Functional analysis of centipede development supports roles for Wnt genes in posterior development and segment generation
SUMMARY
The genes of the Wnt family play important and highly conserved roles in posterior growth and development in a wide range of animal taxa. Wnt genes also operate in arthropod segmentation, and there has been much recent debate regarding the relationship between arthropod and vertebrate segmentation mechanisms. Due to its phylogenetic position, body form, and possession of many (11) Wnt genes, the centipede Strigamia maritima is a useful system with which to examine these issues. This study takes a functional approach based on treatment with lithium chloride, which causes ubiquitous activation of canonical Wnt signalling. This is the first functional developmental study performed in any of the 15,000 species of the arthropod subphylum Myriapoda. The expression of all 11 Wnt genes in Strigamia was analyzed in relation to posterior development. Three of these genes, Wnt11, Wnt5, and WntA, were strongly expressed in the posterior region and, thus, may play important roles in posterior developmental processes. In support of this hypothesis, LiCl treatment of S. maritima embryos was observed to produce posterior developmental defects and perturbations in AbdB and Delta expression. The effects of LiCl differ depending on the developmental stage treated, with more severe effects elicited by treatment during germband formation than by treatment at later stages. These results support a role for Wnt signalling in conferring posterior identity in Strigamia. In addition, data from this study are consistent with the hypothesis of segmentation based on a “clock and wavefront” mechanism operating in this species.
INTRODUCTION
The Wnt genes have highly conserved roles in posterior development across the Animalia (Ryan and Baxevanis 2007; Martin and Kimelman 2009; Niehrs 2010). This role can be divided into (i) conferring posterior identity on a part of the embryo; and (ii) controlling subsequent growth and development of the posterior (Petersen and Reddien 2009).
Studies on diverse animal taxa attest to the importance of the Wnt genes in posterior development. These include studies on poriferans (Adamska et al. 2007; Windsor and Leys 2010), cnidarians (Kusserow et al. 2005; Lee et al. 2006; Momose et al. 2008; Duffy et al. 2010), platyhelminthes (Gurley et al. 2008), and nematodes (Maloof et al. 1999; Silhankova and Korswagen 2007). In vertebrates, Wnt signalling is involved in establishing anteroposterior polarity (Liu et al. 1999; Kiecker and Niehrs 2001; Lekven et al. 2001), and later contributes to posterior development (Bang et al. 1999). In arthropods, Wnt signalling is involved in posterior identity and development; including roles for Wnt8 in the posterior development of the spider Parasteatoda tepidariorum (McGregor et al. 2008), and multiple Wnt genes operating in the posterior development of the beetle Tribolium (Bolognesi et al. 2008b, 2009; Beermann et al. 2011).
In both vertebrates and arthropods, posterior development is best understood within the context of their segmented body plans and of segment development. In vertebrates, the segments develop via a “clock and wavefront” generative mechanism, based on dynamic waves of oscillating gene expression (the clock) in the posterior of the embryo, which become fixed into permanent stripes of expression as they reach a determination front (the wavefront) a certain distance from the posterior end (Pourquié 2003). Wnt signalling, along with the FGF and Notch pathways (Jiang et al. 2000; Jouve et al. 2000), is vital in this process, involved in regulating both the oscillatory gene expression of the clock and the wavefront of determination (Aulehla and Pourquié 2008; Gibb et al. 2009).
Wnt signalling is important in sequential segmentation, as found in most arthropod taxa, and for the formation of segmental boundaries (Janssen et al. 2010). Moreover, arthropod segmentation systems exhibit certain similarities to vertebrate ones. In the spiders Cupiennius salei and Parasteatoda tepidariorum, dynamic Delta expression suggests a role for a Notch-signalling based oscillator in segment generation (Damen et al. 2000; Stollewerk et al. 2003; Schoppmeier and Damen 2005; Oda et al. 2007). In the beetle Tribolium castaneum, recent evidence based on dynamic gene expression suggests the activity of a clock-like segment generation mechanism (El-Sherif et al. 2012; Sarrazin et al. 2012). Evidence from the hemipteran bug Oncopeltus fasciatus and the cockroach Periplaneta americana is consistent with oscillation-based segmentation (Angelini and Kaufman 2005; De Robertis 2008; Pueyo et al. 2008), though Notch-Delta signalling does not seem to be required for segmentation in the cricket Gryllus bimaculatus (Kainz et al. 2011). In fact, dynamic gene expression involving Notch signalling and Wnt activity seems to be common to many arthropods and may represent an ancestral mechanism of posterior patterning (McGregor et al. 2009). Also, studies of segmentation in annelids have revealed some commonalities with that in arthropods (Prud'homme et al. 2003; de Rosa et al. 2005; Rivera and Weisblat 2009). The possibility of a common evolutionary origin for segmentation in these distantly-related phyla remains a matter of debate (Patel 2003; Tautz 2004; Couso 2009; Chipman 2010; Roth and Panfilio 2012).
