Promises and challenges in insect–plant interactions
Abstract
There is tremendous diversity of interactions between plants and other species. These relationships range from antagonism to mutualism. Interactions of plants with members of their ecological community can lead to a profound metabolic reconfiguration of the plants’ physiology. This reconfiguration can favour beneficial organisms and deter antagonists like pathogens or herbivores. Determining the cellular and molecular dialogue between plants, microbes, and insects, and its ecological and evolutionary implications is important for understanding the options for each partner to adopt an adaptive response to its biotic environment. Moving forward, understanding how such ecological interactions are shaped by environmental change and how we potentially mitigate deleterious effects will be increasingly important. The development of integrative multidisciplinary approaches may provide new solutions to the major ecological and societal issues ahead of us. The rapid evolution of technology provides valuable tools and opens up novel ways to test hypotheses that were previously unanswerable, but requires that scientists master these tools, understand potential ethical problems flowing from their implementation, and train new generations of biologists with diverse technical skills. Here, we provide brief perspectives and discuss future promise and challenges for research on insect–plant interactions building on the 16th International Symposium on Insect–Plant interactions (SIP) meeting that was held in Tours, France (2–6 July 2017). Talks, posters, and discussions are distilled into key research areas in insect–plant interactions, highlighting the current state of the field and major challenges, and future directions for both applied and basic research.
Introduction
The study of insect–plant interactions is at the core of a vibrant community of scientists encompassing a broad range of biological questions from molecular to ecosystem level, all united by evolutionary biology. Interdisciplinary research is of major importance for understanding complex interactions between plants and insects. This research field has been revolutionized recently by new technologies and analytical approaches, including next-generation sequencing (NGS) and gene-editing technology (e.g., Clustered Regularly Interspaced Short Palindromic Repeats, CRISPR-Cas9). Advances have also been made in in vivo imaging and high-resolution chemical analyses. Molecular biology, genomics, chemistry, physiology, behavioural studies, and other approaches can now be conducted and their results integrated under controlled laboratory conditions and natural settings. This should enable us to achieve a more comprehensive understanding of complex ecological networks, the physiological, ecological, and evolutionary dynamics of these interactions, and the genetic basis of traits, and to test hypotheses that were previously unanswerable.
Deciphering molecular mechanisms underlying insect–plant interactions require understanding insect and microbial effectors on the one hand and plant responses on the other. However, plants have to interact with multiple biotic partners ranging from parasites to mutualists. The signalling networks that are activated by plants in response to parasitic, herbivorous, and beneficial organisms inevitably overlap. The regulation of the adaptive response of the plant must be finely balanced between defence and acquisition of benefits (Giron et al., 2013; Endara et al., 2017). But the ability to perceive and manipulate plant signals provides insects or pathogens novel adaptive capacities, enabling, for example, the ability to expand to new ecological niches (Fordyce, 2010). Our understanding of insect–plant interactions requires a deeper knowledge on the cellular and molecular dialogue between plants and insects but also the study of their ecological and evolutionary implications. Molecular biologists, geneticists, ecologists, and evolutionary biologists are joining forces to reach this goal and to integrate the molecular basis of insect–plant interactions into an ecological framework. Characterization of multitrophic interactions can reveal novel perspectives on the complexity of induced signalling networks that have evolved between plants and their attackers. Combining the understanding of molecular mechanisms of insect–plant interactions with phylogenetics and evolutionary genomics can help to uncover adaptations that allowed ecological diversification, specialization, and speciation.
Ultimately, discovering how plants defend themselves against phytophagous (or herbivore) insects and how insects adopt an appropriate adaptive response will benefit agriculture and forestry. It is increasingly clear that these agricultural systems rely on ecosystem services provided by natural ecosystems (Rusch et al., 2017). As natural and agricultural ecosystems face major environmental challenges, such as from climate change, the latest research can help understand and ameliorate the environmental crisis that we are now facing. Recent progress in thermal ecology is revealing how ecological interactions will be shaped by global changes and how we potentially mitigate deleterious effects.
Current investigations of plant–insect interactions hold promise for us to gain a better understanding of the functional, ecological, and evolutionary impacts of insect–plant interactions, with implications and relevance for both applied and fundamental research. This paper builds on the latest SIP meeting (2–6 July 2017, Tours, France) and provides brief perspectives on several key research areas in insect–plant interactions (Figure 1), including the current state of the field, discussions of important questions and challenges, and possible future directions and priorities. We start from the finely tuned mechanisms operating at the scale of the interaction between a plant and its aggressor, that is, the insect effectors and the plant responses. Then, this mechanistic background is put into the context of evolutionary ecology of multitrophic networks. We highlight two important fields of research that are moving forward our understanding of complex networks: (1) community ecology and phylogenetics, by revealing hidden biotic links, and (2) evolutionary genomics, by depicting the mechanisms leading to specialization and speciation. Finally, the main global abiotic and biotic pressures acting on these plant–insect networks open up new challenges for scientists working on plant–insect interactions: climate change and crop pest pressure. These two pressures are major drivers of change in the mechanisms of interactions between plants and insects. Several recent reviews have addressed the emerging key role of insect symbionts in insect–plant interactions (Frago et al., 2012; Biere & Bennett, 2013; Douglas, 2013; Sugio et al., 2015; Giron et al., 2017; Shikano et al., 2017) and the SIP meeting had a dedicated session sponsored by the EU COST action FA1405 (http://www.cost-fa1405.eu/). Rather than repeating some of their conclusions, a few key perspectives are briefly highlighted below.
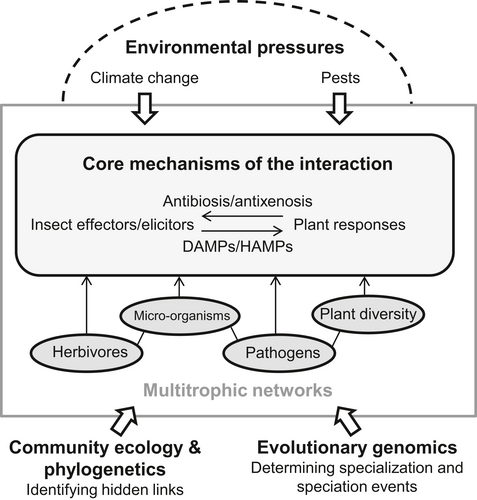
The mechanisms at the core of the interaction: insect effectors and plant responses
Current state of the field
How plants respond to insect herbivores has important consequences for the interacting players themselves as well as for the interactions between the responding plants and other plant-associated organisms (Sugio et al., 2015; Franco et al., 2017). Plants recognize herbivores through damage-associated molecular patterns (DAMPs) and herbivore-associated molecular patterns (HAMPs), also called elicitors (Dangl & Jones, 2001; Erb et al., 2012; Choi & Klessig, 2016). Upon DAMP and HAMP recognition, diverse defensive mechanisms are activated, aiming at reducing the damage of the herbivores through antibiosis (intoxication) and antixenosis (deterrence). Some herbivores have evolved the capacity to inhibit these responses and manipulate the plant's metabolism through the injection of effectors into the plant (Hogenhout & Bos, 2011; Kaloshian & Walling, 2016). Effectors from aphids and spider mites, for instance, have been shown to suppress plant defence signalling and responses, thereby increasing the performance of the herbivores (Atamian et al., 2013; Naessens et al., 2015; Schimmel et al., 2017). Highly specialized herbivores such as galling insects can inject growth hormones into their host plants to create resource sinks and extended phenotypes with unique morphologies (Figure 2). Current advances in the field have led to the identification of insect elicitors and effectors and have allowed for a more detailed understanding of the induced plant responses. However, several important questions remain open, as detailed below.

