Habitat factors associated with Fopius caudatus parasitism and population level of its host, Ceratitis cosyra
Abstract
Biotic and abiotic factors affect herbivores and their natural enemies and understanding of their requirements may permit habitat modification enabling conservation biological control. Ceratitis cosyra Walker (Diptera: Tephritidae), an African-native fruit fly pest is mostly parasitized by the parasitoid wasp Fopius caudatus Szépligeti (Hymenoptera: Braconidae). To assess F. caudatus habitat, the wasp parasitism levels and infestation of its fruit fly host were examined in Sarcocephalus latifolius (Smith) Bruce (Rubiaceae), a shrub of which the fruits are among the preferred hosts of C. cosyra and F. caudatus. Fruit-collection site descriptions, including plant species presence, were analysed in relation to the target insect abundances (emergence from target fruit). Ceratitis cosyra and F. caudatus emerged from all sites; nonetheless, their population levels were associated with both abiotic and biotic factors, of which some can be manipulated. Several factors, such as cultivation level, topography, and vegetation coverage, were correlated with F. caudatus parasitism. Ceratitis cosyra infestation level was correlated with factors such as density of S. latifolius, vegetation cover, cultivation practices, temperature, altitude, rainfall pattern, and stoniness. Proximity to other fruit fly host plants correlated with both pest abundance and F. caudatus parasitism level of the fruit fly. The findings that insects’ interactions and abundance are influenced by habitat structure and that parasitism is positively related to natural habitat indicates the importance of maintaining natural habitats in closeness to cultivated areas with the aim of enhancing pest suppression by parasitoids. Further studies should attempt to identify how plant species composition in and around orchards could affect the management of tephritid fruit fly pests.
Introduction
Habitat characteristics – such as, among others, the availability of shelter, water, and food, vegetation composition, temperature regime, and environmental disturbance – affect parasitoid and herbivore presence and abundance (Partel et al., 1996). In addition, the distance between habitats, the density of plant species, predation, and competition affect the plant-herbivore-natural enemy interaction (Kruess & Tscharntke, 1994; Frankl et al., 2004; Romeis et al., 2005; Rohrig et al., 2008). Pesticide application on arable crops and/or in the surrounding fields further disturbs parasitoid populations (Wang et al., 2005). On top of this, the influence of all these factors is not uniform and may be species specific (Landis et al., 2000; Chaplin-Kramer & Kremen, 2012).
Biological management methods of tephritid fruit flies through the use of parasitoids, specifically Opiinae wasps (family Braconidae), are well-studied and biological control of pest fruit flies is achieved by Opiinae parasitoids in, for example, Hawaii, USA (Miranda et al., 2008; Vargas et al., 2012). Diachasmimorpha longicaudata (Ashmead), Fopius vandenboschi (Fullaway), Fopius arisanus (Sonan), and Psyttalia concolor (Szépligeti) (all Hymenoptera: Braconidae) are some of the species released as exotic species for the specific management of Bactrocera dorsalis (Hendel) and Ceratitis capitata (Wiedemann) (both Diptera: Tephritidae) (Miranda et al., 2008; Vargas et al., 2012). Since long there has been a focus on these exotic parasitoids, whereas studies assessing the potential of native/local Braconidae parasitoids as biocontrol agents for fruit flies are rare (Ovruski et al., 2000). Yet, parasitism in tephritid fruit flies such as Anastrepha spp. can reach 76%, by native parasitoids (Aluja et al., 2003) and parasitism by Fopius caudatus Szépligeti in fruits infested by Ceratitis cosyra Walker can reach 30% (Vayssières et al., 2010b, 2012; Badii et al., 2016). The African-native fruit fly C. cosyra is additionally parasitized by the minor parasitoids Psyttalia cosyrae (Wilkinson) and Psyttalia perproxima (Silvestri) (Vayssières et al., 2010b), members of the P. concolor species complex (Billah et al., 2008; Rugman-Jones et al., 2009). Fopius caudatus is among the most abundant parasitoids of known fruit-infesting tephritid flies in Africa and mainly parasitizes C. cosyra (Vayssières et al., 2010b, 2012). Parasitism by F. caudatus is affected by host plant species and reaches 10% on average in mango, Mangifera indica L. (Anacardiaceae), 10–56% in coffee, Coffea spp. (Rubiaceae), and about 30% in African peach, Sarcocephalus latifolius Bruce (Rubiaceae) (Steck et al., 1986; Vayssières et al., 2010b; Badii et al., 2016). Fopius caudatus has been considered as classical biocontrol agent in, for example, Hawaii and Israel (Wharton et al., 2000; Argov & Gazit, 2012; Bokonon-Ganta et al., 2019). The presence of the parasitoid F. caudatus has been documented in relation to fruit species; yet, information about the factors that govern its distribution and parasitism is scarce. Remarkably few habitat requirements for fruit fly parasitizing species – other than host flies, host fruits (Rousse et al., 2005; Quilici & Rousse, 2012), and climatic conditions (Rousse et al., 2009; Lane et al., 2018) – are known to interact with the abundance and parasitism of released braconid wasp species. Thus, the role of native braconid parasitoids can be further explored, including investigations to broader understanding of their habitat requirements. Conservation biological control (CBC) aims to maximize the impact of existing natural enemies and has proven effective in many crop/pest systems to reduce agricultural losses due to pest insects by providing habitat and resources to enhance survival and/or physiological and behavioural performance of natural enemies (Cullen et al., 2010). Habitat manipulations might occur at small scale as creation of shelter habitats, or at large landscape scale on regional, national, or continental scale (Griffiths et al., 2008; Jonsson et al., 2008).
