Temperature and humidity acclimation increase desiccation resistance in the butterfly Bicyclus anynana
Abstract
Desiccation resistance, that is, the ability to reduce water loss, is an ecologically important trait relevant to all terrestrial organisms, which may constrain species distributions. Nevertheless, relatively few studies have investigated plastic capacities in desiccation resistance. We here investigate plastic responses in body mass change, used as a proxy of desiccation resistance, to variation in temperature and relative humidity in the tropical butterfly Bicyclus anynana (Butler) (Lepidoptera: Nymphalidae). Our results indicate that butterflies acclimated to a higher (27 °C) compared with a lower temperature (18 °C) and a lower (50%) compared with a higher (90%) relative humidity displayed a decreased loss of body mass, and therefore likely a loss of body water (27 °C: 11%, 18 °C: 15%; 50% r.h.: 14%, 90% r.h.: 18%). Thus, mass loss was reduced under conditions indicating increased desiccation risk, suggesting adaptive phenotypic plasticity. Effects were most pronounced during the first 24 h after acclimation, indicating quick and transient responses to environmental conditions. As anthropogenic climate change is predicted to increase the magnitude and frequency of heat and drought periods, we argue that more studies on plastic capacities in traits relating to desiccation resistance are needed to better understand species responses.
Introduction
All organisms rely on access to water, as biochemical reactions as well as transport take place within aqueous solutions (Han et al., 2008; Sassi & Hasson, 2013). Thus, keeping body water at constantly high levels poses a principal challenge for all terrestrial organisms, due to the much higher water content of the tissue as compared with the surrounding medium air (Edney, 1977; Pelletier, 1994). Consequently, water loss is a severe threat, with water availability and humidity representing important constraints on species distributions (Hadley, 1994; Chown et al., 2011). Due to their small body mass, resulting in a high surface area-to-volume ratio, terrestrial insects are particularly susceptible to desiccation, and their evolutionary success is at least partly based on their ability to tolerate low humidity (Chown & Nicolson, 2004; Chown et al., 2011).
Animals lose water through passive diffusion, respiration, and defecation, with loss rates being affected by activity, temperature, and relative air humidity (Pelletier, 1994; Chown et al., 2011). Insects possess two main strategies to deal with desiccation: tolerance and resistance (Edney, 1977). Desiccation tolerance is the ability to tolerate the loss of a significant proportion of body water. For example, terrestrial arthropods in arid environments are able to survive water losses of up to 40–67% of their body mass (e.g., scorpions, insects, mites; Toolson & Hadley, 1977; Arlian & Veselica, 1979). Desiccation resistance is a mechanism to avoid dehydration. In insects, diffusion through the cuticle is the most important factor affecting water loss (Edney, 1977; Stinziano et al., 2015). Consequently, desiccation resistance often involves specialised cuticles including epicuticular waxes (Bazinet et al., 2010; Chown et al., 2011; Stinziano et al., 2015; Arcaz et al., 2016).
Water loss rates differ widely among species and developmental stages (Edney, 1977; Pelletier, 1994; Sassi & Hasson, 2013). However, to what extent species and individuals have the ability to adjust desiccation resistance plastically is not particularly well understood. This is partly due to inconsistent results with regard to the effects of environmental factors (Chown et al., 2011; Kleynhans & Terblanche, 2011). Furthermore, although there is a substantial body of literature on plastic responses in thermal tolerance (e.g., Fischer et al., 2010; Sgro et al., 2016), comparable studies on desiccation resistance are relatively rare and largely restricted to dipterans (e.g., Hoffmann, 1990; Sjursen et al., 2001; Holmstrup et al., 2002; Hoffmann et al., 2005; Elnitsky et al., 2008; Leinaas et al., 2009), although this trait may be ecologically as important as thermal tolerance. The latter is especially true within the current era of anthropogenic climate change, during which heat and drought spells are predicted to increase in frequency, concomitantly increasing desiccation risk (IPCC, 2014).