Future promises and challenges
Understanding how plants recognize herbivores, how herbivores manipulate plants, and how these two processes interact to generate plant response signatures is of major importance for our understanding of plant-herbivore interactions. Furthermore, understanding the molecular mechanisms and ecological consequences harbours potential for application in conservation and agriculture (see section ‘Pest pressure on agriculture and forestry’). We currently see two major types of challenge that need to be addressed in order to advance the field: (1) a deeper mechanistic understanding of elicitor/effector action, and (2) a broader appreciation of the modulation of plant responses to insects by other (micro)organisms and environmental factors.
- To develop functional characterization of elicitors/effectors. Whereas microbial pathogen effectors have been extensively studied, the study of effectors in herbivores has only recently attracted attention. Most of the work has been carried out with aphids and the Hessian fly, Mayetiola destructor (Say), for which genomes are available (Legeai et al., 2010; Zhao et al., 2015). Among genes identified and annotated, many herbivore proteins are predicted for secretion in aphids. Functional characterization of these proteins and identification of effectors are proceeding at a relatively slow pace. Current technology of targeted silencing of herbivore genes is inefficient and new approaches that target multiple putative effector genes are needed. Recent advances in the use of CRISPR-Cas9, a new gene-editing technology (Doudna & Charpentier, 2014), or exogenous application onto plants of herbivore-targeted double stranded RNA, similar to that used for plant pathogens, may provide future solutions (Wang & Jin, 2016; Sun et al., 2017).
- To identify molecular targets of insect elicitors and effectors. So far, we do not know how plants perceive elicitors, and we are only beginning to understand the targets of insect effectors. At this symposium, groups working on aphid (Atamian et al., 2013) or mite (Schimmel et al., 2017) effectors presented data identifying plant targets. Understanding and manipulating these targets will greatly enhance our understanding of the importance of elicitors and effectors in insect–plant interactions. Plant cell walls are the first barrier encountered by most plant pathogens and have to be degraded in order to allow penetration and colonization. Therefore, the degradation of the main cell wall components such as pectin and cellulose is essential for phytoparasitic organisms. Pectin is a highly abundant component of the plant cell wall contributing to the wall's protective function against phytopathogen attack. Therefore, hydrolysing the pectin backbone would help insects to get access to their host and to feed on it. Whereas unknown proteinaceous effectors promote herbivory in hemipteran insects and mites, pectolytic enzymes such as polygalacturonases (PGs) promote herbivory in wood-feeding insects, such as beetles (McKenna et al., 2016). Direct evidence for the role of PGs was demonstrated by silencing mustard leaf beetle PGs using RNA interference effecting growth and development of the insect (Kirsch et al., 2012, 2014). However, the molecular mechanisms of pathogen-induced plant manipulations are still poorly understood for most species, and sequencing their genomes, transcriptomes, or proteomes can reveal the effector repertoires of important plant parasites and help identifying genic modifications that contribute to their plant-parasitic lifestyle.
- To understand links between the induced signalling cascades and the diversity of plant responses to herbivores. In response to attack, plants evolved inducible defence systems. Two plant hormones, salicylic acid (SA) and jasmonic acid (JA), are controlling most of defence responses, but how a single hormonal cascade can create specificity and diversity remains unknown (Thaler et al., 2012). Including effector-mediated signalling and the manipulation of growth hormones into models of defence signalling will likely help to explain some of the specificity that is observed in nature. Plants and herbivores do not exist in isolation, but interact with a multitude of other organisms in nature (see section ‘Integrating core mechanisms into multitrophic interactions and ecological networks’). Microbes, but also natural enemies such as parasitoids, can strongly influence plant responses, possibly by changing the cocktail of elicitors and effectors that are injected into the plant. Understanding the mechanisms and consequences of these modulators is an important future challenge. If overcome, it will help to understand and predict the outcomes of insect–plant interactions in nature. To date, most research deciphering how herbivore oral secretions are perceived by the plants and trigger induced plant defences were conducted without considering that, in some instances, herbivores may harbour symbiotic bacteria or may be parasitized or infected by pathogens. It becomes clear that microbes associated with plants and insects can profoundly influence insect–plant interactions, favouring or improving insect fitness by suppressing plant defences and detoxifying defensive phytochemicals. Acevedo et al. (2017) investigated the effects of bacteria from oral secretions of the fall armyworm, Spodoptera frugiperda (JE Smith), on herbivore-induced defences in host plants. Among the bacterial isolates from the oral secretions identified in field-collected caterpillars, two isolates (Pantoea ananatis and Enterobacteriaceae-1) were shown to modulate anti-herbivore defences (JA-regulated defence transcripts) in host plants. In addition to harbouring symbiotic bacteria, the majority of insects may be parasitized by parasitoids that could manipulate the immunity of their herbivore host but also the immunity of the host plant of the herbivore. For example, the parasitoid Microplitis croceipes (Cresson) suppressed protein synthesis in the labial glands of its host Heliothis virescens (Fabricius) and, thus, affected the expression of key effectors such as glucose oxidase (GOX). Salivary GOX has been shown to elicit JA-regulated defences in tomato (Tian et al., 2012; Wang et al., 2017).
Integrating core mechanisms into multitrophic interactions and ecological networks
Current state of the field
Plants are members of complex, species-rich communities of associated organisms, including pollinators, herbivores, and pathogenic and non-pathogenic microorganisms that consume plant tissues or products, as well as carnivores and microorganisms that are associated with plant-consuming organisms (Stam et al., 2014). Plants may interact directly and indirectly with these members of the associated community, and multitrophic interactions are an important force shaping reticulate interaction webs (Wootton, 1994; Carvalheiro et al., 2014) that may also include competitive, facilitative, and other interactions (Bascompte & Jordano, 2012; Stam et al., 2014). Moreover, recent studies show that the phenotype of each organism may be influenced by microorganisms (Douglas, 2015), adding further complexity to multitrophic networks.
Recently, there has been an increase in the use of network analysis to describe relationships among plants and associated organisms (Bascompte & Jordano, 2012). This powerful analytical tool has been particularly well developed for the study of various types of mutualistic plant–animal interactions (Bascompte et al., 2003), such as plant–pollinators (Olesen & Jordano, 2002), plant–fruit dispersers (Nogales et al., 2016), and plant–mycorrhiza associations (Toju et al., 2014). Its importance is not only related to its efficiency in quantifying relevant parameters in complex systems, but also to its utility in examining how these parameters change across gradients or in response to perturbations, such as predicting community resilience to disturbance (Pocock et al., 2012). Recent developments of network analyses in community ecology include the incorporation of traits, such as evolutionary (Guimarães et al., 2011) or floral traits (Kantsa et al., 2017), as well as the study of multitrophic interactions (Leppänen et al., 2013; Vilela et al., 2014), all adding substantially to basic and applied goals in community ecology. For example, specialization, one of the most important concepts in multitrophic interactions (Forister et al., 2015), has been quantified, using network approaches, and compared across latitudinal gradients (Schleuning et al., 2012). However, two clear shortcomings of the network approach for community ecology and multitrophic interactions are: (1) appropriate temporal and spatial scales of interactions are not considered, thus we are left with ‘metawebs’ (Poisot et al., 2012) that do not actually exist in any one point in space or time (Scherrer et al., 2016), and (2) natural history is not sufficiently incorporated such that published webs do not accurately reflect actual interactions (Ballantyne et al., 2015) – for example, a parasite visiting a flower is often counted as a pollinator when only visitation is used to characterize pollinator webs (Figure 3).