Several issues require further investigation in relation to the roles of Wnt signalling in arthropods. What role does Wnt signalling play in (i) establishing posterior identity, (ii) controlling posterior growth and development, (iii) establishing a wavefront of segment determination, and (iv) controlling oscillatory gene expression in segment generation? Additionally, how do these processes interact? Considering the immense diversity of the arthropods, considerable variation in developmental mechanisms operating in this group is likely. Therefore, a comparative approach is necessary (Murat et al. 2010). It is important to examine the Wnt signalling system in phylogenetically diverse arthropod taxa, and the species examined should represent a wide range of morphological disparity.
Considering that most of the studies of arthropod development performed to date have focused on insects with complex trunk morphologies and reduced complements of Wnt genes, useful insights can be obtained by examining development in an arthropod with relatively low segment diversity, simple trunk morphology, and many Wnt genes, which is phylogenetically distant from the well-examined insects. So, our chosen study species is Strigamia maritima, a geophilomorph centipede. Previous study of Strigamia development points towards a segmentation system that shares many features with other arthropods (Kettle et al. 2003; Chipman et al. 2004a,2004b; Chipman and Akam 2008). Wnt gene activity in S. maritima has also been investigated, revealing that these genes operate in forcipular development (Hayden and Arthur 2013). Recent work (Hayden and Arthur 2014) has examined in detail the expression of the Wnt genes throughout the embryonic development of Strigamia and has established the subfamily affinities of each of these genes. The present study builds on those previous ones, and deals with Wnt expression as it relates to posterior development.
While the phylogenetic position and morphology of S. maritima make it a valuable study species in which to examine certain developmental and evolutionary questions, it has so far proved impossible to develop functional developmental techniques for this species, or, indeed, for any myriapod. The thick chorion of Strigamia eggs and the pressurized material within make microinjection highly impractical, if not impossible, and have frustrated multiple attempts by several experimenters. Accordingly, the functional approach taken here is to make use of lithium chloride, which acts as an uncompetitive inhibitor of GSK3β, thereby activating the canonical Wnt pathway (Klein and Melton 1996; Hedgepeth et al. 1997; Clément-Lacroix et al. 2005). While this technique is not as specific as RNAi perturbation of Wnt pathway components, it is currently the only feasible approach in this type of animal. Lithium chloride has been used to perturb Wnt signalling in a wide range of taxa, both arthropod (including Drosophila melanogaster) and other (including Xenopus laevis and the sea urchin Lytechimus variegatus (Cooke and Smith 1988; Sherwood and McClay 1997; Berger et al. 2005). The LiCl-treated centipede embryos studied here were subjected to a number of analyses, examining both LiCl-mediated phenotypic changes and LiCl-mediated changes in the expression of marker genes.
METHODS
Embryo sampling and preparation
As the life history of S. maritima is not conducive to captive breeding (Lewis ), all eggs required were sampled from a population at Gentian Hill (coordinates: 53°15′N 9°07′W) in Galway, Ireland in May and June of 2010 and 2011. Sampling and embryo culture were performed as described in Chipman et al. (2004b). Embryos were fixed for 24 h in 4% paraformaldehyde (PFA) for in situ hybridization.
Gene identification and amplification
The Wnt genes examined in this paper were previously identified and assigned to their respective subfamilies (Hayden and Arthur 2014).
Whole-mount in situ hybridization
Hybridization probes were produced by means of in vitro transcription with the incorporation of digoxigenin-ddUTP, using a premixed DIG RNA labeling mix (Roche, Switzerland). In situ hybridization was performed with a conventional protocol, adapted from that developed by Kettle et al. (2003) and further refined by Brena et al. (2006). The antibody utilized was an anti-DIG antibody coupled with alkaline phosphatase (Roche); the chromogenic substrate used was NBT/BCIP (Roche).
