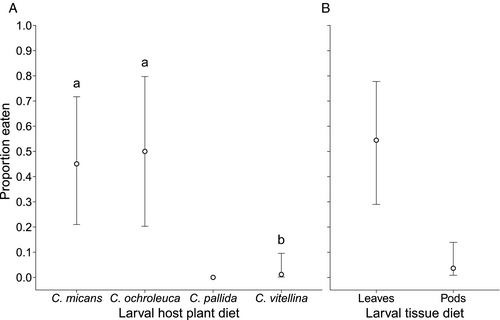
Both plant diet (χ2 = 23.94, d.f. = 3, P<0.001) and tissue diet (leaves or pods; χ2 = 21.45, d.f. = 1, P<0.001) were significant upon model simplification and were therefore retained in the model to explain predation of U. ornatrix by L. erythrognatha. Moth sex (χ2 = 0.03, d.f. = 1, P = 0.88) and spider sex (χ2 = 0.02, d.f. = 1, P = 0.89) did not help explain predation and were therefore dropped from the model. The highest probability of predation was observed when U. ornatrix was fed on C. ochroleuca (0.50; 95% CI: 0.20–0.80) followed by C. micans (0.45; 0.21–0.72) and C. vitellina (0.01; 0.001–0.10) (Figure 4A). The odds ratio of feeding on C. micans vs. C. ochroleuca was 0.82, on C. vitellina vs. C. ochroleuca it was 0.01, and on C. vitellina vs. C.micans 0.01 (Table S9). Therefore, feeding on C. vitellina led to 99% decrease in the odds of predation compared to feeding on C. micans or C. ochroleuca. When fed on C. pallida all U. ornatrix individuals tested were released intact, and therefore I could not fit this factor level in the model or estimate a confidence interval or the odds ratios relative to the other host plant diets. Feeding on pods made the moths much better defended against predation, with a probability of predation of 0.04 (95% CI: 0.01–0.14) compared to 0.55 (0.29–0.78) when fed on leaves. This corresponds to an odds ratio of 0.03, or a 97% reduction in the odds of being eaten by L. erythrognatha (Figure 4B).
Standardised effect sizes
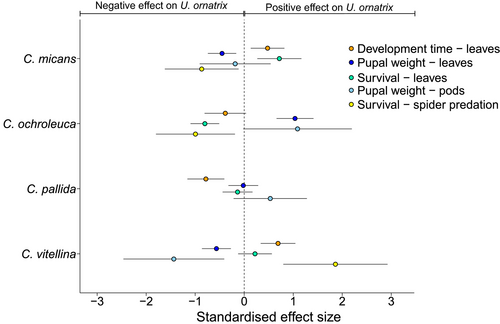
For every analysis where host plant diet was significant, standardised effect sizes were calculated for each plant treatment relative to the overall mean for each experiment. This allowed visualisation of the overall effects of each plant species on U. ornatrix (Figure 5). The effects for all species straddled zero, but the variability in effect sizes among the species differed. Crotalaria vitellina and C. ochroleuca showed large variation in effect sizes, whereas C. pallida effects were all closer to zero (except for defence against predation, for which it gave 100% defence, and no effect size could be calculated).
DISCUSSION
Understanding the evolution of herbivore diet breadth requires the study of herbivores with different ranges of host plant use (Singer, 2008). It has been hypothesised that the specialization on a genus or on chemically similar plants could be driven by plant quality, whereas enemy-free space drives differences within this restricted group (Singer et al., 2004a). Here I have provided results that partially support this hypothesis and point strongly towards differences in defence obtained by U. ornatrix when feeding on different Crotalaria species. However, I have also shown important trade-offs in various components of physiological efficiency of host plant use. These results suggest that each host plant species provides specific costs and benefits that could maintain multiple host plant use by U. ornatrix.
The physiological efficiency hypothesis posits that herbivore host plant use is driven by selection for the use of plants that increase fitness by increasing survival, reducing development time, and increasing reproductive allocation (Scriber, 1983; Traxler & Joern, 1999). Utetheisa ornatrix showed clear differences in performance on the four host plants tested, but each aspect of development was affected differently. Feeding on C. ochroleuca led to a large cost in survival, but the larvae that survived to pupation were much heavier, whereas larvae that developed on C. micans or C. vitellina survived well and developed much more quickly but resulted in smaller pupae. As pupal weight is strongly correlated with adult fecundity in U. ornatrix, this difference could represent 50–75% increase in fecundity in individuals that survive to become adults after feeding on C. ochroleuca (LaMunyon, 1997). Therefore, even when considering only physiological efficiency there is a trade-off in host plant use which could maintain multiple plant use within U. ornatrix populations. Though we know that different host plants can confer different benefits to herbivores (Singer, 2001), such explicit trade-offs among components of physiological efficiency are rarely reported (Hong et al., 2019). These trade-offs in benefits and costs could be common and have been proposed as an explanation for diet mixing (Singer, 2001). Furthermore, they imply that physiological adaptation to host plants could involve complex patterns of selection on multiple herbivore traits to deal with plant nutritional quality, chemical defence, and physical defence (Agrawal, 2020).
The enemy-free space hypothesis emphasises that herbivores feeding on different host plants will be subjected to differing pressures from natural enemies and this could be the major driving force for host plant use (Bernays, 1988; Bernays & Graham, 1988). For herbivores that sequester plant defensive compounds, feeding on different host plants could result in differing levels of herbivore chemical defence (Damman, 1987; Denno et al., 1990; Ohsaki & Sato, 1994; Camara, 1997; Mira & Bernays, 2002; Müller & Arand, 2007). Utetheisa ornatrix sequesters PAs from its Crotalaria host plants (Rossini et al., 2003; Bezzerides et al., 2004), and different PA chemical structures confer different levels of defence (Silva & Trigo, 2002). The results I present here correspond with previous reports of adult defence conferred by different Crotalaria host plants against spider predation. In particular, U. ornatrix obtains high levels of defence from C. pallida and C. vitellina, especially when feeding on pods, whereas C. micans and C. ochroleuca confer lower levels of defence (Martins et al., 2015).