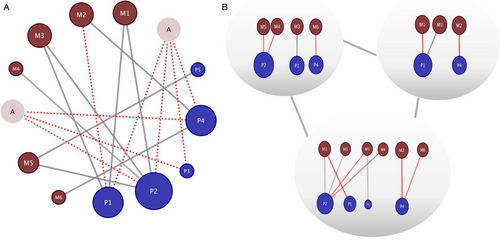
Modern manipulative experimental studies have taken two complementary approaches – autecological approaches starting with individual interactions in laboratory or mesocosm settings, and synecological approaches initiated by studies of entire multitrophic webs in the field. For instance, field studies have shown how the genotype-mediated phenotype (e.g., phytochemical mixtures of individual host plant species; Richards et al., 2015) or damage-induced plasticity in a plant's phenotype can influence which associated organisms colonize a host (Kessler et al., 2004; Poelman et al., 2010). Such synecological approaches can be complemented with autecological studies addressing the specific traits that influence long-lasting, community-wide effects. This may lead to the identification of specific plant genes (Kessler et al., 2004), herbivore characteristics (Turlings et al., 1990), herbivore-associated organisms (Poelman et al., 2011; Chung et al., 2013) or plant-associated microorganisms (Pineda et al., 2013) that influence multitrophic interactions. The latter may occur in different plant tissues, such as seeds (Hernandez-Cumplido et al., 2016) or roots (Rasmann et al., 2005), or in different tissues that may influence or interact with each other (Soler et al., 2013). Thus, although studies of plant–pollinator interactions and studies of plant-herbivore interactions have often been carried out independently, more integrative studies combining these interactions and their consequences are now emerging (Kessler & Halitschke, 2009; Lucas-Barbosa et al., 2016).
Another recent focus of multitrophic interactions research is to assess the effects of abiotic conditions, such as temperature and drought, on multitrophic interactions (Pineda et al., 2013; Becker et al., 2015; Pincebourde et al., 2017), which is relevant in the context of both climate change (see section ‘Global changes put the heat on insect–plant interactions’) and understanding of how multitrophic interactions vary along gradients, such as latitude and elevation (Descombes et al., 2017). Multitrophic interactions have traditionally been investigated at smaller scales, such as an individual plant or a local plant patch (Stam et al., 2014). However, multitrophic interactions at these smaller scales are embedded in a landscape of small food webs that are connected to each other via dispersal and other processes (Poisot et al., 2012). The effects of this spatial context should be included in studies of multitrophic interactions (Aartsma et al., 2017).
Future promises and challenges
During the last decade, empirical studies, meta-analyses, and ecological modelling approaches have greatly enhanced our knowledge of multitrophic interactions of plants with their associated communities. These studies have expanded the research focus from simple interactions to complex multi-interaction studies in a community ecology and networks context. Future challenges include the following:
- To integrate species interactions and related traits in multitrophic-multitrait systems at the scale of the entire community. For example, networks of interacting metabolites within a plant species (e.g., see the previous section) can be linked to networks of interacting arthropods in an entire community to examine how networks at various scales interact in dynamic systems (Richards et al., 2015). Ideally, such approach should map the various effectors and elicitors from each partner acting across the networks. This requires multidisciplinary approaches (Kantsa et al., 2017) and the training of biologists that can collaborate in studies addressing different levels of biological integration (Baldwin, 2012; Richards et al., 2015, 2016; Baude et al., 2016).
- To investigate the role of microorganisms in multitrophic interactions among macroorganisms. Every organism is a community in itself, consisting of a dynamic collection of micro- and macroorganisms (Gilbert et al., 2012) leading to various consequences for multitrophic interactions between plants and their associated community (Frago et al., 2012; Giron et al., 2013, 2017; Douglas, 2015; Sugio et al., 2015; Sanders et al., 2016). Investigating the effects of micro- and macroorganisms associated with plants or insects on insect–plant interactions will be an important next step in the development of the study of multitrophic interactions.
- To connect plant defence and reproduction. Integrating studies of plant defence and plant reproduction will help to understand the factors that influence interactions of plants with various interactants (Lucas-Barbosa et al., 2016; Chrétien et al., 2018). One important challenge nowadays is to understand pollinator decline (Biesmeijer et al., 2006), or even insect declines in general (Hallmann et al., 2017; Vogel, 2017) and the decline of ecosystem services such as pollination. The extent to which these declines can be linked to modification of the balance between plant defences and reproduction remains largely an open question.
- To extend studies to multiple spatial scales. To fill the scale gap, studies of multitrophic interactions must examine very small spatial and temporal scales at which interactions between organisms occur, as well as simultaneously extending to the landscape and broader geographical and evolutionary scales (e.g., Trøjelsgaard & Olesen, 2016; Galmán et al., 2018). Currently, it is not clear how much sampling is necessary for accurate estimates of network parameters at any scale, nor what are the relationships between local interaction networks and larger-scale network properties (Poisot et al., 2012; Fründ et al., 2016).
- To transfer knowledge towards key societal issues. Multitrophic interactions are relevant to a number of important applied issues, including agricultural practices (see section ‘Pest pressure on agriculture and forestry’), biological conservation, and restoration of ecosystem services in deteriorated habitats. For example, modern restoration efforts incorporate basic chemical ecology and plant–insect interaction theory to better manage for potential pests of trees planted for ecosystem restoration (Massad et al., 2011). Moreover, investigating the genetic architecture underlying plant responses to multiple attackers from different kingdoms is instrumental for breeding of crops that are resilient to environmental conditions and that are characterized by a species-rich community of attackers (Thoen et al., 2017; see section ‘Pest pressure on agriculture and forestry’). Such approaches will contribute to conserving nature and ecosystem services, as well as developing food production systems that fit in a multitrophic context, rather than eliminating biota from agricultural systems.
The hidden links revealed by community ecology and phylogenetics
Current state of the field
As explained above (section ‘Integrating core mechanisms into multitrophic interactions and ecological networks’), plants host diverse and dynamic insect communities. Those communities are strong agents of selection shaping various plant traits. Understanding the relative importance of biotic and abiotic factors that affect insect community assemblages and species co-occurrence on plants is thus central to our understanding of insect–plant interactions (Trivellone et al., 2017). The recent growing appreciation of the importance of induced plant defences has greatly enriched our view of the dynamic nature of herbivore communities on plants. When attacked by herbivores, plants often respond with drastic changes in their chemical profile, which may enhance or suppress the performance of subsequent (above- and belowground) herbivores (Kessler & Halitschke, 2007; Stam et al., 2014). Such an effect may sometimes persist over an entire season (Stam et al., 2014). Thus, the order of arrival of insects can have major consequences for the resulting insect community on plants. Similarly, a small number of herbivore species may act as keystone herbivores, thereby exerting a major effect on overall community assembly (Poelman & Kessler, 2016). Abiotic factors add another layer of complexity, because climate and resource availability can alter how plants allocate resources to structural and inducible defences (Züst & Agrawal, 2017), as well as insect microbial symbionts, as they can influence how insects interact with their host plants and natural enemies (Frago et al., 2012, 2017).
Although there is strong emphasis on antagonistic insect–plant interactions in the field of community ecology, communities of mutualists can also have a profound impact on plant performance and trait evolution. Many plants limit the identity of species with which they interact by developing mechanisms to filter out less-beneficial partners (Heil et al., 2014), but the conditions favouring such specialization are not well understood. Whereas mutualisms are often studied from the perspective of the benefits partners trade, accounting for costs can also help understand the community ecology of mutualism. For example, TM Palmer and colleagues demonstrate that in protective ant–plant mutualisms, less aggressive (and hence less beneficial) ant species can be important partners of plants under stressful (e.g., drought) conditions because they are less costly to maintain (require less plant resources) than the more aggressive ant partner (Stanton & Palmer, 2011).
Phylogenetics has traditionally been used to infer the ‘pattern’ of evolution in both mutualistic and antagonistic insect–plant interactions, for example, to test whether herbivore phylogeny mirrors host plant phylogeny or to determine the direction of host switches or trait evolution (Kergoat et al., 2017). With the recent sophistication of methods to incorporate timelines to phylogenetic trees and the development of various analytical tools using time-calibrated phylogenies, it is now possible to infer the ‘tempo’ of evolutionary events, and thereby to address a broader range of key evolutionary questions in insect–plant interactions. For example, studies have shown that shifts to novel host plant lineages can result in an initial burst of speciation with subsequent slowdown (Fordyce, 2010), that major climatic events may be associated with diet shift and diversification (Winkler et al., 2009), and that the evolution of plant defence traits can outpace that of herbivore counter-defence (Endara et al., 2017). Studies such as these are beginning to reveal how coevolutionary scenarios envisioned by earlier theoretical models fit real-world examples.