Mounting and microscopy
Intact embryos were examined and photographed under an Olympus SZX 16 stereomicroscope with an attached DP25 or DP71 camera. Strigamia embryos were also physically dissected and flat-mounted. Flat-mounted samples and sections were examined with an Olympus BX51 compound microscope, equipped with DIC and UV illumination capabilities. Images, including multiple image alignments, were taken with the Olympus CellB, CellD, and CellF software packages and later adjusted for color balance, brightness and contrast with the GIMP software package.
Cryo-sectioning
In order to allow high-resolution analysis of the expression of some genes, sectioning of embryos already subjected to in situ hybridization was performed. The samples were dehydrated into MeOH, then transferred stepwise into 20% sucrose as follows: 1× PBS, 1× PBS with 10% sucrose, 0.5× PBS with 10% sucrose, 0.5× PBS with 20% sucrose, 20% sucrose. Samples were then transferred to moulds containing OCT compound (VWR) and frozen, in 2-methyl-butane, cooled with liquid N2. Sections were taken with an MEV SLEE cryotome, at a thickness of 15–20 µM and moved onto gelatin-chromium coated slides. These were then stained with 1% toluidine blue in 70% EtOH, rinsed in ddH2O and allowed to dry. Samples were mounted in glycerol for imaging.
Lithium chloride treatment
Lithium treatment was performed at different developmental stages: treatment during germ-band formation allows for analysis of the roles of Wnt signalling in establishment of posterior identity, while treatment during segmentation and germ-band growth allows for analysis of the roles of Wnt signalling in the later developmental events of segmentation and posterior growth. The embryonic development of S. maritima, at a culture temperature of 13°C, lasts for ~47 days from early cleavage until hatching (Brena and Akam 2012). Lithium chloride treatment was performed by means of the replacement of some or all of the 150 mM NaCl in the normal embryo medium (locust embryo saline, LES) with LiCl. Having first performed preliminary experiments to establish suitable concentrations for treatment, two different treatment media were prepared, one with 50 mM LiCl and one with 150 mM LiCl. Preliminary studies were also conducted into the use of other “small molecule” compounds (Niclosamide, Quercetin, and dichloroacetic acid) that are known to perturb Wnt signalling. These compounds were applied via addition to the embryonic culture medium, but do not seem to pass through the Strigamia chorion.
Lithium treatment was performed by placing the eggs in the LiCl medium, sandwiched between filter papers, and allowing lithium to pass into the egg via diffusion. The period of interest in this study stretched from the formation of the blastoderm (stage 2.1) to the end of the main period of segmentation (stage 5). When embryos are cultured at 13°C, this period lasts for 13 days (Brena and Akam 2012). Embryos were treated either for five days at the start of the experimental period (early treatment), or for the last five days of the period (late treatment), or for the entire 13-day period (constant treatment). A negative control group was cultured in standard LES under the same conditions (no treatment). After the experimental period, all embryos were fixed for 24 h in 4% PFA. To determine the developmental stage of embryos, clutches collected from the field site were cultured in 1X LES under filter paper and one embryo from each clutch was examined under a stereomicroscope to ascertain its approximate stage. In situations where the examined egg was not yet at approximately stage 2.1, the clutch was allowed to develop further before being examined again. In situations where the clutch examined was close to stage 2.1, one egg was removed from the clutch, fixed for 24 h in 4% PFA, stained with DAPI, and examined under UV light to more precisely determine its current developmental stage. Having thus confirmed the exact developmental stage of the egg in question, the clutch from which it was taken, based on the close synchrony of Strigamia clutch development (Brena and Akam 2012), can be considered to be very close in developmental stage to the single sampled egg. In this manner, a high degree of confidence that all embryos were treated at the appropriate stage could be established.
