Socio-economic burden of disease: Survivorship costs for renal cell carcinoma
Abstract
Objective
The objective of this study is to assess the risk-stratified 10-year socio-economic burden of renal cell carcinoma (RCC) follow-up costs after initial treatment in Germany from 2000 to 2020.
Methods
A micro-costing method considering direct and indirect medical expenditure associated with follow-up procedures was employed to calculate survivorship costs per patient. The frequencies of physician–patient visits, examinations and diagnostic tests were extracted from guidelines, whilst expenses were sourced from literature and official scales of tariffs. Societal costs were calculated based on three perspectives: patients, providers and insurers.
Results
Mean societal 10-year follow-up costs per patient amounted to EUR 3,377 (95%CI: 2,969–3,791) for low-risk, EUR 3,367 (95%CI: 3,003–3,692) for medium-risk and EUR 4,299 (95%CI: 3,807–4,755) for high-risk RCC in 2020. Spending increased by +32% from 2000 to 2020 for low-risk RCC, whilst medium-and high-risk RCC expenditure was cut by −39% and −22%, respectively. Patients shouldered 27%, providers 43% and insurers 35% of costs in 2020. Resources were consumed by medical imaging (52%), physician-patient consultations (31%), travel expenses (17%) and blood tests (1%).
Conclusion
Results highlight the economic burden cancer survivorship poses for society. Cancer survivors require individualised, evidence-based and insurance-covered follow-up schedules to permit the early detection of side-effects, metastasis and secondary malignancies.
1 INTRODUCTION
Renal cell carcinoma (RCC) is the 6th (men) and 11th (women) most common malignancy in Germany, responsible for more than 5,000 deaths per year (Robert Koch-Institut, 2021). Declining mortality rates due to targeted therapies ultimately resulted in a growing number of RCC survivors requiring follow-up—especially in France, Austria, Scandinavian countries and Germany (Ferlay et al., 2018; Levi et al., 2008). Between 2000 and 2017, the age-standardised mortality rate per 100,000 inhabitants declined from 6.5 to 4.6 (−29%) for men and 3.0 to 2.0 (−33%) for women in Germany (Robert Koch-Institut, 2021). During the same time, the age-standardised incidence rate declined by only −20% for men and −13% for women. Consequently, the 10-year prevalence of RCC surged by +27% to 61,172 patients for men and +16% to 36,619 patients for women between 2004 and 2017 (Figure 1). As a result, there is a pertinent rationale to provide adequate care for RCC survivors and assess the socio-economic burden of cancer survivorship costs during follow-ups.
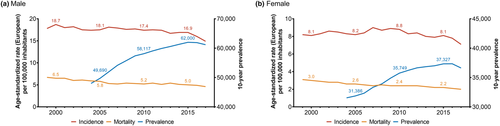
The rising 10-year RCC relative survival rate of 72% in women and 69% in men is most likely attributable to improved early diagnostics and novel therapeutic options in advanced stages (Robert Koch-Institut, 2021). Since 2006, the US Food and Drug Administration approved several anti-neoplastic agents with distinct therapeutic targets that significantly improved overall and progression-free survival for RCC patients. Among these count multi-target tyrosine kinase inhibitors (sunitinib, sorafenib and bevacizumab), mammalian target of rapamycin (mTOR) targeted drugs (temsirolimus and everolimus) and immune checkpoint inhibitors (avelumab, nivolumab and ipilimumab) (Mihaly et al., 2012). Especially treatments regimens combining targeted therapies and immune checkpoint inhibitors significantly improved overall survival in metastatic RCC (Chakiryan et al., 2021).