With the increasing ease of reconstructing phylogenetic relationships using DNA sequence data, the use of phylogenetic information in the study of community ecology has become widespread. For example, studies show that plant anti-herbivore traits are more or less independent of phylogeny and, thus, are evolutionarily labile, with frequent shifts in defence strategies as plants colonize new habitats. Similarity in herbivore communities among plant species is often correlated with similarity in defences rather than with plant phylogeny, so host selection by herbivores appears to be more evolutionarily constrained (Endara et al., 2017). Phylogenetic studies have also shown that patterns of tropical herbivorous insect diversity are driven not only by plant species diversity but also by a hidden niche axis generated by parasitoids (Condon et al., 2008, 2014). Comparative phylogenetic analyses have also shown that Wolbachia endosymbionts could play a role in how insects manipulate their host plant metabolism (Gutzwiller et al., 2015).
Finally, advances in molecular methods are helping to have a deeper understanding of plant–herbivore interactions. For instance, plant DNA can be extracted from herbivorous beetles and identified using DNA barcodes (Jurado-Rivera et al., 2009; García-Robledo et al., 2013). DNA barcoding has helped to reveal the full complexity of tropical plant–herbivore food webs and how the level of specialization varies between feeding guilds (Novotny et al., 2010). DNA barcodes have also shown that global estimates of herbivore diversity are heavily biased due to dearth of sampling in the tropics (Lees et al., 2013). In addition to traditional Sanger sequencing, the use of NGS amplicon sequencing allows the parallel acquisition of DNA barcodes from hundreds of specimens (Shokralla et al., 2014) and the characterization of communities of both insects and their associated microbial symbionts simultaneously (Gibson et al., 2014). The recent development of metabarcoding and environmental DNA barcoding is being used to unveil hidden interactions that are missed using traditional methods. For instance, metabarcoding technology has shown that pollinators are much more generalist than expected from visit surveys (Pornon et al., 2017).
Future promises and challenges
We outline four key questions that we consider important in the field of community ecology and phylogenetics.
- To combine the effects of multiple factors. How are insect communities on plants affected by the multiple factors that influence community structure? Experimental analyses of the drivers that shape insect communities on plants commonly assess each in isolation (induced defences, plant chemistry, plant genotype, abiotic stress, presence and identity of neighbouring plant species, etc.). Combining the effects of multiple factors can be challenging, but will yield important insights into how these drivers interact to affect community structure. Ultimately, this multifactorial approach should be integrated with the multitrait vision explained above to reach a complete understanding of the community structure dynamics (see section ‘Integrating core mechanisms into multitrophic interactions and ecological networks’).
- To compare beneficial and detrimental interactions. How do the communities of antagonistic and mutualistic insects differ? Antagonism and mutualism have historically been studied in near isolation in the field of insect–plant interactions, but there are processes common to the two communities that help improve our knowledge of community ecology.
- To integrate ‘deep history’ into the study of insect–plant interactions. How did past climatic and geological events affect the evolutionary outcomes of insect–plant interactions? Major climatic and geological events are often accompanied by major extinctions or new adaptive radiations, but whether major evolutionary transitions in insect–plant interaction (host plant family shifts, evolution of novel chemical defence, shift to novel feeding mode, etc.) occur after such climatic/geological events remains unclear. Recent developments in analytical platforms provide an opportunity to address these outstanding questions.
- To merge approaches in community ecology and phylogenetics. What determines the species richness of insects associated with plants, and how do such determinants vary along latitudinal gradients (see also section ‘Integrating core mechanisms into multitrophic interactions and ecological networks’)? Explaining the extraordinary diversity of herbivorous insects continues to be a major challenge in ecology and evolution. Studies based on phylogenies emphasize the role of key innovation or key colonization, whereas ecological studies identify the importance of available niches. Merging community ecology and phylogenetics approaches should thus bring major advancements in our understanding of the factors determining species richness and the latitudinal gradient in biological diversity.
Evolutionary genomics of specialization and speciation events
Current state of the field
Most herbivorous insect species feed on plants of single or closely related clades, some being adapted to a single plant species (Futuyma & Agrawal, 2009; Forister et al., 2015). Therefore, specialization to their host plants is a dominant feature of phytophagous insect biology. This specialization pattern not only involves host plant as a feeding source but also as a multi-dimensional ecological niche of the insect, providing resources and conditions for its development, reproduction, nutrition, and protection (Kergoat et al., 2017). Since they colonized land, plants and insects have been engaged in an evolutionary arms race, which has led to the evolution of sophisticated plant defence mechanisms on the one hand (Howe & Jander, 2008; War et al., 2012), and adaptive responses in the herbivorous insects through a wide range of behavioural, ecological, and physiological mechanisms on the other hand (Simon et al., 2015). This arms race is also a major driver of diversification, as plants and herbivorous insects contribute to more than half of current estimated biodiversity (Mullen & Shaw, 2014; Wiens et al., 2015). In parallel, some herbivorous insects have developed mutualistic interactions with their host plants, as in the case of pollination, creating more ecological opportunities and possibilities for species diversification (Schatz et al., 2017). Insect–plant interactions offer many opportunities to study general mechanisms facilitating the organism's adaptation to the environment and the consequences of this for speciation and patterns of diversification (Figure 4; Gloss et al., 2016).

Evolutionary genomics (i.e., the study of genomic changes over the course of evolution enabled by ‘omics’ technologies) has revolutionized the field of adaptation, by enabling better establishment of links between genotypes and phenotypes, and by reconciling macro- and micro-evolutionary approaches to the study of adaptation and speciation. This is particularly true for studies on insect–plant interactions where major advances have been made on the mechanisms underlying adaptation to host plants in herbivorous insects, and on how host adaptation can trigger insect diversification (Matsubayashi et al., 2010; Nosil & Feder, 2011). Many herbivorous insects cause dramatic damage to plants, either directly or indirectly (e.g., as vectors of plant diseases). Therefore, evolutionary genomic approaches to insect–plant interactions also offer the potential to bring the necessary knowledge and a robust conceptual and theoretical framework to the development of sustainable strategies for control of crop pest insects, such as those relying on enhanced plant defences (see section ‘Pest pressure on agriculture and forestry’). Here, we briefly highlight three lines of research where we think insect–plant interactions have benefitted enormously from evolutionary genomics, allowing major achievements in knowledge acquisition and application (Figure 4).
In recent years, decisive progress has been made on the molecular mechanisms underlying plant choice and exploitation by herbivorous insects. Candidate gene studies, genome-wide association methods without any a priori knowledge, and comparative genomics have provided solid evidence for the involvement of key genes and functions in groups of insect herbivores. Chemosensory genes (e.g., olfactory, gustatory, and ionotropic receptors) have been identified as key components of host plant choice (Goldman-Huertas et al., 2015; Karageorgi et al., 2017). Effector proteins triggering or suppressing plant defences have been characterized in oral secretions from a range of arthropods including dipterans, hemipterans, lepidopterans, and acarines (see section ‘The mechanisms at the core of the interaction: Insect effectors and plant responses’; Elzinga & Jander, 2013; Naessens et al., 2015; Zhao et al., 2015). Specific insect enzymes allowing digestion of plant nutrients or neutralization of toxic compounds were also identified as conferring a key role in plant adaptation (Bass et al., 2013; Alyokhin & Chen, 2017). In many cases, these characterized genes belong to gene families, highlighting the importance of gene expansion in the evolution of insect diet breadth (Bass et al., 2013; Missbach et al., 2014; Zhao et al., 2015). Overall, these studies enabled the discovery of a diversity of mechanisms underlying plant adaptation in herbivorous insects, aiding to our understanding on how they function beyond model species. Studies on the mechanisms of plant defences against herbivorous insects have continued to flourish at an unprecedented pace, uncovering the complexity of the plant defence system in response to herbivory (Hogenhout & Bos, 2011; Kaloshian & Walling, 2016). In some systems, identification of mechanisms and pathways involved in molecular insect–plant interactions has allowed unveiling the evolutionary arms races between plants and insects. This is the case for the evolution of glucosinolate defences in the Brassicales, which parallels the evolution of the nitrile-specifier family in the associated Pierinae (Edger et al., 2015). In this association, the increase in chemical defence complexity in the plants and the counter adaptation mechanisms in the butterflies were, thus, shown to be facilitated by gene and genome duplications.