Against this background, we here investigate plastic responses in the loss of body mass, used as a proxy for desiccation resistance, to variation in temperature and relative humidity, comprising two key extrinsic factors influencing survival (Lyons et al., 2014). We use the tropical butterfly Bicyclus anynana (Butler) (Lepidoptera: Nymphalidae), a species inhabiting a highly seasonal environment with alternating wet-warm and dry-cool seasons, such that it relies heavily on phenotypic plasticity to master associated challenges (Brakefield, 1997; Lyytinen et al., 2004). Temperature induces, for instance, plastic responses in growth and development, reproduction, and survival in this species (Fischer et al., 2010, 2014; Klockmann et al., 2017). We predict that acclimation at a higher temperature and at a lower relative humidity, both indicating increased desiccation risk, increase subsequent desiccation resistance by reducing mass loss. We further explore sex differences in desiccation resistance, predicting that females are the more desiccation-resistant sex as has been found in some other insects (Sassi & Hasson, 2013; Lyons et al., 2014).
Material and methods
Study organism and insect rearing
Bicyclus anynana is a tropical fruit-feeding butterfly ranging from southern Africa to Ethiopia (Larsen, 1991). As an adaptation to alternate wet-dry seasonal environments and the associated changes in resting background and predation, this species exhibits striking phenotypic plasticity (two seasonal morphs; Lyytinen et al., 2004). Reproduction is confined to the favourable wet season during which food and oviposition plants are abundantly available and 2–3 generations occur (Brakefield & Reitsma, 1991; Brakefield, 1997). The dry season, which lasts for at least 6 months, is characterised by a lack of rain, low relative humidity, and cool temperatures (Brakefield, 1997). Concomitantly, neither food nor suitable hostplants are available, and therefore reproduction is halted until the first rains indicate the beginning of the next wet season (Brakefield & Reitsma, 1991; Brakefield, 1997).
In 1988, a laboratory stock population was established at Leiden University, The Netherlands, from over 80 gravid females collected at a single locality in Malawi. From the Leiden stock population, a laboratory population was established in 2007 at Greifswald University, Germany, from which all animals for this experiment originated. Several hundred adults are reared in each generation, maintaining high levels of heterozygosity at neutral loci (van't Hof et al., 2005). All animals used in the experiments described below were reared under wet season conditions (27 °C, 70% r.h., L12:D12 photoperiod) on young maize plants within large population cages (50 × 50 × 50 cm). Unless otherwise stated, these conditions were used throughout.
Experimental design
To investigate phenotypic plasticity in desiccation resistance we performed two experiments, one using thermal and one using humidity acclimation. Both experiments involved an acclimation and a subsequent testing phase. Butterflies were randomly allocated to acclimation treatments on the day of adult eclosion. Consequently, body mass at eclosion did not differ among treatments (t-test: both P>0.16). During acclimation, all butterflies were fed with banana and water ad libitum, whereas neither was provided during the testing phase. For each butterfly, we scored body mass (1) ca. 6 h after adult eclosion (eclosion mass), (2) after the acclimation period (acclimation mass), and (3) 24 h after the start of the testing phase (test mass), using a Sartorius LE225D microscale (Sartorius, Goettingen, Germany). In the first experiment, additionally the mass 48 h after the start of the testing phase was measured. This was not done in the second experiment as effects of acclimation were largely confined to the 1st day after acclimation in experiment 1 (see below). On the basis of these data, we calculated the mass changes during acclimation and during the testing phase. Thus, we used mass loss during a 24-h testing phase as a proxy of water loss, assuming that mass changes during a period without access to food or water are primarily driven by water loss. Minimising water loss is in turn considered a key factor increasing desiccation resistance. We thus used standard procedures in insect ecology (e.g., Pelletier, 1994; Leinaas et al., 2009; Terblanche & Kleynhans, 2009). To additionally score longevity without access to food or water (as a proxy of dehydration survival), we checked twice a day (at 11:00 and 18:00 hours) whether butterflies were still alive.
In experiment 1, butterflies were randomly distributed across two temperatures (18 and 27 °C) for a 3-day acclimation period (relative humidity was kept a favourable level of 70% throughout). Sample size was 43 males and 48 females at 18 °C and 33 males and 57 females at 27 °C. After acclimation, all butterflies were transferred to 22.5 °C and 50% r.h. until death to score mass changes and longevity. The intermediate temperature was used for testing to make sure that both treatment groups experienced the same magnitude of change in environmental conditions (4.5 °C each). During acclimation and testing, all individuals were kept individually in 100-ml plastic cups covered by gauze. For acclimation and the subsequent testing phase, three technically identical climate cabinets were used (MLR-351H; Sanyo, Bad Nenndorf, Germany).