Integrating the results and analysing the ecological trade-offs in host use, each Crotalaria species provides a unique ecological setting that will influence U. ornatrix fitness. Components of U. ornatrix physiological efficiency vary in different directions when feeding on different host plants and they also trade off with the defence obtained. Crotalaria micans and C. vitellina provided benefits in survival to pupation and development time, but at the cost of pupal weight; individuals that fed on C. ochroleuca were heavier, but also suffered costs in survival to pupation, development time, and defence against predation; C. pallida was intermediate in all its effects, except defence against predation, for which it provided the best defence (no predation whatsoever). Contrary to the slow growth/high mortality hypothesis, defence conferred by sequestration from host plants that slow development can also confer greater defence to the adults. This trade-off between exposure to natural enemies and defence sequestration has been shown previously for larvae of different species and is likely to be an important component of host plant use for insects that sequester plant defences or use other plant traits as defence (Dobler & Rowell-Rahier, 1994; Camara, 1997; Müller & Arand, 2007). However, we still know little about how defence conferred by sequestration from different host plants is correlated between the larval and the adult stages. It will be interesting to discover whether greater larval defence corresponds to greater adult defence, or whether they are in conflict (Ballabeni et al., 2001).
There is also one further trade-off that must be considered: access to feeding on seeds, which confers both developmental and defence benefits, comes at the significant cost of boring into the pod. Previous studies had shown a large benefit of feeding on seeds, but these studies provided seeds without the need to bore into the pod, so they did not account for the cost of boring (Ferro, 2001; Ferro et al., 2006; Sourakov, 2015). Combined with previous results that showed costs of boring into pods, the large cost I detected for boring into pods suggests that the benefit of feeding on green seeds within pods is driven by defence and not performance, as it confers a large benefit in protection against adult predators and provides shelter for larvae when predator pressure is high (Ferro et al., 2006; Magalhães et al., 2017). Pod size varies significantly among species: C. micans pods (42 cm3) are 3–4× larger than those of C. pallida (14 cm3) and C. vitellina (9 cm3) (M Pareja, unpubl.). There are no data available for C. ochroleuca, but it appears to have the largest pods (pers. obs.), so the two species that provide lowest chemical defence (C. micans and C. ochroleuca) are likely to provide the greatest defence in larval refuges.
These results lead me to conclude that use of multiple Crotalaria host plants, as well as a mixed diet on leaves and green seeds, by U. ornatrix is maintained by the interaction of food plant quality and defence conferred, as has been observed for other systems (Mira & Bernays, 2002; Singer & Stireman III, 2003; Rodrigues et al., 2010; Rodrigues & Freitas, 2013; Murphy & Loewy, 2015). This interaction between plant quality and defence raises the hypothesis that selection pressure for host plant use exerted by plant quality and predators varies with changes in predator communities and is therefore context-dependent (Katsanis et al., 2016; Singer et al., 2019). In communities where predation pressure is high, U. ornatrix fitness is predicted to be higher on host plants that provide higher defence, whereas in communities where predation pressure is low, greater fitness is predicted from plants that optimise physiology. In communities where predation pressure is intermediate or temporally variable the net effect on fitness is likely to be similar on different host plants (Singer and Stireman III, 2005; Mooney et al., 2012). Therefore, tests of the three necessary hypotheses for detecting enemy-free space (Berdegue et al., 1996) could generate spatially variable results, where different host plants create enemy-free space in some communities but not in others. This variability could further strengthen multiple host plant use at both local and regional scales by creating geographic selection mosaics (Thompson, 2005).
In summary, I have shown that there are several intercrossing trade-offs in performance and defence when U. ornatix feeds on different Crotalaria host plants. Therefore, neither the physiological efficiency nor the enemy-free space hypotheses on their own are sufficient to explain multiple host plant use by U. ornatrix. To further understand U. ornatrix diet breadth, future work should explore how host plant diet affects predation of other life stages and how they affect adult fitness components, in particular through changes in PA-derived male pheromone titres (Conner et al., 1990; Dussourd et al., 1991). These results provide further evidence that a bitrophic perspective is too limited to understand host plant use and that we must further study the ecological trade-offs imposed by tritrophic interactions and context dependency and how they mould herbivore niches (Price et al., 1980; Singer & Stireman III, 2005; Murphy & Loewy, 2015).
ACKNOWLEDGEMENTS
I thank José Roberto Trigo for providing laboratory space that permitted the completion of this study and Carlos H. Martins for valuable advice during the experiments. This study was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP project grant 2008/05558-1 and by the Fundo de Apoio ao Ensino, à Pesquisa e à Extensão—FAEPEX/UNICAMP (grant no. 60412 and PAPDIC 2014/4715). Sampling authorization was granted through ICMBio/SISBIO register 18975, and the research carried out is registered in the SISGEN database under the register AE31300. I do not have any conflicts of interest to declare.
AUTHOR CONTRIBUTION
Martin Pareja: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Funding acquisition (lead); Investigation (lead); Methodology (lead); Project administration (lead); Visualization (lead); Writing—original draft (lead); Writing—review & editing (lead).
Open Research
DATA AVAILABILITY STATEMENT
The data are available in the Zenodo online repository under d.o.i. 10.5281/zenodo.5514386.