Studies in herbivorous insects have played a key role in developing and testing theoretical and conceptual models for speciation. Convincing empirical evidence has accumulated over the past several years that plant specialization by insects may trigger divergent selection in herbivorous insect populations that can further increase genomic differentiation at some barrier loci, eventually leading to reproductive isolation and speciation (Peccoud et al., 2009; Martin et al., 2013; Soria-Carrasco et al., 2014; Riesch et al., 2017).
Future promises and challenges
- To intensify comparative genomics studies and the use of quantitative genetics. Conceptually and theoretically grounded approaches that include large-scale genome sequencing, transcriptomics, proteomics, experimental evolution, and genome-wide association hold great promise to extend our knowledge on the mechanisms that underlie adaptations to plant hosts of model and non-model herbivores (Gloss et al., 2016). Comparative genomics between generalist and specialist insects will be particularly valuable in relevant systems as such studies may allow to better link patterns of genomic evolution (e.g., gene expansions) with insect host range and feeding strategy (Simon et al., 2015). More quantitative genetics studies are also needed to better understand the genomic architecture underlying the evolution of host shifts, specialization, and generalism in insects. This is of paramount importance to examine potential interactions between loci controlling insect traits for plant specialization, and to characterize the genomic regions under the quantitative trait loci (QTLs) for further gene discovery and functional validation (Kanvil et al., 2015).
- To link genotype to phenotype through functional studies. Several key genes and their functions that are putatively involved in the evolution of host range have recently been identified and more will inevitably be discovered in the near future. A limiting factor for deciphering the specific role of a gene in its interaction with the host plant is the lack of functional information. Many genes of unknown function are identified in these studies, which rely on gene ontology analysis from gene prediction databases. Thus, functional analyses of these genes are needed to substantiate their involvement and role in host plant selection and exploitation. Integrative approaches linking -omics technologies, such as transcriptomics, proteomics, and metabolomics, offer great potential to pinpoint candidate genes for further functional characterization. This, combined with emerging tools in evolutionary genomics, is increasingly accessible for non-model organisms (e.g., CRISPR-Cas9 genome editing), allowing functional validation of candidate genes (Koutroumpa et al., 2016). Similarly, plant defence strategies against insects are being characterized in an increasing number of systems (War et al., 2012; Züst & Agrawal, 2016). Increased knowledge in key mechanisms of insect–plant interactions may help to elucidate the evolutionary histories of the arms races between antagonists and subsequent effects on diversification patterns.
- To implement knowledge for the development of sustainable agricultural strategies. Deciphering the functions and genes involved in insect–plant interactions will undoubtedly offer new targets for controlling crop pest insects. For example, knowledge of the functions of saliva proteins will help to select plant genotypes that are less sensitive to those protein functions. Similarly, identifying chemosensory genes involved in host plant recognition by insects may provide the basis for screening potential attractants or repulsive plant volatiles in order to elaborate push-pull strategies (Bruce et al., 2015; Zhang et al., 2017).
- To combine approaches on various biological systems. Coupling population genomic, phylogeographic, and phylogenetic (phylogenomic) analyses across taxonomic levels (e.g., species complex, genus, family) on insect taxa with different host range and feeding strategies has to be generalized to accumulate more robust evidence on the role played by plant specialization on diversification and speciation in herbivorous insects (Kergoat et al., 2017). This will allow for a better understanding of the evolutionary dynamics of insect–plant interactions, and further our knowledge on how genetic differentiation accumulates throughout the genome over the course of the speciation process (Nosil et al., 2017).
- To integrate insect symbionts in the study of insect–plant interactions. Herbivorous insects host microbial communities and there is growing evidence that they expand the insect's ability to exploit plants and modulate plant primary and secondary metabolisms and/or defences against parasites. Evolutionary genomics of insect–plant interactions should, thus, explicitly consider the diversity, dynamics, and roles of microbial communities associated with insects and their host plants, as their contribution to insect adaptation and diversification has been largely ignored (Kaiser et al., 2010; Gutzwiller et al., 2015; Sugio et al., 2015; Giron et al., 2017; Shikano et al., 2017).
Global changes put the heat on insect–plant interactions
Current state of the field
Plants and insects are the two most successful groups of multicellular organisms on the planet, and they frequently interact strongly with one another – most obviously when insect herbivores eat plant tissues or when insect adults carry pollen between flowers, but also in more complex, communication-based networks of trophic relationships among insects or in large-scale effects of insect-transmitted pathogens of plants. Because plants and insects are primarily ectotherms, a comprehensive background of the biophysics and thermal biology of at least some species in both groups is readily available. Here, we first give a brief overview of the state of the field, focusing on areas of relative strength. We then turn to areas of uncertainty, which arise from many sources, and which prevent us from establishing strong frameworks capable of predicting the future of insect–plant interactions during climate change.
Plants are filters of environmental conditions (Figure 5). The thermal budget of plant organs, individual plants, and plant covers has been well studied (Gates, 1980; Campbell & Norman, 1998; Nobel, 1999). Briefly, the ecophysiology of plants interacts strongly with environmental factors; for example, irradiance increases surface temperature, whereas transpiration through stomata lowers leaf temperature. Leaf temperature can be higher than ambient air temperature, in particular in temperate areas, whereas the reverse is also true in subtropical regions (Linacre, 1967; Pincebourde & Woods, 2012). Diverse physiological, behavioural, and biochemical processes, including numerous life-history traits (e.g., developmental rate) of arthropods are highly sensitive to temperature (Chown & Nicolson, 2004; Kingsolver, 2009). A few studies have related insect performance to measured leaf temperatures, and they have all demonstrated a strong impact (e.g., Pincebourde et al., 2007, 2017; Potter et al., 2009; Saudreau et al., 2013). The evolution of thermal biology traits is driven by environmental conditions and by the degree to which insects experience leaf surface temperature (Woods, 2013; Caillon et al., 2014). The strength of this link may, however, change over time for an organism as it grows larger during development. Increases in body size push them further out of the leaf surface boundary layer, which determines the interaction strength between leaf temperature and the insect (Woods, 2013).
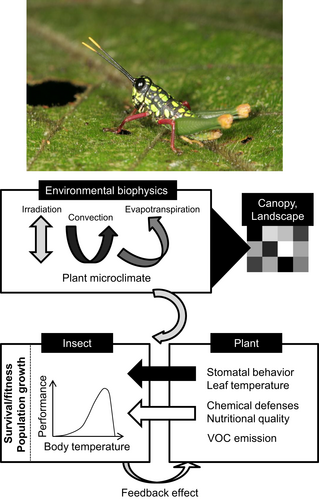
Biophysical mechanisms matter for insect–plant interactions (Figure 5; Pincebourde et al., 2017). Niche models often ignore these microclimatic conditions at the location of the plant and the insect (Potter et al., 2013). Typical methods for modelling species distributions or population dynamics and how these processes might respond to climate change include species distribution models (SDMs) or environmental niche models (ENMs) (Elith & Leathwick, 2009; Buckley et al., 2010). However, they seldom model the specific microclimate at the insect–plant interface. By contrast, rather coarse spatial or temporal estimates of the niche are provided based on coarse underlying climate datasets, which in turn may introduce considerable inherent bias (Potter et al., 2013). Moreover, the distribution of weather stations across the world is highly uneven, and the raw climatic data can be sporadic (Dillon et al., 2010). This means that some regions rely more heavily on spatial interpolation than others and it is not known whether this matters for fine-scale microsite interactions. In addition, when integrated into such models, the focal organism's biology or key traits are typically kept static although plasticity of both behavioural and physiological traits is well documented, either in terms of magnitude, timing, or persistence or some combination thereof (Sgrò et al., 2016). Furthermore, few, if any, studies have considered modelling the interactions among the major processes (e.g., nutrition, plant defences, temperature, plant hydration status) that interact to determine microclimate conditions (Pincebourde & Woods, 2012) and hence determine the population dynamics at the insect–plant microsite, while accounting for these various dynamic processes (but see Pincebourde & Casas, 2015; Kleynhans et al., 2018). Mechanistic, semi-deterministic, agent-based or process-based modelling frameworks, including integral projection models (IPMs), are designed to handle such dynamic complexity in a spatiotemporally explicit manner and, with growing computational power, this, therefore, becomes an increasingly tractable problem (e.g., Coulson et al., 2017).