Examination of embryonic phenotypes
The embryos from LiCl treatment experiments, after fixation, were cleared and treated with DAPI, allowing visualization of nucleic acids. DAPI treatment was performed at a concentration of 15 μg/ml for 30 min, followed by at least five successive washes in glycerol/PBS. For each embryo, it was first noted whether it had survived and formed a germ-band during the experimental period. The phenotypes of the surviving embryos were then examined. On the basis of the focus of the present study being on posterior identity/development and on segmentation, four criteria were chosen for analysis. The first three of these related to posterior identity and development: (a) the degree of truncation of the germ-band; (b) the curving of the germ-band; and (c) the size increase of posterior structures. A fourth criterion examined was (d) defects in segmentation. For each embryo, a score from 0 (normal phenotype) to 4 (most extreme phenotype) was assigned. The criteria used for each category of phenotypic change are summarized in Table 1.
| Criterion | Severity | Phenotype |
|---|---|---|
| Germ-band truncation | 0 | Germ-band length normal |
| 1 | Germ-band length decreased 25% | |
| 2 | Germ-band length halved | |
| 3 | Germ-band length decreased 75% | |
| 4 | Germ-band length <500 μm | |
| Germ-band curve | 0 | Germ-band entirely straight |
| 1 | Slight irregularity in germ-band shape | |
| 2 | Germ-band bends over 30° | |
| 3 | Germ-band bends over 60° | |
| 4 | Germ-band entirely torqued | |
| Posterior size | 0 | Posterior normal |
| 1 | Posterior size increased by less than half | |
| 2 | Posterior region size increased by half | |
| 3 | Posterior region size doubled | |
| 4 | Posterior region size increased 4-fold | |
| Segmentation | 0 | Segmentation normal |
| 1 | Disordering of <10% of segmental boundaries | |
| 2 | Disordering of ~25% of segmental boundaries | |
| 3 | Over half of segmental boundaries malformed | |
| 4 | No normal segments |
Statistical analysis
The results of the phenotypic scoring were subjected to statistical analysis. As the phenotypic scores represent categorical data, Chi-squared contingency table tests were employed, including both four-way and pairwise comparisons of the relevant samples. All statistical analyses were performed with the Minitab 15 software package.
RESULTS
Early posterior Wnt expression
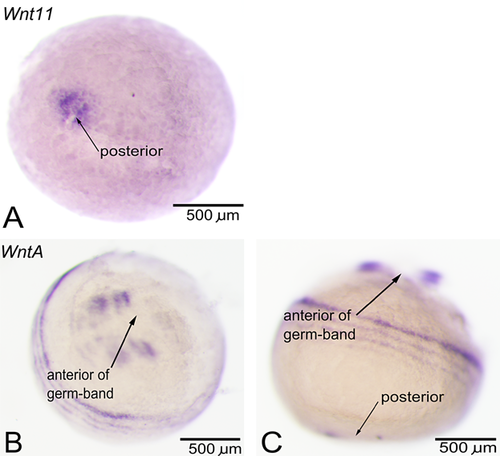
The expression of all 11 Wnt genes present in Strigamia was examined in relation to the establishment of posterior identity. Other aspects of Wnt expression have been dealt with in a separate publication (Hayden and Arthur 2014). One Wnt gene, Wnt11, was implicated in posterior identity, as it seems to be the only Wnt gene expressed in the posterior of the embryo during early (blastoderm stage) development. In the blastoderm (at stage 2.1), this gene is expressed as a broad domain at the presumptive future posterior end (Fig. 1A). Later, this expression retreats into a smaller region surrounding the proctodeum, as the germ-band forms. Because this expression of Wnt11 is present in the future posterior of the embryo before any anteroposterior polarity is visible, this gene is a likely candidate for the establishment of initial anteroposterior polarity. Of additional interest is the expression of WntA, with its dynamic waves of expression as rings surrounding the embryo's posterior end (Fig. 1, B and C). However, this expression is quite transient and fades away by stage 5. This expression may point towards a role for WntA in the control of the putative oscillatory mechanism.
Posterior Wnt expression during germ-band growth
All 11 Wnt genes present in Strigamia were examined in relation to possible roles in posterior growth and development. Nine Wnt genes are expressed at the posterior of the embryo during germ-band growth. Three of these, Wnt2, Wnt9, and Wnt16, only display very low transcript levels near the proctodeum. Four Wnt genes, Wnt1, Wnt6, Wnt10, and Wnt11, are expressed in a small domain surrounding the proctodeum during stages 4–5 (Fig. 2, A–D). In contrast to the small size of this expression domain, the region of posterior growth seems to be much larger (Brena and Akam 2012).