Insect population responses to their physical and chemical environment have been the focus of many basic and applied studies in insect ecology (Villani et al., 1990; Letourneau et al., 2011; Karp et al., 2018), although there has been little research attention devoted to CBC in management of tephritid fruit flies (Zamek et al., 2012). To be able to efficiently manipulate the environment in favour of specific braconid parasitoids for tephritid management, knowledge is needed about factors that impact them. Temperature influences the development and longevity of P. cosyrae (Mohamed et al., 2006), whereas fruit species and varieties influence F. caudatus parasitism rate (Vayssières et al., 2010a,b). Pesticide use affects the population growth of braconids such as F. arisanus, D. tryoni, and Psyttalia fletcheri Silvestri (Wang et al., 2005). Success of habitat management is likely to depend on both the composition of the local food web and the extent to which suitable and limiting resources are provided to the target natural enemy (Jonsson et al., 2010). Knowledge of plant species and habitat characteristics that affect parasitism of tephritid pests is important for biological control. Therefore, we aimed at defining habitat factors that determine parasitism by F. caudatus, one of the most abundant native parasitoids of C. cosyra, to guide further experimental studies of habitat manipulation effects.
Materials and methods
Study area characteristics
The study was conducted in the Republic of Benin, West Africa. A description of each of the 30 fruit-collection sites was made, comprising information about climactic characteristics, soil features, vegetation structure, land use and agricultural practices, and hydrography (summarized in Table 1). The climatic variables of the fruit-collection sites were precipitation, temperature, relative humidity, and rainfall pattern, which were previously used to define climatic zones in Benin (Adomou et al., 2006; Akoègninou et al., 2006). The temperature and relative humidity values for each climatic zone were the annual range, that is, the averages of minimum and maximum values for the last 5 years. The precipitation used was the average per year, for the last 5 years. The plant formation was appreciated in terms of savannah, fallow, woodland, etc. For vegetation stratification, we roughly distinguished three storeys: tree, shrub, and herb layers. The cover of each layer was visually estimated in terms of the percentage of the total site area being covered by trees, shrubs, and herbs following the Braun Blanquet’s approach (Kent, 2012). The density of S. latifolius was measured by estimating the number of S. latifolius trees on the site. Soil description accounted for type of soil, topography, and litter level. Land use and agricultural practices information was based on the main activities during the time of fruit collection and the previous 5 years, collected through a survey among the producers. Information about agricultural management included chemical fertilization and phytosanitary treatment. Hydrography was described with estimation of proximity of a river, water presence, and existence of stagnant water in the rainy season.
| Descriptive factor | Modalities | ||||
|---|---|---|---|---|---|
| Extra low | Low | Medium | High | Extra high | |
| Climate | |||||
| Temperature (°C) | 25.0–29.0 | 21.2–32.5 | 20.8–34.1 | ||
| Relative humidity (%) | 26–82 | 46–87 | 69–97 | ||
| Rainfall pattern (per year) | Unimodal, 1 rainfall season | Bimodal, 2 rainfall seasons | |||
| Precipitation (mm/year) | 900 | 1200 | 1300 | ||
| Altitude class (m) | <100 | 100–200 | 200–300 | 300–400 | 400–500 |
| Soil | |||||
| Topography | Flat | Gently slope | Slope | ||
| Soil typea | Clayey soil | Silty soil | Sandy soil | ||
| Litter rate | Practically no litter | Low quantity ground litter | ground cover by litter | ||
| Stony | Low stones coverage | Medium stone coverage | |||
| Vegetation | |||||
| S. latifolius density (ha−1) | 1–5 | 5–10 | 10–20 | 20–40 | >40 |
| Tree coverage | 2 = 5–25% | 3 = 25–50% | 4 = 50–75% | ||
| Shrub coverage | 2 = 5–25% | 3 = 25–50% | 4 = 50–75% | ||
| Herb coverage | 2 = 5–25% | 4 = 50–75% | 5 = 75–100% | ||
| Land use and agricultural practices | |||||
| Land used | Natural habitatb | Plantationb | Few crops cultivated, mainly cereals | Cultivated (e.g., cereals, vegetables) | Many crops, including cotton |
| Chemical input used | No = no chemical fertilizer or pesticide | Chemical fertilizationc | Chemical fertilization, phytosanitary treatmentc | ||
| Presence of housea | No = no houses in neighbourhood | Yes = houses in neighbourhood | |||
| Hydrography | |||||
| River distance | River within site | River distance <500 m | River distance >500 m | ||
| Flooda | No = never flooded | Yes = flooded part of the year | |||
- a Factors not described by increasing values.