For insects associated with plants, the plant adds another layer of complexity because the plant responds to environmental changes. The great variety of factors at play generates a suite of interrelated direct and indirect effects of environmental changes onto the insect (Pincebourde et al., 2017). For example, changing humidity or irradiation level (after a change in architecture, for instance) causes a change in leaf surface temperatures and, therefore, cascades onto insect performance (Saudreau et al., 2013). Another example: for insect-built structures such as galls or cambium mines, a change in gas composition within the structure – for instance, after a sudden variation in plant assimilation rate – might make the insect less tolerant to high temperatures (Pincebourde & Casas, 2016). Indeed, this dovetails with a major research focus area for arthropod thermal physiology – the link between an organism's metabolic supply and demand and whether it sets thermal tolerance in a deterministic fashion (e.g., Boardman & Terblanche, 2015; reviewed in Verberk et al., 2016).
In addition, the thermal biology of insects provides important insights into how insects tolerate high temperatures while feeding on their host plant. Tolerance to high temperature depends largely on the physiological state of the insect, including its nutritional or hydration status (e.g., Bujan & Kaspari, 2017; Mitchell et al., 2017) and age (Bowler & Terblanche, 2008). Therefore, any influence of the environment on the plant nutritional quality is likely to modulate the thermal sensitivity of the phytophagous insect (Clissold et al., 2013). The thermal sensitivity of insects usually varies when feeding on host plants that differ in their nutritional quality (Kleynhans et al., 2014). Finally, climate change also includes a gradual increase in atmospheric CO2 concentration. Although plant biologists have been working a lot on the effect of increasing atmospheric CO2 on plant growth and productivity (Bazzaz, 1990), comparatively little is known about the impact of CO2 on insects (Nicolas & Sillans, 1989). Although the direct effect of increasing atmospheric CO2 on the insect may be negligible (Kerr et al., 2013), the indirect impact via the consequences on plant ecophysiology can be dramatic (Zavala et al., 2013; Pincebourde et al., 2017). Increasing CO2 modifies both plant nutritional status and levels of chemical defences (Bidart-Bouzat & Imeh-Nathaniel, 2008), and it also reduces stomatal conductance and transpiration rate (Field et al., 1995), which in turn raises leaf surface temperature (Jarvis, 1976). The survival of insects likely relies on their thermal biology and ability to sustain the novel thermal environment created at the leaf surface by a CO2-enriched atmosphere. Overall, the biophysical approach of modelling the heat budget of organisms is promising as it can integrate the many factors that might play a role in the response of plants and insects to global climate change.
Future promises and challenges
We highlight five avenues that we think deserve more attention in the short term. This list is not exhaustive, but our aim is to prioritize actions that we consider are needed to improve our ability to accurately quantify the effects of global climate changes on insects associated with plants.
- To estimate the insect–plant microclimate. Although the microclimatic conditions established by plants have been measured and modelled for decades, lack of coherent and comprehensive databases on the temperature of leaves or other plant organs across latitudes, altitudes, and biomes is currently a major limitation (but see Michaletz et al., 2015). Evolutionary hypotheses on a macroecological gradient of leaf temperatures remain largely untested (Helliker & Richter, 2008; Pincebourde & Woods, 2012; Michaletz et al., 2015; Dong et al., 2017), making it difficult to infer any macroecological gradients of insect responses to environmental changes. Furthermore, technological progresses should make it easier in the near future to collect a large amount of microclimatic data, either via high-resolution remote sensing (e.g., satellites, unmanned aerial vehicles; Faye et al., 2016) or micro-robotics (Floreano & Wood, 2015).
- To develop methods of trait measurement. High-quality, high-resolution data are needed not only on microsites but also on the thermal biology traits of herbivores. Recent advances (Terblanche et al., 2011) demonstrated that methodology can have a huge influence on the outcomes of thermal biology assays and careful thought is required to make good inferences about the potential detrimental or lethal temperatures for terrestrial invertebrates. The broad-scale compilations of data that are currently popular (e.g., Sunday et al., 2011; Hoffmann et al., 2013) may be inappropriate for inferring fine-scale insect–plant interactions and their responses to climate change (Pincebourde & Suppo, 2016). Perhaps, a mathematical solution can be achieved which allows for easy conversion between thermal limit estimates and their application to different purposes (e.g., comparative studies vs. predictive forecasting). Thus, more detailed consideration of how these traits are measured should be given in climate change impact models, or at least a mathematically robust way to convert any available metric of thermal performance or tolerance into diverse, ecologically relevant contexts. In addition, it is critical to consider the level of phenotypic plasticity of species, at each latitude and for each developmental stage as this can have a profound impact on performance and survival of arthropods. The plasticity of thermal biology traits should be assessed using realistic setups simulating specific environmental conditions (van Loon et al., 2005), and especially since transgenerational effects of temperature can have fitness implications at least in some instances (e.g., Klockmann et al., 2017). The effects of climate on traits such as insect effectors and elicitors (see section ‘The mechanisms at the core of the interaction: Insect effectors and plant responses’) remain largely ignored and untested.
- To experimentally address the multifactorial world. No framework has yet been developed that balances, on the one hand, the responses of the plant to variation in temperature and CO2 in terms of nutrition vs. defences for herbivores, and on the other hand, the responses of insect phenotypes to physical and chemical changes in their microhabitat (Pincebourde et al., 2017). The particular case of insects that live within the plant tissues (gallers, miners, borers) can provide insight on links between gas composition within their microsite and their warming tolerance limits (Pincebourde & Casas, 2016). Finally, the influence of variance of all factors, and the co-variance among them, should be considered as they are likely to matter for the dynamics of insect–plant interactions (Wetzel et al., 2016; Koussoroplis et al., 2017).
- To develop approaches to analysing fine-scale temporal and spatial variability. We know little about how spatial or temporal configurations of thermal patches affect the responses of insects to environmental changes (Pincebourde et al., 2016; but see Sears et al., 2016; on reptiles). Plants create a high heterogeneity (not just in temperature) at small scales from within a leaf (Saudreau et al., 2017), among leaves (Pincebourde et al., 2007), to within and among canopies (Leuzinger & Körner, 2007). Generally, we still have a poor understanding of how insects exploit, or are constrained by, this microsite heterogeneity (Kingsolver et al., 2011; Pincebourde & Woods, 2012; Woods, 2013; Caillon et al., 2014). Studies now need to integrate the displacements of individuals within these heterogeneous matrices of temperatures (Woods et al., 2015).