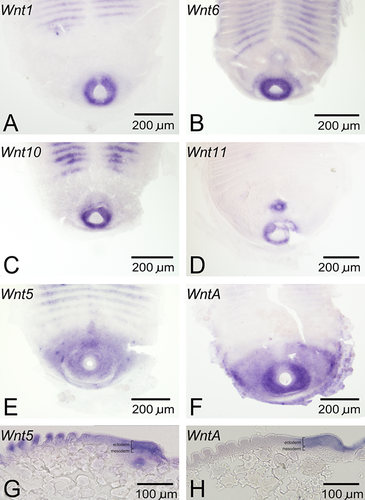
Two Wnt genes, Wnt5 and WntA, are expressed in much more extensive posterior domains (Fig. 2, E and F). These two genes may thus be the best candidates for roles in posterior growth. Considering that WntA is both segmentally expressed and expressed at the posterior of the embryo, the similarities to WntA expression in the millipede Glomeris are noteworthy (Janssen et al. 2010). Additionally, because the edge of the expression domains of both of these genes correspond to the segment-forming region (Chipman and Akam 2008), these genes are suitable candidates for defining a wavefront for the putative segmentation mechanism of Strigamia. When examined in flat-mounted embryos, these two genes appear to be almost completely co-expressed. However, there are subtle differences between them, with the anterior expression boundary of WntA being more clearly defined than that of Wnt5. Moreover, comparison of the expression domains of the two genes in sagittal sections (Fig. 2, G and H) reveals that WntA expression is apparently restricted to the ectoderm, while Wnt5 expression appears to extend into the mesoderm.
Effects of LiCl treatment on phenotype
To test for the role of Wnt signaling in posterior development and segmentation, the Wnt pathway was activated during early (germ band formation) or late (segmentation and germ band growth) Strigamia embryogenesis by treatment with different concentrations (50 mM, 150 mM) of LiCl. In addition, for each LiCl concentration some embryos were treated throughout both early and late periods (constant treatment) while others were left untreated to serve as a negative control. All embryos that survived the treatment period were categorized and scored according to the phenotypic scoring scheme described in Table 1. Sample sizes for each group are given in Table S1. Four categories of phenotypic perturbations were scored: germ-band truncations, changes in germ-band curvature, changes in posterior size, and segmentation defects. The stage at which embryos were treated caused clear differences, while the effect of LiCl concentration was quite minor (hence, embryos treated with different concentrations of LiCl were pooled). Examining the relative proportions of embryos falling into each of the phenotypic categories reveals a number of patterns, as shown in Figure 3.
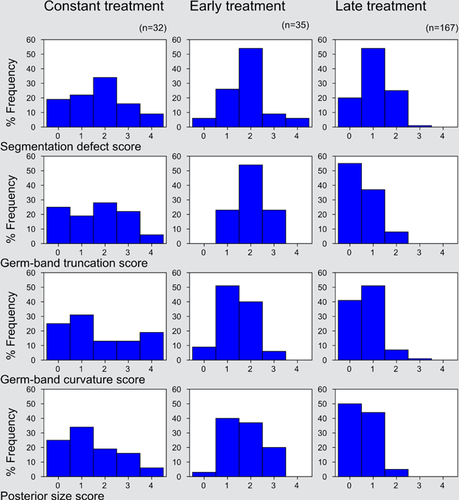
Most prominent among the patterns illustrated here is the difference between the late-treated embryos on the one hand and the early-treated and constant-treated groups on the other. The last two groups show a high degree of similarity in the typical magnitude of defect (though the constant-treated group shows more variation). Also of interest is the lesser degree of difference between the late-treated group and the early-treated and constant-treated embryos in the category of segmentation defects than in the other three categories, all of which relate more directly to posterior identity and development. This is consistent with the establishment of posterior identity before the main phase of segmentation, as might be expected based on analysis of Strigamia development (Brena and Akam 2012).