- b Natural habitat = no crops, houses or plantations, that is, wild area. Plantation = mango, cashew, guava, or papaya plantation.
- c Chemical fertilization = urea, NPK. Phytosanitary treatment, for example, acetamiprid, lambda-cyhalothrin, emamectin benzoate, or cypermethrin.
Plant species present on each site were sampled and sent to the national herbarium of Benin, at the University of Abomey-Calavi for botanical identification. Botanical nomenclature followed Akoègninou et al. (2006). All plant species were categorized by their vegetation type (i.e., tree, shrub, and herb), which was used for calculating diversity indices.
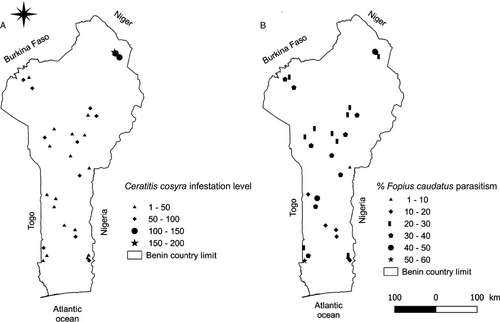
Fruits were collected in 30 sites, within circular plots of 100 m radius each. The sites were distributed throughout the entire country, at least 5 km apart from each other, and their geographical coordinates and altitude were recorded with GPS (global positioning system) (Figure 1). The fruit-collection sites were targeted to be situated in several climatic zones in the country and where the presence of at least three S. latifolius plants within the site area was verified.
Fruit sampling
The documented high F. caudatus parasitism in S. latifolius (Vayssières et al., 2010b, 2012; Badii et al., 2016), its distribution limitation (Trostle Duke, 2005), together with a country-wide distribution of S. latifolius (Orwa et al., 2009) contributed to our choice of the studied C. cosyra host fruit, S. latifolius. Sarcocephalus latifolius is mainly infested by C. cosyra (99%) and rarely by, for example, B. dorsalis (0.4%) and Ceratitis punctata (Wiedemann) (0.7%) (Vayssières et al., 2012). Its fructification period extends from July to October in Benin (Vayssières et al., 2010a; A Adomou, pers. obs.); therefore, collection of S. latifolius was done from 23 July to 30 October 2017. As the mating and oviposition of fruit flies and parasitoids are affected by weather conditions – temperature, light intensity, wind speed, relative humidity, and barometric pressure (Bateman, 1972; Rousse et al., 2009) – fruits were sampled repeatedly from all the sites every 2 weeks. One sample consisted of five S. latifolius fruits. Two samples were taken per site (one from the branches and one from the ground), per collection occasion. Ripe fruits were targeted for sampling, yet rotten and immature fruits were occasionally collected when the number of mature fruits did not attain the desired sample size. Fruits from each sample were packed in paper envelopes, labeled by site. All envelopes were placed in woven plastic bags and sent to the laboratory for incubation, at the latest the following morning.
Incubation of fruit samples
Incubation of the fruits was done in a screen house, at an ambient 26 ± 2 °C and 80 ± 5% r.h. Samples were incubated by placing the fruits on a mesh in a plastic container (200 ml) with a sand layer at the base. The plastic containers were then covered with a thin polyester fabric, tightened with a rubber band. Each incubation container was labeled. The fruits were cut open to ease the emergence of the larvae. The sand used as pupation substrate was sieved each 4 days in order to track the formation of the pupae, until the total decomposition of the incubated fruits and the pupation of all the larvae. Each incubation container was thus monitored for a period of 4–6 weeks. The pupae were collected in Petri dishes whose lid were perforated and covered with fine mesh.
Petri dishes were labeled with the incubation container number from which the pupae were collected. The collected pupae were transferred to the laboratory where they were counted and preserved for the emergence of Tephritidae and Braconidae adults. The pupae were preserved in an insectarium under laboratory conditions at 25 ± 1 °C and 75 ± 5% r.h.
Identification of tephritid fruit flies and parasitoids
All insect emergence was observed every 2 days and documented in a collection file. The emerged flies and parasitoids were counted and then released into various cages according to the species. The non-eclosed pupae from each sample were sorted and dissected in order to evaluate the eventual parasitism, 2 months after the last emergence. Ceratitis cosyra pupae were easily distinguished from B. dorsalis by their size and colour, as C. cosyra pupae were small (2.8 ± 0.07 mm long) and light yellow, whereas B. dorsalis pupae were ca. 3.7 ± 0.1 mm long and brownish. Dissection were done by carefully opening the pupal case and taking it away with a pair of tweezers to see the pupa content (Rull et al., 2009). Parasitoids and fruit flies were fully developed inside most of the non-eclosed pupae, which was assessed by, for example, their long antennae. We considered small third-instar larvae or pupae (female pupae with their ovipositor visible) to be F. caudatus, possibly in diapause, if they had not emerged within the expected development time (Aluja et al., 1998; Murillo et al., 2015). Black liquid inside the pupal case was considered to point at C. cosyra; fruit flies are not known to undergo diapause in tropical and subtropical areas (Fletcher, 1987).