In experiment 2, butterflies were randomly allocated to two relative humidity treatments (50 and 90%) for a 3-day acclimation period (favourable temperature of 27 °C throughout). Sample size was 40 males and 34 females at 50% r.h. and 23 males and 37 females at 90% r.h. Afterwards, all butterflies were transferred to 70% r.h. and 27 °C until death. Comparable to above, an intermediate humidity was used for testing (20% change in relative humidity each). Otherwise, the experiment followed the above design, except that mass loss during testing was measured after 24 h only. The temperatures and humidities chosen are ecologically realistic and are regularly experienced by the butterflies in their natural environment. The higher temperature and humidity was chosen to mimic wet season, the lower ones reflect dry season conditions (Brakefield, 1997).
Data analysis
Data were analysed using analyses of covariance (ANCOVAs) with acclimation treatment and sex as fixed factors and body mass as covariate. Testing for normal distribution (Kolmogorov–Smirnov test) and variance homogeneity (Levene test) did not reveal major violations of ANOVA requirements. Thus, no transformations were applied to the data. Pair-wise comparisons were performed employing Tukey's honestly significant difference (HSD) test for unequal sample sizes. All statistical analyses were performed using STATISTICA v.8.0 (StatSoft, Tulsa, OK, USA). Throughout the text, least square means ± 1 SEM are given.
Results
Effects of thermal acclimation
After acclimation, butterflies acclimated to 27 °C (70.9 ±0.8 mg) had on average a significantly higher mass than those acclimated to 18 °C (64.4 ± 0.8 mg; Table 1). This temperature effect though was significant in females only (females: 84.5 ± 1.2 mg at 27 °C > 70.2 ± 1.3 mg at 18 °C; males: 57.4 ± 1.6 mg at 27 °C ≈ 58.6 ±1.5 mg at 18 °C; Tukey's HSD after ANOVA, significant temperature*sex interaction; Table 1). Females (77.4 ± 1.0 mg) were on average significantly heavier than males (58.0 ± 1.3 mg). The mass difference between acclimation temperatures resulted from an overall mass gain at 27 °C (3.4 ± 0.8 mg) but a mass loss at 18 °C (−3.2 ± 0.8 mg). Males actually lost similar amounts of mass at both temperatures, whereas females gained mass, with mass gain being significantly higher at 27 than at 18 °C (Tukey's HSD after ANOVA, significant interaction; Figure 1A). Overall, mass changes amounted to −9.6 ± 1.3 mg in males and 9.8 ± 1.0 mg in females.
| Variable | Factor | MS | d.f. | F | P |
|---|---|---|---|---|---|
| Acclimation mass | Acclimation temperature | 0.0018 | 1 | 31.3 | <0.0001 |
| Sex | 0.0057 | 1 | 97.2 | <0.0001 | |
| Acclimation temperature*sex | 0.0026 | 1 | 43.9 | <0.0001 | |
| Eclosion mass | 0.0065 | 1 | 111.1 | <0.0001 | |
| Error | <0.0001 | 176 | |||
| Mass change | Acclimation temperature | 0.0018 | 1 | 31.3 | <0.0001 |
| Sex | 0.0057 | 1 | 97.2 | <0.0001 | |
| Acclimation temperature*sex | 0.0026 | 1 | 43.9 | <0.0001 | |
| Eclosion mass | 0.0006 | 1 | 10.7 | 0.0013 | |
| Error | 0.0001 | 176 | |||
| Mass loss 24 h | Acclimation temperature | 0.7 × 10−4 | 1 | 8.8 | 0.0035 |