The scores obtained were then analyzed statistically, as follows. First, they were pooled, with scores from 0 to 1 and from 2 to 4 being combined, creating a binary distinction between unaffected or nearly unaffected embryos and moderately or severely affected embryos for each of the four categories of phenotypic change. Next, scores were analyzed by means of overall Chi-square contingency table tests to establish the existence of significant differences among the four test groups (early-treated, late-treated, constant-treated, and untreated). After that, a series of pairwise comparisons was performed, as shown in Table 2. All four categories of phenotypic change measured showed the same pattern, with a highly significant result for all comparisons except for the comparison of early-treated and constant-treated embryos.
| Comparison between: | χ2 | d.f | P |
|---|---|---|---|
| Seg-early, seg-late, seg-constant, seg-untreated | 79.352 | 3 | <0.001 |
| Seg-early, seg-late | 23.938 | 1 | <0.001 |
| Seg-early, seg-constant | 0.615 | 1 | 0.433 |
| Seg-late, seg-constant | 14.157 | 1 | <0.001 |
| Seg-early, seg-untreated | 72.857 | 1 | <0.001 |
| Seg-late, seg-untreated | 26.389 | 1 | <0.001 |
| Seg-constant, seg-untreated | 60.254 | 1 | <0.001 |
| Tru-early, tru-late, tru-constant, tru-untreated | 117.445 | 3 | <0.001 |
| Tru-early, tru-late | 71.461 | 1 | <0.001 |
| Tru-early, tru-constant | 3.235 | 1 | 0.072 |
| Tru-late, tru-constant | 33.94 | 1 | <0.001 |
| Tru-early, tru-untreated | 72.857 | 1 | <0.001 |
| Tru-late, tru-untreated | 6.977 | 1 | 0.008 |
| Tru-constant, tru-untreated | 45.703 | 1 | <0.001 |
| Cur-early, cur-late, cur-constant, cur-untreated | 74.212 | 3 | <0.001 |
| Cur-early, cur-late | 33.857 | 1 | <0.001 |
| Cur-early, cur-constant | 0.026 | 1 | 0.872 |
| Cur-late, cur-constant | 29.621 | 1 | <0.001 |
| Cur-early, cur-untreated | 44.835 | 1 | <0.001 |
| Cur-late, cur-untreated | 6.977 | 1 | 0.008 |
| Cur-constant, cur-untreated | 42.242 | 1 | <0.001 |
| Pos-early, pos-late, pos-constant, pos-untreated | 102.033 | 3 | <0.001 |
| Pos-early, pos-late | 63.034 | 1 | <0.001 |
| Pos-early, pos-constant | 1.825 | 1 | 0.177 |
| Pos-late, pos-constant | 33.907 | 1 | <0.001 |
| Pos-early, pos-untreated | 58.286 | 1 | <0.001 |
| Pos-late, pos-untreated | 4.75 | 1 | 0.029 |
| Pos-constant, pos-untreated | 38.848 | 1 | <0.001 |
- Seg, segmentation defects; Tru, truncation defects; Cur, curvature defects; Pos, posterior size defects.
Normal embryonic phenotypes are shown in Figure 4, and can be compared with the phenotypes produced by LiCl treatment (Fig. 5). Readily apparent differences were seen between late-treated embryos and early or constantly treated embryos. Typical late-treated embryos are displayed in Figure 5, A–C. Characteristic phenotypic changes include mild to moderate defects in segmentation (segmentation defect scores of 1 or 2 being most common), with the usually straight segmental boundaries becoming undulating and branching (Fig. 5A). The germ-band was also slightly truncated, usually by ~25% (Fig. 5B). In addition, the germ-band was slightly curved (Fig. 5C) and the proctodeum and the surrounding posterior region increased in size (Fig. 5C).
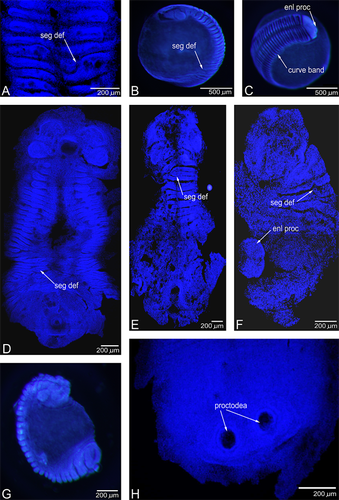
Embryos subjected to either early treatment (a 5-day period from germ-band formation onwards) or constant treatment (a 13-day period from germ-band formation onward) displayed more severe phenotypic defects, as seen in Figure 5, D–G. The posterior region, including the proctodeum, was increased in size to a greater degree than in late-treated embryos (Fig. 5, E and F). The germ-band was shortened to a fraction of its normal length (Fig. 5, D–G), with the embryo curving in on itself and enclosing a small portion of the egg yolk, instead of the whole egg, as is normal (Fig. 5G). In both early and late-treated embryos, those treated with 150 mM LiCl showed slightly more severe defects than those treated with 50 mM LiCl. The various phenotypic effects displayed a strong tendency to co-occur, with severely affected embryos scoring highly on all four of the phenotypic measures described here. The trends towards truncation of the germ-band, lateral curvature of the germ-band, and increase in posterior size all point to a role for Wnt gene activity in posterior development and identity, while the segmental defects suggest a role for Wnt gene activity in segment generation. Additional phenotypic effects were observed in a small number of embryos, with the most striking of these being the presence of two proctodeums (Fig. 5H).