Data description
The sampling design consisted of two random samples (tree and ground) repeated 8× from 30 collection points (sites) across the country. We obtained 470 samples instead of 480 as planned due to fruit unavailability. As several solitary braconid species parasitize C. cosyra (Mohamed et al., 2006), the sum of all emerging Braconidae wasps (F. caudatus and Psyttalia spp.) was used to calculate the C. cosyra infestation. We hence expressed the level of C. cosyra infestation per fruit sample as the summation of emerged and dissected C. cosyra, F. caudatus, and Psyttalia spp. obtained per sample. The low number of Psyttalia spp. did not allow further analysis in relation to the site’s descriptive factors. Fopius caudatus parasitism in each sample was expressed as the totality of emerged F. caudatus and the dissected parasitoid individuals from non-eclosed pupae divided by the infestation per sample.
Statistical analysis
To test the effect of environmental factors and plant species on the dependant variables, that is, the infestation of C. cosyra and F. caudatus parasitism, a generalized additive model (GAM) (Wood, 2017) was performed to obtain as far as possible a parsimonious model that reduced overdispersion of adjusted data. The GAM assumes that relationships among variables are not restricted to any shape and then uses a non-linear smooth function to estimate these relationships between the covariates and the outcome. Thus, the model was fitted using a negative binomial distribution which offered the best fit among the models tested. Fopius caudatus parasitism was modeled with an offset function (log of C. cosyra infestation) to account for the amount of variation in the response (count number of pupa). The goodness of fit was evaluated through the ability of the model to reduce the global deviance in comparison to the total effective degree of freedom. A stepwise regression was thereafter performed on the fitted model to determine, among the set of all covariates, which covariate significantly contributed to explain the variability in F. caudatus parasitism and C. cosyra infestation level.
Habitat diversity was expressed through the Shannon diversity index, and was calculated based on the number of species in each vegetation type. Pearson correlation was used to access the degree of association between the diversity and both C. cosyra infestation and F. caudatus parasitism. All analyses were carried out with R software v.3.5.1 (R Core Team, 2018). We used the QGIS v.2.18 (QGIS, 2017) software to project the fruit-collection sites, their C. cosyra infestation level, and the F. caudatus parasitism on the map of Benin.
Results
Inventory of Tephritidae and Braconidae species
Braconidae species were identified based on available identification keys (Wilkinson, 1927; Wharton & Gilstrap, 1983; Carmichael et al., 2005; Wharton, 2007; Billah et al., 2008; Rugman-Jones et al., 2009). The main parasitoid species that emerged was the native F. caudatus with an average parasitism rate of 24.4 ± 1.16% (mean ± SE; Table 2). Fopius caudatus was present in C. cosyra-infested S. latifolius fruits in all collection sites in Benin. Other Opiinae species belonged to the P. concolor complex; based on the mean (± SE) ovipositor length of 3.5 ± 0.1 mm, we considered the Psyttalia specimens to be P. cosyrae rather than P. perproxima (Billah et al., 2008).
| Family | Species | Feature | Total number | Mean (± SE) number/sample | Mean (± SE) % |
|---|---|---|---|---|---|
| Tephritidae | Thirithrum spp. | Emerged | 9 | 0.02 ± 0.01 | 0.04 |
| Bactrocera dorsalis | Emerged | 57 | 0.14 ± 0.04 | 0.02 | |
| Ceratitis cosyra | Emerged | 17131 | |||
| Dissected | 1278 | ||||
| Total | 18230 | 73.61 | |||
| Infestation level | 24698 | 63 ± 4 | 99.73 | ||
| Braconidae | Psyttalia spp. | Emerged | 331 | 1.0 ± 0.15 | 1.34 |
| Fopius caudatus | Emerged | 5050 | 15 ± 1 | ||
| Dissected | 988 | ||||
| Total | 6052 | 24.43 ± 1.16 | |||
| Parasitism rate | 29.48 ± 1.27 |
Out of the 470 samples collected, 83% was infested by C. cosyra, which was the main tephritid species emerging from the fruits (99.7%) (Table 2). Ceratitis cosyra infestation level and F. caudatus parasitism varied among the fruit-collection sites, ranging from 2 to 187 C. cosyra per sample (Figure 1A) and from 1 to 60% parasitism (1–33 F. caudatus per sample) (Figure 1B).