| Sex | 1.1 × 10−4 | 1 | 15.1 | 0.0002 | |
| Acclimation temperature*sex | <0.1 × 10−4 | 1 | 0.6 | 0.46 | |
| Acclimation mass | 2.1 × 10−4 | 1 | 27.2 | <0.0001 | |
| Error | 0.1 × 10−4 | 176 | |||
| Mass loss 24–48 h | Acclimation temperature | 0.2 × 10−5 | 1 | 0.9 | 0.33 |
| Sex | 3.3 × 10−5 | 1 | 16.9 | <0.0001 | |
| Acclimation temperature*sex | 0.8 × 10−5 | 1 | 4.1 | 0.044 | |
| Acclimation mass | 4.3 × 10−5 | 1 | 21.8 | <0.0001 | |
| Error | 0.2 × 10−5 | 176 | |||
| Longevity | Acclimation temperature | 4.4 | 1 | 9.8 | 0.0020 |
| Sex | 2.7 | 1 | 6.1 | 0.015 | |
| Acclimation temperature*sex | 1.9 | 1 | 4.3 | 0.039 | |
| Acclimation mass | 6.4 | 1 | 14.4 | 0.0002 | |
| Error | 0.4 | 176 |
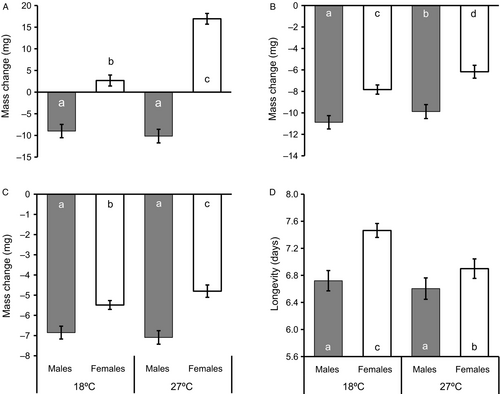
During the first 24 h of testing, mass loss was significantly higher in individuals acclimated to 18 °C (9.4 ± 0.3 mg; 14.6%) than to 27 °C (8.0 ± 0.3 mg; 11.3%), and in males (10.4 ± 0.6 mg; 17.9%) than in females (7.0 ± 0.4 mg; 9.0%) (Table 1, Figure 1B). Mass loss was positively related to initial mass (r = 0.241, P<0.001; n = 181). The overall pattern remained similar during the 2nd day of testing, though significant effects of acclimation temperature were restricted to females here (Tukey's HSD after ANOVA; significant interaction), which again indicated a higher mass loss after acclimation to 18 °C (Figure 1C). Mass loss was once more significantly higher in males than in females, and in heavier compared with lighter individuals (r = 0.161, P<0.01; n = 181). Longevity was in general significantly longer in animals acclimated to 18 °C (7.1 ± 0.1 days) than to 27 °C (6.8 ± 0.1 days), and in females (7.2 ± 0.1 days) than in males (6.7 ± 0.1 days). Note though that effects of temperature were significant in females only (Tukey's HSD after ANOVA; significant interaction; Figure 1D). Longevity was positively related to body mass (r = 0.616, P<0.001; n = 181).
Effects of humidity acclimation
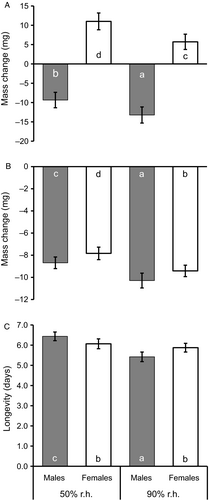
After acclimation, butterflies acclimated to 50% r.h. (59.0 ± 1.3 mg) were significantly heavier than those acclimated to 90% r.h. (55.3 ± 1.3 mg), and females (66.5 ± 1.6 mg) were significantly heavier than males (47.8 ± 1.6 mg; Table 2). The mass difference between humidity treatments resulted from a slight mass gain at 50% r.h. (0.8 ± 1.3 mg) but a mass loss at 90% r.h. (−3.8 ± 1.3 mg). As above, males lost mass (−11.3 ± 1.6 mg) whereas females (8.4 ± 1.6 mg) gained mass during acclimation (Figure 2A). During testing, mass loss was significantly higher in individuals acclimated to 90% r.h. (−9.9 ± 0.4 mg; 17.9%) than in those acclimated to 50% r.h. (−8.3 ± 0.3 mg; 14.1%), but did not differ across sexes (Figure 2B). Mass loss was positively related to initial body mass (r = 0.323, P<0.001; n = 134). Longevity was in general significantly longer in animals exposed to 50% r.h. (6.3 ± 0.1 days) than to 90% r.h. (5.7 ± 0.1 days) during acclimation. Note though that this effect was significant in males only (Tukey's HSD after ANOVA; significant interaction; Figure 2C). Longevity was positively related to body mass (r = 0.459, P<0.001; n = 156).