Effects of LiCl treatment on Abd-B expression
Abdominal-B (Abd-B) is a Hox gene expressed at the extreme posterior of the Strigamia embryo (Brena et al. 2006) that can serve as a marker for posterior identity. It is therefore expected that LiCl-mediated changes in anteroposterior identity would be reflected by changes in the Abd-B expression domain. In embryos treated with LiCl, this region of expression is significantly extended to cover a number of the posterior-most leg-bearing segments (Fig. 6B; compare with untreated embryo in Fig. 6A). However, this transformation is only seen in embryos treated from early development. As LiCl treatment mimics ubiquitous Wnt ligand presence, this extension of the Abd-B expression domain provides further evidence that Wnt signalling confers posterior identity.
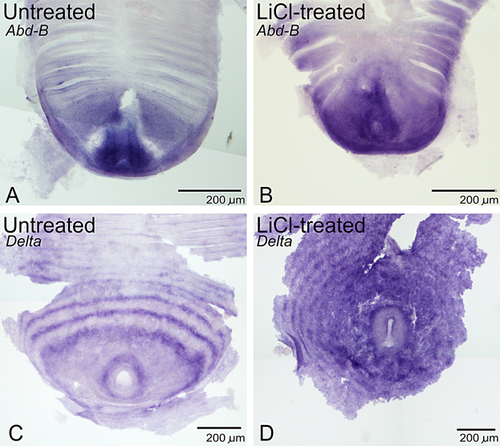
Effects of LiCl treatment on Delta expression
Delta encodes a ligand for the Notch receptor, making it a vital component in the Notch signalling system that may be important in Strigamia segment patterning (Artavanis-Tsakonas et al. 1999; Chipman and Akam 2008). Posterior Delta expression takes the form of rings surrounding the proctodeum, which have been interpreted as dynamic waves of expression moving out from the proctodeum (Chipman and Akam 2008). This Delta expression is altered in LiCl-treated embryos with segmentation defects. With LiCl treatment, these waves of expression at the posterior of the embryo become disorderly in form (Fig. 6D; compare with untreated embryo in Fig. 6C). This suggests that LiCl-mediated disruption of segmentation may at least partly be mediated by the alteration of a Delta-based segment generation mechanism. The perturbation of posterior waves of Delta bears some similarity to a phenotype observed in Parasteatoda, where Wnt8 RNAi (perturbing Wnt signalling) causes the failure of Delta transcripts to “clear” from the posterior region of the spider embryo (McGregor et al. 2008). Together with possible dynamic gene expression in both taxa (Damen 2007; Chipman and Akam 2008), this indicates similarity of posterior development and segmentation mechanisms in spiders and centipedes.
DISCUSSION
Wnt gene activity in posterior identity, growth and development
The evidence presented here, together with expression data from other studies (notably Chipman and Akam 2008), can be used to build a tentative model for Wnt activity in the linked processes of posterior development and segmentation in Strigamia, as follows. Wnt signalling is involved in the initial establishment of posterior identity that is required to allow germ-band development. Functional evidence for this derives from the effects of LiCl treatment during early germ-band formation, causing disruption of the establishment of anteroposterior identity. Additionally, early Wnt11 expression in the future posterior is consistent with this model and indicates that Wnt11 may be the Wnt gene involved in this process. Wnt11 is also expressed at the posterior pole of the embryo in the early development of Glomeris, another myriapod (Janssen et al. 2010). It is also noteworthy that its early expression bears a clear similarity to the early expression of Wnt1, Wnt8, and WntA in Tribolium (Bolognesi et al. 2008a) and of Wnt8 in Parasteatoda (McGregor et al. 2008). Considering that Wnt8 seems to be absent from the genome of Strigamia (Chipman et al. 2014), one interesting possibility is that the role of Wnt8 in establishing posterior identity has been assumed by Wnt11 in Strigamia. After this initial establishment of the anteroposterior axis, Wnt signalling is also important in posterior growth and development during germ-band extension. While there are many Wnt genes with small zones of expression near the proctodeum, two Wnt genes, Wnt5 and WntA, are more broadly expressed there and, thus, are particularly strong candidate genes for involvement in posterior growth and development.