- To decipher how the environment modulates the chemical communication between the plant and its herbivores, predators, and parasitoids. Environmental factors can influence the emission of volatile organic compounds (VOCs; Wilson et al., 2015). The mechanisms of VOC emission, however, should be studied more deeply – we barely know if VOCs are emitted through stomata (Harley, 2013), which respond to temperature (Jarvis, 1976). VOC-based communication systems between plants, herbivores, predators, and parasitoids can be important to plant and insect fitness (Kessler & Heil, 2011), but they are inherently noisy systems (Wilson et al., 2015). Climate change is likely to have three classes of effects on olfactory signalling (Wilson et al., 2015). First, changes in local microclimates are likely to alter body temperatures of insects, which in turn may alter how they receive and process olfactory information. Second, changes in leaf temperatures will differentially alter fluxes through the biochemical pathways that produce VOCs, and likely will alter the vapour–pressure ratios of VOCs right at the leaf surface. In addition, changes in local hydrological cycles may alter relative stomatal opening, and many important VOCs may be emitted primarily through stomata. Third, changes in local communities of plants may alter the chemical background against which the VOCs of any particular plant are emitted. All of these effects represent climate-driven injection of noise into signalling systems. At present, we have a poor understanding of how robust and resilient signalling systems are in the face of these kinds of disturbances.
Pest pressure on agriculture and forestry
Current state of the field
In spite of rapid advances in technology and scientific understanding, pest pressure is expected to increase in the future in agriculture and forestry (Bebber et al., 2014; Wingfield et al., 2015). There are many reasons for this. Since the 1950s, pest management in many parts of the world has relied heavily on pesticides (Karabelas et al., 2009). Many of them are no longer effective because pests have evolved resistance (Sparks, 2013; Lombardo et al., 2016), whereas others have come under regulatory scrutiny and, as a result, have been removed from the market (Karabelas et al., 2009). Developing new pesticides that have a reduced risk of environmental hazard is becoming increasingly difficult and expensive (Sparks, 2013). Intensification and broad-spectrum pesticides also adversely affected populations of natural enemies (Rusch et al., 2010). Changes in plant cultivars, cultural practices, and climate are turning formerly benign insects into pests (Lüthi et al., 2015; Sallé et al., 2017). Climate change is helping pests to move into new regions (Paradis et al., 2008). Novel insect–plant interactions also arise from biological invasions (Bebber et al., 2014; Brockerhoff & Liebhold, 2017). Some of these new host interactions are made possible by new associations between insects and symbiotic microbes (Wingfield et al., 2016). An example of the addition of a symbiont elevating a species to ‘superbug’ pest status is the sweet potato whitefly, Bemisia tabaci (Gennadius). The rapid evolutionary ‘leap’ the whitefly was able to make via infection by a Rickettsia bacterium, which is a facultative symbiont, consisted of better survival and reproductive fitness on host plants (Himler et al., 2011). Microbial symbionts can also contribute to the expansion of pest host range (Hansen & Moran, 2014). Therefore, the ever-changing mixture of old and new plant–pest and plant–pest–symbiont interactions makes the task of the pest manager increasingly difficult. This difficulty is likely to be exacerbated by the rapid turnover of agricultural practices which further generate opportunities to study the population dynamics of insect–plant interactions under unsteady and highly perturbed conditions. But this new opportunity of research has not been taken yet.
Future promises and challenges
Scientists can rely on novel strategies and tools to design future resistant plant genotypes and stands, and to delay or prevent pest adaptation to resistant plant genotypes. These are a few challenges that face scientists who study insect–plant interactions with hopes of translating their knowledge into solutions for pest management.
- To quantify benefits and costs of plant diversification. To mitigate the negative effects of intensification in crops and woody species, there is ongoing interest in the pest regulation services associated with plant diversification. Diversification can be considered at various scales: the landscape scale, the stand or crop scale when combinations of different species or genotypes are being used, the plant scale if cultural practices promote the structural diversity, and the molecular scale through the ‘stacking’ of different resistance genes within a single elite genotype. Promoting this diversification at various scales can help to delay insect adaptation to resistant host genotypes, such as Bt crops (Jin et al., 2015; Carrière et al., 2016). Plant diversity at the stand or landscape levels can also reduce pest damage on focal hosts through associational resistance, which can occur via reduced ability of pests to locate their hosts, modifications of host nutritional quality, and/or resistance level, or increased multitrophic interactions (Barbosa et al., 2009; Letourneau et al., 2011). For instance, defoliation of broadleaved trees generally decreases with the number of tree species in a stand (Guyot et al., 2016). Although it seems to occur less frequently than associational resistance, plant diversity may also promote focal plants’ susceptibility to pests through associational susceptibility (Barbosa et al., 2009; Jactel et al., 2017). For example, trees with generalist defoliators (e.g., birch) can suffer from higher damage when mixed with other tree species as a result of dietary mixing, which enhances the performance of the herbivores (Wein et al., 2016). There is still much to be learned about factors affecting associational resistance and susceptibility. Several practical issues must be addressed in order to predict the optimal mixture of plant diversification for pest management services (Jactel et al., 2017). What is the most relevant plant taxonomic level? What is the most relevant spatial scale? What are the temporal dynamics of the management services especially in perennial crops or forests? It must also be noted that, although diversification is generally associated with increased plant protection, there might be downsides for farmers and growers who achieve reduced economies of scale, by rededicating land, labour, and capital to less profitable plant production (Schroth & Ruf, 2014).
- To translate knowledge of insect–plant interactions from model systems. Eighteen years ago, Arabidopsis thaliana (L.) Heynh. was the first plant to have its genome sequenced (Arabidopsis Genome Initiative, 2000). The fact that this paper has now been cited over 8 000× illustrates the usefulness of this model system for plant biology, with plant biotic interactions being an important aspect. Several other model systems for plant biotic interactions stand out for the contributions they have made to insect–plant interactions, for example, Nicotiana attenuata Torr. ex S. Watson, Brassicaceae other than Arabidopsis, wild parsnip (Pastinaca sativa L.), and milkweed (Asclepias spp.). Each has created tools for exploring genetics, genomics, cell biology, physiology, systems biology, plant defence via allelochemicals, and insect adaptation to chemical defences. While research continues in these model systems, we are also beginning to see the research and technology ‘roadmaps’ developed from these model plant systems transferred to plants that are important for forestry and agriculture (e.g., wheat; Harris et al., 2015).
- To embrace plant and insect -omics. For scientists who want to translate knowledge into management tactics, ‘roadmaps’ provided by model systems show that genomics is a good place to start. The availability of fast, low-cost sequencing technology means that the genome of every major crop plant, and of an increasing number of cultivated trees, has either been or now is being sequenced. A question addressed in all genome papers is: what does the genome tell us about the challenges the species has faced throughout its history and how has it adapted to these challenges? In plant genomes, there can be evidence of biotic challenges, such as Resistance genes, as was the case for A. thaliana (Yi & Richards, 2007). As genomes become available for more and more genotypes within the plant species and transcriptomics adds another layer of discernment, a sense emerges of the traits the plant has evolved for protection against biotic stress. Likewise, genomes and transcriptomes are becoming available for related plant species, again revealing traits important for defence. Taken together, this knowledge draws the spectrum of traits that might be utilized by plant breeders to build a broadly resistant plant. A sense of how large the pool of plant species might be for finding useful traits comes from a recent study by Krattinger et al. (2016), who found that a durable multipathogen Resistance gene in wheat conferred partial resistance to a blast fungal pathogen in rice. In parallel with plant model systems, there are now many deeply studied ‘model’ molecular systems for agents of biotic stress (see also section ‘The mechanisms at the core of the interaction: Insect effectors and plant responses’). Most of these are in plant pathology, which was quicker to embrace genetics and the various -omics than ‘plant entomology’. Entomologists have an animal model that provides a ‘roadmap’: plant-parasitic nematodes, for example, root knot and cyst nematodes. Insect genomics and the various other -omics, which are really just starting for herbivores relative to plant pathogens, will have important benefits for pest management. In insect genome papers, a question relevant for pest management is: what is the genomic signature of living a such-and-such lifestyle? For insect herbivores, the host plant is, perhaps, the greatest challenge, as was evident in the pea aphid, the first insect herbivore to have a published genome (The International Aphid Genomics Consortium, 2010) and the Hessian fly, the second insect herbivore to have a published genome (Zhao et al., 2015). The insect adaptations for living with a particular plant become candidate targets for the pest management tactics of the future.