| Variable | Factor | MS | d.f. | F | P |
|---|---|---|---|---|---|
| Acclimation mass | Relative humidity | 0.0005 | 1 | 4.1 | 0.045 |
| Sex | 0.0065 | 1 | 49.9 | <0.0001 | |
| Humidity*sex | <0.0001 | 1 | <0.1 | 0.92 | |
| Eclosion mass | 0.0085 | 1 | 64.9 | <0.0001 | |
| Error | 0.0001 | 153 | |||
| Mass change | Relative humidity | 0.0008 | 1 | 6.3 | 0.013 |
| Sex | 0.0073 | 1 | 55.3 | <0.0001 | |
| Humidity*sex | <0.0001 | 1 | 0.2 | 0.70 | |
| Eclosion mass | 0.0016 | 1 | 12.3 | 0.0006 | |
| Error | 0.0001 | 153 | |||
| Mass loss 24 h | Relative humidity | 8.0 × 10−5 | 1 | 10.3 | 0.0017 |
| Sex | 1.00 × 10−5 | 1 | 1.3 | 0.26 | |
| Humidity*sex | <0.10 × 10−5 | 1 | <0.1 | 0.98 | |
| Acclimation mass | 9.00 × 10−5 | 1 | 11.5 | 0.0009 | |
| Error | 0.80 × 10−5 | 129 | |||
| Longevity | Relative humidity | 13.9 | 1 | 9.6 | 0.0023 |
| Sex | <0.1 | 1 | <0.1 | 0.89 | |
| Humidity*sex | 6.6 | 1 | 4.6 | 0.035 | |
| Acclimation mass | 21.9 | 1 | 15.2 | 0.0002 | |
| Error | 1.4 | 151 |
Discussion
We here measured changes in body mass, likely in the first place driven by water loss, as a proxy of desiccation resistance. Our results on thermal and humidity acclimation suggest adaptive phenotypic plasticity in desiccation resistance in B. anynana, as acclimation to a higher temperature as well as to a lower humidity decreased subsequent mass loss, in line with our a priori predictions (cf. Kleynhans et al., 2014). Thus, desiccation resistance seems to respond plastically to temperature in a way similar to thermal resistance traits in our study organism (Fischer et al., 2010; Klockmann et al., 2017). Previous studies on insects revealed contradictory evidence (Chown et al., 2011; Kleynhans & Terblanche, 2011). For instance, Gibbs et al. (1998), Terblanche et al. (2005), Leinaas et al. (2009), and Parkash & Ranga (2014) did not find an effect of acclimation temperature on water loss, whereas others did so (e.g., Hoffmann et al., 2005; Renault et al., 2005; Terblanche & Chown, 2006). Interestingly, even detrimental effects of heat hardening on desiccation resistance have been reported (Bubliy et al., 2012). The reasons for such differences are currently unknown, but may be related to differences in ecology and physiological properties of the species investigated, or to the costs involved in plastic responses (Bubliy et al., 2012). For instance, species inhabiting highly seasonal environments such as B. anynana may generally rely more strongly on phenotypic plasticity than other organisms. Another complication here might be population-specific plastic responses, as has been found in our study organism (de Jong et al., 2010, 2012). Taken together, our current knowledge on thermal plasticity in desiccation resistance is still quite limited and deserves further attention.
In contrast to thermal acclimation, plastic responses in desiccation resistance as induced by different levels of humidity have not been frequently investigated thus far, but most studies show effects similar to the ones reported here (e.g., Terblanche & Kleynhans, 2009; Bazinet et al., 2010; Parkash & Ranga, 2014; Parkash et al., 2014).
In our study, effects of acclimation temperature on mass loss were most pronounced during the first 24 h following acclimation, whereas later effects (24–48 h following acclimation) were weaker and restricted to females. This suggests that the means providing increased desiccation resistance respond very quickly to changes in environmental conditions. In insects, several mechanisms may contribute to a reduction in water loss under dry conditions (Edney, 1977; Hadley, 1994; Chown & Nicolson, 2004). This may involve behavioural, physiological, biochemical, or morphological adjustments such as a change in respiration or evaporation through the integument (Hadley, 1994; Gibbs et al., 1997, 2003). Potential candidate mechanisms include the expression of heat shock proteins, being upregulated not only under heat but also under desiccation stress (Michel & Starka, 1987; Hoffmann, 1990; Wang et al., 2011), a reduction in metabolic rate (Hoffmann & Parsons, 1989; Gibbs et al., 1997, 2003; Matzkin & Markow, 2009; Wang et al., 2011; Teets et al., 2012), and changes in osmolytes or epicuticular waxes (Bazinet et al., 2010; Chown et al., 2011; Parkash et al., 2014; Stinziano et al., 2015; Arcaz et al., 2016).