The effects of treatment with the GSK3 inhibitor LiCl, during the developmental period under study here, including truncation of the germ-band, duplication of the proctodeum, and the change in Abd-B expression domain, provide support for such an interpretation. As GSK3 has additional functions in development, not all defects seen after GSK3 inhibition by LiCl are necessarily due to activation of the Wnt pathway. However, the correlation of the phenotypic perturbations with expression of various Wnt ligands suggests that LiCl acts predominantly as a Wnt agonist in these experiments, and strongly implicates Wnt activity in conferring posterior identity.
The activity of multiple Wnt genes in the posterior development of Strigamia bears a resemblance to Wnt gene activity in the beetle Tribolium and is consistent with the presence of ancient Wnt-based posterior development mechanisms in the ancestral arthropod (Shimizu et al. 2005; Bolognesi et al. 2008b). Certain parallels can be drawn between the effects of LiCl treatment in Strigamia and axin RNAi in Tribolium (Fu et al. 2012), as both act to increase the strength of Wnt-mediated posteriorization and cause anterior expansion of posterior markers. However, in contrast to axin RNAi in Tribolium, LiCl treatment in Strigamia does not inhibit anterior structure formation. This may be due to the differences in approach between the different treatments (one up-regulating a posterior factor, the other down-regulating an anterior factor) or to differences in posterior development in the two systems concerned. Additionally, the increase in size of the posterior region seen in LiCl treated Strigamia embryos (Wnt pathway activation) may be contrasted with the decrease in size of the posterior region of spider embryos after Wnt8 RNAi (Wnt pathway downregulation) and is consistent with Wnt signalling promoting posterior development in both cases (McGregor et al. 2008).
Wnt gene activity in Notch-based segment generation in Strigamia
When considered in the context of previous work describing oscillatory gene expression in Strigamia (Chipman and Akam 2008), the data presented here provide circumstantial evidence consistent with “clock and wavefront” segmentation. Furthermore, this mechanism seems to be regulated by Wnt signalling, as evidenced by the effects of LiCl treatment on segment formation and the LiCl-mediated disruption of wave-like Delta expression. The functions of Wnt signalling in segmentation could be mediated by their (a) controlling the wavefront of determination in a putative clock and wavefront mechanism or (b) acting to control oscillatory gene expression involved in segmentation. The posterior expression of Wnt5 and WntA is consistent with the former. The LiCl-mediated extension of the posterior germ-band can best be interpreted as waves of gene expression travelling further from the posterior via ectopic Wnt pathway activation, again supporting activity via the former mechanism. However, the disruption of Delta expression after LiCl treatment (and possible disruption of the oscillator mechanism) and the early expression of WntA in a wave-like pattern are more consistent with the latter. Thus, Wnt signalling may be involved in both processes.
CONCLUSIONS
The data presented here provide the basis for a model in which Wnt signalling plays multiple roles in the linked processes of posterior development and segmentation in Strigamia, as follows. Localized Wnt11 expression helps to establish posterior identity. Then, Wnt5 and WntA operate in posterior growth and development, while also playing roles in the control of the wavefront of determination for a Notch-based segmental oscillator. WntA also seems to operate in the oscillation system. Given Strigamia's phylogenetic position, the work described in this paper also provides support for the operation of Wnt signalling in the ancestral posterior development system of arthropods and has highlighted the importance of the study of non-model species. The further development of functional techniques in such non-model species has much to teach us about evolutionary and developmental processes.
Acknowledgments
We extend our thanks to Carlo Brena and Jack Green for the kind gifts of plasmids containing Delta and Abdominal-B sequences. We thank anonymous reviewers for useful comments on an earlier version of this manuscript. We are grateful to The Irish Research Council for Science, Engineering and Technology for an IRCSET EMBARK Fellowship (to LH).