- To keep up with new technologies. Gene editing is a revolutionary technology that will have major impacts on pest management. The new gene-editing technology CRISPR burst on the biology scene just a few short years ago (Doudna & Charpentier, 2014). CRISPR allows scientists to target genes in an organism's genome conveniently and precisely and provides opportunities to tinker with many genes at one time. There can be off-target effects such as mutations in protein-coding genes that are not targeted by the RNA-guided nucleases (Fu et al., 2013), but it is expected that these problems will be resolved. CRISPR has already made significant contributions to understanding the function of individual genes and how genes work together or against each other to produce plant, disease, and pest phenotypes (see sections ‘The mechanisms at the core of the interaction: Insect effectors and plant responses’, ‘The hidden links revealed by community ecology and phylogenetics’, and ‘Evolutionary genomics of specialization and speciation events’). CRISPR has already been used to manipulate Resistance genes in crops such as wheat and rice (Wang et al., 2014, 2016). CRISPR is also creating knowledge about insects that could be used to inform selection of candidate plant traits for plant resistance to insects. In 2017, CRISPR was used to manipulate genes involved in recognition of olfactory cues in ants, thereby altering behaviour and brain development in ways that adversely affected ant fitness (Trible et al., 2017). Other new technologies that are expected to have relevance for pest management are RNA interference (RNAi; Kupferschmidt, 2013) and gene drive, an idea that was theoretical until new CRISPR technology was discovered. Using CRISPR and knowledge of selfish elements in genomes, it is proposed that a genetic trait could be ‘driven’ into a population at a rate that is much faster than when occurring through normal Mendelian inheritance. Gene drive has been proposed to help save endangered species like birds (Esvelt et al., 2014), eradicate unwanted species like mosquitoes, or more simply to eliminate ‘bad’ traits from a species, such as the ability to transmit human diseases, such as malaria or dengue. Scientific groups in the USA initially gave the go-ahead for exploring gene drive in ‘carefully controlled field trials’ (National Academies of Sciences, Engineering, and Medicine, 2016), although there are warnings about field trials being too risky (Esvelt & Gemmell, 2017).
- To join research communities assembling around valued plant species. Research communities are now being assembled around highly valued plant species. Assembling the community often begins when collaborations develop among members of a genome initiative. If the plant community persists after publication of the genome, it creates a forum for interdisciplinary discussions and sharing of expertise and technology. It is critical that scientists studying insect–plant interactions be part of these plant scientist communities. Participation ensures that arthropods (both as antagonists and mutualists of plants) are considered when engaging the process of building the ideal plant and going about tinkering with features of the plant that might be ‘perfected’: protection against large numbers of harmful pathogens, promotion of pollinators and beneficial symbionts, more efficient utilization of nutrients and water, and maximizing benefits of photosynthesis. As the perfect plant is created, it is likely that conflicts will arise. Two possible examples for plant biotic interactions are as follows: (1) a conflict between ‘perfecting’ the plant for protection against herbivores vs. pathogens, or (2) a conflict between ‘perfecting’ the plant for protection against herbivores vs. promotion of pollinators. An example of a research community that has assembled around a valued plant species is The Wheat Initiative (wheatinitiative.org), whose goal ‘Coordinating global research for wheat’ relies on the grassroots creation of Expert Working Groups. One of these Expert Working Groups (‘Control of wheat pathogens and pests’) recently released an overview of diverse wheat pests (arthropods, nematodes, and rodents), prospects for new pest management technologies, the impact of climate change on wheat insect pests, and wheat pest management case studies in New Zealand and Australia (Eigenbrode & Macfadyen, 2017; Harris et al., 2017a,b; Horrocks et al., 2017).
- To develop relationships with plant breeders. Plant breeders decide which traits will be incorporated into elite cultivars and they only do this if they are aware of the trait and if there is strong evidence of its benefits. The traits that might be engineered into plants are not just traits that confer direct resistance, for example, Resistance genes and toxins, but also traits that confer indirect resistance, for example, traits that attract natural enemies (see also section ‘The mechanisms at the core of the interaction: Insect effectors and plant responses’). Cabbage genotypes differ in their ability to attract natural enemies (Poelman et al., 2009). Wheat was engineered to produce an aphid alarm pheromone that was expected to force aphids off of plants and attract parasitoids to plants (Bruce et al., 2015). Genotypes with enhanced resistance or tolerance to phytophagous insects are among the most effective, economical, and sustainable methods of pest management (Mitchell et al., 2016). It is, therefore, imperative that entomologists develop relationships with plant breeders, who will want to know which insects are a particular concern and who will tell entomologists where pest resistance sits within the hierarchy of traits that are of concern for the plant breeder. Creating a new plant cultivar can now be achieved in a fraction of the time it took even 10 years ago (Lombardo et al., 2016). High throughput genotyping and phenotyping are both playing important roles (Goggin et al., 2015). A three-step approach, MutRenSeq, combines chemical mutagenesis with exome capture and sequencing allows for rapid cloning of disease-resistance genes (Steuernagel et al., 2016). Having a cloned resistance gene means that plant breeders now have perfect genetic markers for tracking the desirable gene through the process of its transfer to elite cultivars via marker-assisted selection.
- To keep up with regulatory issues and advocate for pest management strategies that are more sustainable. What regulatory restrictions will be brought to bear on CRISPR gene editing is being decided at this very moment. Typically, each country will decide for itself. It seems likely that regulatory agencies in many countries will exempt certain ‘smaller’ CRISPR crop modifications from the regulations that were put in place for transgenic GM crops. It should be noted that agriculture and related fields reliant on plants are not what is driving regulations at this time. Rather it is the promise that CRISPR holds for human health. A recent article in the Wall Street Journal (2018) addressed the disparities between the United States and China regarding governmental concerns about the possible hazards of CRISPR. China is now going ahead with human trials. With human health driving the debate about CRISPR, it is possible that the regulatory climate for CRISPR plants may, at least to some degree, follow in the footsteps of what is allowed for CRISPR animals, including our own species.
Conclusions
Despite the diversity of questions and problems addressed in the field of insect–plant interactions, many challenges remain. The first concerns the necessity to develop and master new technologies (e.g., CRIPSR-Cas9). This is clearly a limiting factor in our quest to identify the role of genes in specific ecological interactions and in our ability to manipulate biological systems. The rapid development of technologies provides valuable tools, but much still needs to be done especially in non-model organisms. A second general challenge concerns our ability to go beyond model systems and to transfer laboratory-based knowledge into natural ecosystems, including in multispecies interactions. A third challenge concerns the need to develop studies across various spatial scales, from microclimates to landscape and larger geographical scale, and that encompass various time scales (integrating geological and ecological events). Finally, above all, one of the greatest challenges is to integrate the diversity of factors that shape insect–plant interactions, from multiple factors (biotic and abiotic) to multiple partners (beneficial and detrimental micro- and macroorganisms) and multiple traits (defence, growth, reproduction). Integrating this diversity into experiments and models is far from trivial, and no general framework has yet been developed.
Despite these challenges, the study of insect–plant interactions has a rich future. Forums such as the International Symposium on Insect–Plant interactions promote the training of a new generation of biologists with diverse technical skills but also with a better awareness of how the study of insect–plant interactions can help provide solutions to the major ecological and societal issues ahead of us. The next decade is likely to see major progress in unravelling the mechanisms underlying insect–plant interactions and their functional, ecological, and evolutionary consequences, with implications and relevance for both applied and fundamental research. They will shed light on exciting research topics and hold promise for the preservation of ecosystem services, development of sustainable food production, and adaptation to global change.
Acknowledgements
We thank the participants of the 16th International Symposium on Insect–Plant interactions for many of the ideas put forward in this article. This paper was supported by the sponsors of this meeting, especially the French National Center for Scientific Research (CNRS), the University of Tours, the French National Institute for Agricultural Research (INRA), the Région Centre Val-de-Loire (project no. 2014-00094521), the European Cooperation in Science & Technology (COST) program, Wiley, the British Ecological Society, the MIDI network and the Féri Network.