Descriptive factors in relation to Ceratitis cosyra infestation level and Fopius caudatus parasitism
Among the 18 descriptive factors and their 56 modalities used to describe the fruit-collection sites (Table 1), an assembly of five factors (six modalities) were related to F. caudatus parasitism, whereas 13 factors (18 modalities) were associated with C. cosyra infestation levels (Table 3). All modalities with an absolute t-value >2 and P<0.05 were associated with C. cosyra infestation level or F. caudatus parasitism (Table 3). The factors natural habitat and flat topography were correlated with high F. caudatus parasitism whereas high shrub coverage, low herb and tree coverage, and highly cultivated land were correlated with low parasitism. Infestation was positively related to stoniness, moderate and little cultivation, high temperature, low S. latifolius density, altitude between 300 and 400 m, and low herb coverage. Ceratitis cosyra infestation level was negatively correlated with low and high chemical input used, silty soil, slope, unimodal rainfall pattern, high herb coverage, low temperature, presence of house, and low litter rate (Table 3).
| Estimate | SE | t | Pr(>|t|) | ||
|---|---|---|---|---|---|
| Fopius caudatus parasitism | (Intercept) | −1.47 | 0.22 | −6.75 | <0.001 |
| Flat topography | 0.58 | 0.18 | 3.29 | <0.001 | |
| Natural habitat land | 0.48 | 0.18 | 2.68 | 0.008 | |
| High litter rate | 0.36 | 0.23 | 1.52 | 0.13 | |
| Presence of house | 0.26 | 0.16 | 1.67 | 0.096 | |
| High tree coverage | 0.17 | 0.35 | 0.48 | 0.63 | |
| Low shrub coverage | −0.07 | 0.11 | −0.67 | 0.50 | |
| Little cultivated land | −0.08 | 0.16 | −0.47 | 0.64 | |
| Slope topography | −0.11 | 0.28 | −0.39 | 0.69 | |
| Low litter rate | −0.17 | 0.11 | −1.61 | 0.11 | |
| High herb coverage | −0.25 | 0.14 | −1.80 | 0.073 | |
| Low tree coverage | −0.33 | 0.13 | −2.54 | 0.011 | |
| Moderately cultivated land | −0.35 | 0.31 | −1.15 | 0.25 | |
| Low herb coverage | −0.46 | 0.18 | −2.62 | 0.009 | |
| Very cultivated land | −0.47 | 0.19 | −2.46 | 0.014 | |
| High shrub coverage | −0.55 | 0.19 | −2.90 | 0.004 | |
| Ceratitis cosyra infestation level | (Intercept) | 10.06 | 2.14 | 4.70 | <0.001 |
| Stony area | 8.21 | 2.31 | 3.55 | <0.001 | |
| Moderately cultivated land | 5.03 | 1.57 | 3.21 | 0.001 | |
| Little cultivated land | 4.43 | 1.04 | 4.25 | <0.001 | |
| High temperature | 3.91 | 0.87 | 4.48 | <0.001 | |
| Extra low abundance of S. latifolius | 3.80 | 0.96 | 3.95 | <0.001 | |
| Altitude class 300–400 m | 3.56 | 0.82 | 4.35 | <0.001 | |
| Low herb coverage | 0.95 | 0.39 | 2.41 | 0.016 | |
| High litter rate | 0.79 | 0.51 | 1.57 | 0.12 | |
| Low abundance of S. latifolius | 0.74 | 0.35 | 2.09 | 0.038 | |
| Altitude class 400–500 m | 0.40 | 0.56 | 0.71 | 0.48 | |
| High abundance of S. latifolius | 0.00 | 0.28 | −0.01 | 0.99 | |
| Very cultivated land | −0.18 | 0.54 | −0.32 | 0.75 | |
| Altitude class 100–200 m | −0.57 | 0.66 | −0.86 | 0.39 | |
| Extra high tree coverage | −0.60 | 0.40 | −1.49 | 0.14 | |
| Flat topography | −0.63 | 0.85 | −0.74 | 0.46 | |
| Clayey soil | −0.75 | 0.54 | −1.38 | 0.17 | |
| Low tree coverage | −0.91 | 0.39 | −2.30 | 0.022 | |
| Natural habitat land | −1.12 | 0.57 | −1.97 | 0.050 | |
| High chemical input used | −1.18 | 0.48 | −2.45 | 0.015 | |
| Low litter rate | −1.48 | 0.34 | −4.30 | <0.001 | |
| Presence of house | −1.55 | 0.57 | −2.71 | 0.007 | |
| Low temperature | −1.71 | 0.52 | −3.29 | <0.001 | |
| High herb coverage | −1.93 | 0.59 | −3.30 | 0.001 | |
| Altitude class 0–100 m | −2.41 | 1.37 | −1.76 | 0.080 | |
| Unimodal rainfall pattern | −3.03 | 0.87 | −3.50 | <0.001 | |
| Slope topography | −3.31 | 1.16 | −2.85 | 0.005 | |
| Silty soil | −6.40 | 1.66 | −3.86 | <0.001 | |
| Low chemical input used | −7.22 | 2.02 | −3.58 | <0.001 |
Plant species in relation to Fopius caudatus parasitism and Ceratitis cosyra infestation level
In total 474 plants were collected from all sites, comprising 179 identified species. Plant species richness varied from 8 to 27 species depending on the sites, yet the Shannon diversity indexes, based on vegetation type (i.e., tree, shrub, and herb) did not correlate with the level of C. cosyra infestation (r = –0.01, P = 0.8) and F. caudatus parasitism (r = –0.03, P = 0.6). Among the collected plant species, 43 were present in at least 10% of the sites (Table S1). These species were considered for correlation analysis between the target insects and the plant species presence. Analysis showed that 16 plant species were related to F. caudatus parasitism and 20 plants were associated with infestation, and both positive and negative correlations with the presence of certain plant species were observed (Table 4).