With the introduction of expensive new targeted agents, the question of financial viability came into focus (Benedict et al., 2011). Consequently, several studies began to evaluate the cost-effectiveness of new therapy regimens for RCC (Choueiri et al., 2020; Hoyle et al., 2010; Lister et al., 2017; Redig et al., 2019; Thompson Coon et al., 2010). Whilst some health economic studies consider new adverse events caused by these novel drugs (Deniz et al., 2019; Redig et al., 2019), the healthcare costs arising from prolonged survivorship due to follow-up visits are often neglected. This study employs a detailed micro-costing approach to estimate the socio-economic burden for the German healthcare system. Direct and indirect medical expenses arising for patients, providers and insurers during RCC follow-up were estimated from 2000 to 2020.
1.1 Renal cell carcinoma guidelines
Every year, the European Association of Urology (EAU) publishes and updates RCC follow-up guidelines (Carballido et al., 2000; Ljungberg et al., 2010, 2020). The EAU recommends follow-up schemes that are tailored to a patient's risk-stratification. This risk-stratification should be based on pre-existing classification systems like the University of California, Los Angeles (UCLA) Integrated Staging System (UISS) (Capogrosso et al., 2018; Patard et al., 2004). Clinical studies conducted by Beisland et al. (2016) demonstrate the benefit of structured and risk-stratified follow-up protocols which resulted in a significantly longer overall survival for patients. However, there is an ongoing controversial debate about the optimal duration of structured follow-ups, considering late metastases occurring after 5 years tend to be solitary and, therefore, if detected early, treated in curative intend. Risk-stratified RCC follow-up guidelines issued by the EAU from 2000 to 2020 are summarised in Table S1.
1.1.1 Low-risk renal cell carcinoma
Patients with low-risk RCC are recommended to have a physical examination, blood examination including serum creatinine yearly for the first 3 years after initial treatment and every 2 years thereafter. Additionally, ultrasound of the abdomen, kidneys and renal bed should be performed after 6 months followed by annual computed tomography (CT) scans of the chest and abdomen until the third year and every 2 years thereafter.
1.1.2 Medium-risk renal cell carcinoma
Patients with low-risk RCC are recommended to have an annual physical examination and blood examination including serum creatinine for the first 3 years after initial treatment and every 2 years thereafter. In contrast to patients with low-risk RCC, a CT scan of the chest and abdomen should be performed after 6 months and yearly until the third year. Subsequently, CT scans should be performed every 2 years.
1.1.3 High-risk renal cell carcinoma
Patients with high-risk RCC are recommended to have physical examinations and blood tests including serum creatinine every year. CT imaging should be performed after 6 months followed by a yearly scan until the third year. Subsequently, CT scans of the chest and abdomen should be performed every 2 years.
2 METHODS
An established costing methodology was employed to calculate the socio-economic burden of RCC survivorship in Germany (Michaeli et al., 2021, 2022; Michaeli & Michaeli, 2022). In accordance with the EAU guidelines' risk-stratification, we estimated costs across three distinct patient groups: low-, medium-, and high-risk RCC. Since EAU guidelines focus on a 10-year follow-up period, the same time horizon was examined. The employed micro-costing method considered direct and indirect medical expenditure associated with follow-up procedures to calculate survivorship spending per patient. All relevant three perspectives—patients, providers and insurers—were included to approximate the socio-economic burden in 2000, 2010 and 2020.
2.1 Patient perspective
From the patient perspective, opportunity costs relevant for RCC were assessed. Time consumption was extracted from literature and validated by an experienced oncologist (Table 1). The analysis is based on a ratio of 88% statutory and 12% privately insured patients to resemble the dual German healthcare system. Hence, the higher hourly income of privately insured patients (2000: EUR 23.90; 2010: EUR 30.17; 2020: EUR 37.78) relative to statutory insured patients (2000: EUR 18.49; 2010: EUR 23.39; 2020: EUR 28.81) was implemented into the calculations (Destatis, 2021). Opportunity costs were estimated by calculating patients' lost productive caused by RCC follow-up visits.