Although mass loss during testing was positively related to initial mass, males lost a substantially higher proportion of their mass than the in general heavier females. The general pattern of a sex difference in mass loss under food and water deprivation prevailed in both experiments, but attained significance in the thermal acclimation experiment only. Other studies also indicated that females are the more desiccation-resistant sex (Sassi & Hasson, 2013; Lyons et al., 2014). A higher water loss in males is presumably linked to their smaller size and a concomitantly higher surface area-to-volume ratio. Moreover, physiological differences may also contribute to sex differences, such as a higher male activity and a concomitantly higher metabolic rate (e.g., Fischer et al., 2004a). In addition, females may have been selected for more efficient strategies to avoid water loss, owing to (1) the need for water for egg production, (2) the loss of body water due to oviposition, and/or (3) a generally higher (desiccation) stress resistance, as females are expected to benefit more strongly from longer lifespans than males. The latter notion rests on the finding that female lifespan is often positively related to realised fecundity (Honek, 1993; Bauerfeind & Fischer, 2007, 2008). In contrast, male mating opportunities are often strongly skewed towards the beginning of the flight period, especially in species in which female remating is relatively rare as is the case here (Brakefield et al., 2001). In such systems long lifespans in males will yield very little fitness returns, which may also explain the generally longer lifespans of females versus males in B. anynana and other butterflies (see also Bauerfeind & Fischer, 2005; Bauerfeind et al., 2009; Karl & Fischer, 2009).
During acclimation, thus despite having access to food and water, males generally lost mass, presumably as an effect of ageing and the concomitant use of storage reserves, for instance to sustain flight activity. Females, in contrast, gained mass within the same period of time, which is most likely caused by oocyte production and maturation. Bicyclus anynana butterflies eclose without any mature eggs and oocyte maturation is critically dependent on adult nutrition (Fischer et al., 2004b; Bauerfeind & Fischer, 2005). Thus, females certainly produced substantial numbers of egg during acclimation. As they did not have the chance to mate and oviposit, an accumulation of eggs is expected which has likely resulted in increased body mass. The higher female mass gain at 27 °C compared with 18 °C is most likely caused by higher feeding rates at the higher temperature. In addition, oocyte maturation is itself temperature-dependent and thus quicker at higher temperatures (Steigenga & Fischer, 2007). With regard to humidity treatments, the overall higher mass of butterflies acclimated to 50% r.h. compared to 90% r.h. suggests detrimental effects of high humidity, though the underlying mechanisms are hitherto unknown.
The notion of high humidity being detrimental is also supported by the fact that, overall, lifespan was longer in animals exposed to 50% r.h. compared to 90% r.h. However, this finding contradicts others as low rather than high humidity typically reduces lifespan (e.g., Han et al., 2008; Pappas et al., 2008). Note that the relative humidities used here did not involve stressfully low levels, which may explain the deviation from the above results. Furthermore, the animals acclimated to 90% r.h. were afterwards transferred to 70% r.h., and their subsequently – as well as during the acclimation period – increased water loss may have reduced lifespan (cf. Terblanche & Kleynhans, 2009). The shorter lifespans of the animals acclimated at 27 vs. 18 °C, on the other hand, most likely reflect the higher metabolic rates during acclimation at the former temperature, resulting in increased rates of ageing (e.g., Chen et al., 2005; Karlsson & Wiklund, 2005).
To conclude, our study revealed evidence that desiccation resistance (measured as mass loss here) is a phenotypically plastic trait, which is readily and quickly responding to variation in temperature and humidity. Desiccation resistance is undoubtedly an ecologically important trait, as it may constrain species distributions (Hadley, 1994; Chown et al., 2011). This is particularly true for terrestrial insects, comprising a major fraction of biodiversity (Chown & Nicolson, 2004; Chown et al., 2011). Anthropogenic climate change will increase the magnitude and frequency of heat and drought spells, and thus desiccation risk (IPCC, 2014). As thus far results on acclamatory responses in desiccation resistance are contradictory (Chown et al., 2011), we suggest that more studies on plastic responses in this trait would greatly facilitate a better understanding of species responses to the expected changes in selection pressure.
Acknowledgements
We acknowledge financial support provided by Greifswald University.