| Plant species | Family | Life forma | Fopius caudatus parasitism | Ceratitis cosyra infestation level | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | t | Pr(>|t|) | Estimate | SE | t | Pr(>|t|) | |||
| (Intercept) | −4.29 | 0.67 | −6.43 | <0.001 | −2.43 | 1.56 | −1.56 | 0.12 | ||
| Borassus aethiopum Mart. | Arecaceae | T | −1.28 | 0.53 | −2.43 | 0.015 | −1.70 | 0.53 | −3.21 | 0.001 |
| Terminalia laxiflora Engl. & Diels | Combretaceae | T | −1.00 | 0.22 | −4.63 | <0.001 | 1.48 | 0.58 | 2.57 | 0.011 |
| Spermacoce filifolia Schumabch. & Thonn. | Rubiaceae | H | −0.86 | 0.31 | −2.74 | 0.006 | −4.42 | 0.71 | −6.27 | <0.001 |
| Prosopis africana (Guill. & Perr.) Taub. | Fabaceae | T | −0.80 | 0.35 | −2.31 | 0.022 | −4.98 | 0.79 | −6.32 | <0.001 |
| Monechma ciliatum Jabcq. Milne-Redh | Acanthaceae | H | −0.79 | 0.19 | −4.15 | <0.001 | −0.70 | 0.30 | −2.33 | 0.021 |
| Phyllanthus muellerianus (Kuntze) Exell | Phyllanthaceae | S | −0.78 | 0.27 | −2.88 | 0.004 | −1.25 | 0.34 | −3.69 | <0.001 |
| Maranthes polyandra Benth. Prance | Chrysobalanaceae | T | −0.71 | 0.27 | −2.59 | 0.010 | ||||
| Mangifera indica L. | Anacardiaceae | T* | −0.47 | 0.20 | −2.32 | 0.021 | ||||
| Cochlospermum planchonii Hook.f. | Cochlospermaceae | H | −0.45 | 0.22 | −2.05 | 0.042 | −1.09 | 0.29 | −3.78 | <0.001 |
| Terminalia avicennioides Guill. & Perr. | Combretaceae | T | 0.33 | 0.15 | 2.15 | 0.032 | ||||
| Piliostigma thonningii (Schumach.) Milne-Redh. | Fabaceae | S | 0.48 | 0.24 | 2.01 | 0.045 | ||||
| Gmelina arborea Roxb. ex Sm. | Verbenaceae | T | 0.56 | 0.20 | 2.82 | 0.005 | −1.76 | 0.30 | −5.81 | <0.001 |
| Elaeis guineensis Jacq. | Arecaceae | T | 0.65 | 0.32 | 2.03 | 0.043 | 2.62 | 0.84 | 3.11 | 0.002 |
| Chamaecsista mimosoides L. Greene | Fabaceae | H | 0.76 | 0.28 | 2.75 | 0.006 | ||||
| Eucalyptus camaldulensis Dehn. | Myrtaceae | T | 1.63 | 0.45 | 3.67 | <0.001 | 1.36 | 0.55 | 2.50 | 0.013 |
| Vitellaria paradoxa C.F.Gaertn. | Sapotaceae | T* | 2.96 | 0.79 | 3.76 | <0.001 | 6.11 | 1.31 | 4.66 | <0.001 |
| Indigofera. leprieurii Baker | Fabaceae | H | 2.14 | 0.60 | 3.54 | <0.001 | ||||
| Combretum collinum Engl. & Diels | Combretaceae | S | −3.26 | 0.54 | −6.00 | <0.001 | ||||
| Rourea coccinea Schumach. & Thonn. Benth | Connaraceae | S | 2.39 | 0.85 | 2.83 | 0.005 | ||||
| Acacia hockii De Wild. | Fabaceae | S | 2.09 | 0.46 | 4.50 | <0.001 | ||||
| Anacardium occidentale L. | Anacardiaceae | T* | −1.49 | 0.55 | −2.73 | 0.007 | ||||
| Albizia lebbeck De Wild. | Fabaceae | T | 5.51 | 1.03 | 5.34 | <0.001 | ||||
| Daniellia oliveri Rolfe Hutch. & Dalziel | Fabaceae | T | 2.28 | 0.55 | 4.15 | <0.001 | ||||
| Parkia biglobosa Jabcq. R.Br. ex G.Don | Fabaceae | T | −2.56 | 0.53 | −4.79 | <0.001 | ||||
| Azadirachta indica A.Juss. | Meliaceae | T | 3.31 | 0.50 | 6.58 | <0.001 | ||||
| Crossopteryx febrifugea (G.Don) Benth. | Rubiaceae | T | 4.44 | 0.79 | 5.61 | <0.001 | ||||
| Tectona grandis L.f. | Verbenaceae | T | 0.78 | 0.22 | 3.47 | <0.001 | ||||
Discussion
That C. cosyra and F. caudatus were present in the whole country indicated that the temperature, humidity, and precipitation range in the various climatic zones were not limiting factors for the distribution of the wasp and its host. Biotic and abiotic factors correlating with C. cosyra infestation levels differed from those linked to F. caudatus parasitism, and interestingly some factors were associated with the two insects in a contrasting manner. Abiotic factors, such as temperature, rainfall pattern, and altitude, interacted with C. cosyra infestation level but not with F. caudatus parasitism, whereas topography interacted with both insects. Biotic factors, such as land use and vegetation type cover, were inversely associated with infestation and parasitism. Natural habitat, high tree, high herb, and low shrub coverage were correlated with high F. caudatus parasitism and contrastingly to low infestation by C. cosyra. Infestation was also related to plant by host density of S. latifolius, agricultural methods, and soil characteristics. Some of these factors might be included in further investigations of their effect in habitat manipulation for fruit fly management exploiting CBC.