| Private health insurance: GOÄ (EUR) | Social health insurance: EBM (EUR) | Time consumption (minutes) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2010 | 2020 | 2000 | 2010 | 2020 | Physician | Assistant | Patient | References | Distribution | |
| Physician-patient consultation | 20.10 | 20.10 | 20.10 | 49.59 | 40.13 | 46.81 | 7.6 | 5 | 7.6 | (Irving et al., 2017) | Gamma |
| Physical examination | 34.86 | 34.86 | 34.86 | -a | -a | -a | 15.8 | - | 15.8 | (Irving et al., 2017) | Gamma |
| Blood taking | 4.19 | 4.19 | 4.19 | 0.00 | 0.00 | 0.00 | - | 5 | 5 | (Irving et al., 2017) | Normal |
| Serum creatinine | 4.69 | 4.69 | 4.69 | 0.41 | 0.40 | 0.40 | - | - | - | (Irving et al., 2017) | NA |
| Serum haemoglobin | 4.69 | 4.69 | 4.69 | 0.26 | 0.25 | 0.25 | - | - | - | (Irving et al., 2017) | NA |
| Ultrasound abdomen, kidney and renal bed | 102.56 | 102.56 | 102.56 | 21.94 | 15.60 | 15.91 | 30 | - | 30 | (Cleveland Clinic, 2021) | Gamma |
| Chest X-ray | 47.21 | 47.21 | 47.21 | 12.66 | 15.07 | 16.04 | 10 | 15 | 10 | (RSNA & ACR, 2021) | Normal |
| Chest CT scan | 241.31 | 241.31 | 241.31 | 121.54 | 65.36 | 64.38 | 10 | 25 | 10 | (Irving et al., 2017) | Normal |
| Abdominal CT scan | 272.79 | 272.79 | 272.79 | 121.54 | 81.14 | 79.55 | 10 | 30 | 31 | (U.S. National Library of Medicine, 2021) | Normal |
| Travel time | - | - | - | - | - | - | - | - | 60 | (Irving et al., 2017) | Gamma |
| Waiting period | - | - | - | - | - | - | - | - | 45 | (Radtke, 2019) | Gamma |
| Travel cost | - | - | - | - | - | - | - | - | 40 | (Randelhoff, 2011) | Gamma |
| Parking cost | - | - | - | - | - | - | - | - | 45 | (Randelhoff, 2011) | Gamma |
- Notes: Reimbursement rates for private and statutory health insurance were extracted from the respective reimbursement catalogue: Gebührenordnung für Ärzte (GOÄ) and Einheitlicher Bewertungsmaßstab (EBM) (Brück, 1998; Kassenärtzliche Bundesvereinigung, 2000, 2010, 2020). Time consumption for physicians, medical assistants and patients was extracted from relevant literature. CT, computed tomography; NA, not applicable.
- a The statutory insurance reimburses a fixed rate which entails physician-patient consultation and examinations. For simplicity, this rate is represented under physician-patient consultation rate.
2.2 Provider perspective
From the provider perspective, opportunity costs for all relevant healthcare providers involved in follow-ups of patients with RCC were assessed. Due to budgeting decisions and reimbursement restrictions in Germany for statutory insured patients, there remains an inconsistency between the provider's bill of medical services and performed medical tasks. Time consumption was extracted from the aforementioned literature (Table 1). Opportunity costs were again estimated based on the average salary and the worked/productive hours (Curtis et al., 2010; Curtis & Burns, 2020; Netten & Curtis, 2000). Opportunity costs per hour of patient contact rose throughout the analysed time horizon for both physicians (2000: EUR 136.20; 2010: EUR 215.72; 2020: EUR 286.81) and medical assistants (2000: EUR 31.86; 2010: EUR 41.98; 2020: EUR 47.24). Data were extracted from reports of the Personal Social Services Research Unit issued by the University of Kent, which annually assesses healthcare unit costs in a standardised manner for more than 20 years. Costs were collected from these reports to minimise artificial calculation-derived expenditure variations on results. Their detailed cost calculation considers physicians' and nurses' average salaries and worked hours alongside expenses for salary oncosts, qualification, overheads, capital overheads, travel and adjustments for city size.