Abiotic factors: climate and soil
The analysis showed that temperature was associated with C. cosyra infestation, where high temperature was related to high infestation and low temperature to low infestation. High temperatures are generally observed in the northernmost region of the country and previous studies have reported high C. cosyra populations in the Sudanian zone (north) and low infestation in the Guinean zone (south) showing how minimum temperature and rainfall are negatively correlated with Ceratitis species (Gnanvossou et al., 2017). Ceratitis cosyra optimum temperature is slightly different from that of the most detrimental Tephritidae fruit fly species in Benin, B. dorsalis, which is not commonly found in zones with high temperatures such as Northern Sudan and Sahelian zones (Vayssières et al., 2009; De Villiers et al., 2013). Hence, interspecific competition between B. dorsalis and C. cosyra is low in the warmer areas compared to areas more suitable for B. dorsalis (Geurts et al., 2014; Gnanvossou et al., 2017). Precipitation pattern but not precipitation amount interacted with infestation; the unimodal rainfall pattern was correlated with low infestation. Unimodal rainfall occurs in the Sudanian zone where temperature is high; hence, the results denote that one factor is not singly determining population levels. No interaction between temperature, precipitation, or rainfall pattern and F. caudatus parasitism was observed. Annual precipitation ranges in the various sites were not a limiting factor for F. caudatus presence, yet precipitation has been suggested as a habitat requirement for F. caudatus distribution (Trostle Duke, 2005) and abundance (Vayssières et al., 2010a). The level of precipitation does not have a north–south pattern; some areas in the northern part of the country have as high precipitation as in the south. Climatic suitability studies of the closely related parasitoid species F. arisanus demonstrated that the south of Benin (Southern Guinean zone) along the coast is highly suitable for the wasp, whereas it was predicted that F. arisanus could not survive in the north of Benin (Lane et al., 2018). It is therefore likely that F. arisanus and F. caudatus have different climatic optima and that F. caudatus is more suitable as biocontrol agent in the Sudanian zone. A discrepancy in how abiotic factors correlate with either F. arisanus or its host B. dorsalis (De Villiers et al., 2015; Lane et al., 2018) might explain why biological control can be more efficient in some areas than in others.
Topography was related to both F. caudatus parasitism and C. cosyra infestation: flat areas had high F. caudatus parasitism and undulated areas had low infestation. Also, stony and sandy soil sites were related to high C. cosyra infestation, contrary to sites with clayey and silty soil. Fruit flies complete their development cycle in the soil, and the pupal formation and emergence rate is higher with less compact soil types (Ahmed et al., 2007). The results about topography are puzzling and need to be studied further to understand this correlation.
Biotic factors: natural habitat, cultivation, plant diversity, vegetation cover, and presence
High F. caudatus parasitism was detected in natural habitat areas with low cultivation, whereas C. cosyra infestation level was negatively related to natural areas but also to chemical input. Fruit fly management options, such as GF-120, which is an attractant and insecticide mixture, additionally disturb tephritid parasitoids such as F. arisanus, D. tryoni, and Psyttalia fletcheri Silvestri (Stark et al., 2004; Wang et al., 2005; Vayssières et al., 2009). However, pesticide use, which could have affected our results, was more related to closeness to high-input production systems (i.e., cotton) than to fruit orchards. Land use interacted both with F. caudatus parasitism and C. cosyra infestation, yet F. caudatus parasitism was more positively correlated with natural habitat than C. cosyra. Natural habitat importance for biological control varies depending on type of crop, pest, predator, land management, and landscape structure (Tscharntke et al., 2016). High complexity of the landscape, more often found in wild than in cultivated areas, might increase pest control (Rusch et al., 2013), due to an increase in natural enemy species, higher diversity of refuges, and hosts (Chaplin-Kramer & Kremen, 2012; Rega et al., 2018). Parasitoid and predator species might, however, overlap in functional traits and, hence, higher species diversity does not automatically increase parasitism (Menalled et al., 1999; Karp et al., 2018). An increase in plant species diversity might also increase the overlap in function and not automatically increase the function (nutrients, alternative hosts, overwintering habitat, refuge) for the parasitoid. Yet, overall herbivore suppression, natural enemy enhancement, and crop damage suppression are stronger in diversified cropping systems than in crops with none or few adjacent species (Letourneau et al., 2011). Although the presence of specific crops can facilitate the establishment of parasitoids in the area, some crops might also cause a dispersion of parasitoids into different plant hosts. Many studies show a positive relationship between plant species richness and the diversity of insect pests and natural enemies (Raupp et al., 2001; De Cauwer et al., 2006; Letourneau et al., 2011). Yet how plant diversity affects specific parasitoid species parasitism is less known. We found that plant diversity was neither correlated with C. cosyra infestation level nor with F. caudatus parasitism. However, establishment of parasitoids is generally greater in areas with a rich vegetation with nectar- and pollen-producing plants, than in areas without flowering plants (Tooker & Hanks, 2000; Zhang et al., 2004). Additionally, nectar feeding increases parasitoid longevity and fecundity (Lee & Heimpel, 2008; Nafziger & Fadamiro, 2011). Our results suggest that the parasitoids’ capacity to establish in Benin was not limited by flowering plants; yet, it is possible that certain plants might have an effect on the insects’ abundance and parasitism, which should be studied further.
The number of tree species related to C. cosyra infestation was higher than that of herbaceous plants. Coverage of the various plant life forms (herb, scrub, tree) as well as plant community was related to the two studied insects. Low herb and tree coverage, and high shrub coverage were associated with low F. caudatus parasitism. High herb coverage was likewise negatively related to C. cosyra infestation level. Areas with very high shrub coverage might have a low herb coverage and be poor in diversity of plant species (Báez & Collins, 2008), which might explain why the high coverage of shrubs is related to low F. caudatus parasitism. High parasitism and predation activity by important natural enemy groups (aphid predators, stem borer parasitoids, syrphids, spiders, rape pollen beetle parasitoids) are more often associated with herbaceous habitats than with arboreal habitats (Bianchi et al., 2006).
Resource availability (i.e., suitable hosts) is the principal factor for population fluctuations in fruit flies such as Anastrepha spp. (Celedonio-Hurtado et al., 1995; Aluja et al., 1996). Distance between host fruits and their fructification periods additionally affect fruit fly population level (Vayssières et al., 2015). Wild fruits play a role in the dynamics of fruit fly and parasitoid populations (White & Elson-Harris, 1992; Grové et al., 2017), as larval food, for roosting (Mcquate & Vargas, 2007), and as adult food sources (Nishida, 1958; Furtado et al., 2016). Fopius caudatus emerges from some of the known C. cosyra host fruits (Vayssières et al., 2010b, 2012) and high infestation level and high F. caudatus parasitism were observed in presence of shea tree, V. paradoxa, from which both C. cosyra and F. caudatus emerge (Vayssières et al., 2010b). High infestation was likewise observed when the target host plant (S. latifolius) was not abundant, possibly linked to few concurrent suitable hosts. However, cashew (Anacardium occidentale L.) plantation nearby linked to low C. cosyra infestation maybe due to off-season fruiting of cashew apples (S Mama Sambo, pers. obs.). Mango plantations in the site were not related to C. cosyra infestation in sampled S. latifolius, though F. caudatus parasitism correlated negatively with mango in the vicinity. In order to understand the preference, seasonality, and importance of vegetation composition, it is important to further examine the correlations (or the lack of them) between the wasp and the fruit fly’s host fruits. Another biotic factor that might affect both C. cosyra and F. caudatus is the presence of other competing fruit flies and natural enemies. The presence of, for example, predatory ants and invasive fruit flies potentially affects fruit fly parasitism and fruit fly infestation (Van Mélé et al., 2009; Appiah et al., 2014; Migani et al., 2017).
Conclusion
These first observations of habitat components that interact with F. caudatus parasitism may support further controlled studies of CBC to test manipulation of abiotic and biotic factors for enhanced suitability of the F. caudatus habitat. Several descriptive factors were related to both F. caudatus parasitism and C. cosyra infestation; yet, some of those factors displayed an inverse relationship, indicating that some areas might be more appropriate for fruit flies than for the parasitoid and vice versa. Land use and vegetation characteristics were related to the parasitism of F. caudatus, indicating features of suitable habitat for the wasp population. Infestation and F. caudatus parasitism were furthermore related to the presence of other host fruits, presence of plantation, and specific plant species. It is, however, important to note that causality was not studied, but rather correlation – hence, factors might merely be indicative of habitat suitability. Whether F. caudatus feeding is actually affected by specific plant species/families is important to investigate and studies in cultivated fruit orchards should also test how land use, vegetation characteristics, and composition of surrounding plants could be designed to create a suitable habitat for F. caudatus in order to sustain biological control.
Acknowledgements
We thank all the fruit collectors and the colleagues Pascal Ayelo, Rodolphe Layodé, Fabrice Gbenonsi, Cyrille Akponon, and Marcus Ahokpossi. We appreciate being hosted by the International Institute of Tropical Agriculture (IITA-Benin). This work was supported through the project 229-2013-1978 granted by The Swedish Research Council for Environment, Agricultural Science and Spatial Planning (Formas), and the component Applied Research of the Regional Project of Fruit Flies Management in West Africa (PLMF).