2.3 Insurer perspective
Bills invoiced to the insurer by the provider are regarded relevant for the insurer perspective. Germany's dual healthcare system was remodelled by applying distinct reimbursement rates for social health insurance (SHI) and private health insurance (PHI) funds. SHI funds are billed based on binding reimbursement rates published in a frequently updated catalogue (Einheitlicher Bewertungsmaßstab—EBM) (Kassenärtzliche Bundesvereinigung, 2000, 2010, 2020). Similarly, private insurers are invoiced based on established reimbursement rates in a distinct catalogue (Gebührenordnung für Ärzte—GOÄ) (Brück, 1998). Reimbursement quotes for related services from both scales were extracted for 2000, 2010 and 2020 (Table 1).
2.4 Cost calculation
The recommended frequency of physician visits, examinations and diagnostics was derived from EAU guidelines as described in the introduction. Opportunity costs for providers and patients, resulting from forgone time consumption for follow-ups, were assessed together with the resulting expenditure bill for the insurers (Table 1). All costs were inflation adjusted to 2020 values. Cost development was compared across years, stakeholder, resource use and cost type.
2.5 Sensitivity analysis
Probabilistic sensitivity analysis accounts for discrepancies in the length of physician–patient consultations, examinations and diagnostics. Providers' and patients' time consumption parameters were derived by random sampling from their defined distribution (Table 1). Since reimbursement rates are independent of time consumption in Germany, these parameters are excluded from sensitivity analysis. The analysis estimated costs for 1,000 patients per treatment cohort and year. Cost data were expressed as means with 95% confidence intervals. For the two-factorial analysis of variance, ANOVA with Dunnett's test was applied. A two-tailed probability value <0.05 was considered significant.
3 RESULTS
The overall follow-up related expenditure for RCC survivorship ranged from EUR 3,367 to EUR 4,299 per person in 2020 depending on risk stratification (Figure 2). Whilst societal costs dropped for medium- and high-risk RCC follow-ups, spending for low-risks surged. However, there are differences in expenditure progression between the patients, provider and insurers perspectives from 2000 to 2020.
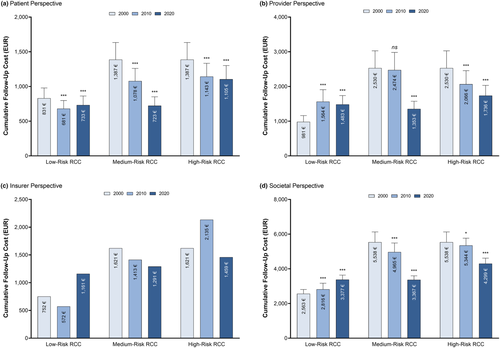
3.1 Patient perspective
Ten-year estimated follow-up costs for patients significantly decreased from 2000 to 2020 (Figure 2a). More precisely, follow-up costs for low-risk RCC dropped from EUR 831 in 2000 to EUR 733 in 2020 (p < 0.001). Similarly, the burden for medium (2000: EUR 1,387; 2020: EUR 723; p < 0.001) and high-risk (2000: EUR 1,387; 2020: EUR 1,105; p < 0.001) RCC follow-up costs was reduced.
3.2 Provider perspective
The assessment of 10-year follow-up costs from 2000 to 2020 revealed a surge for low-risk RCC for providers, whilst costs dropped for medium- and high-risk follow-ups (Figure 2b). Costs for providers grew by +51% for low-risk RCC (2000: EUR 981; 2020: EUR 1,483; p < 0.001). In contrast, providers were relieved of the economic burden by −47% for medium-risk (2000: EUR 2,530; 2020: EUR 1,353; p < 0.001) and even −71% for high-risk RCC (2000: EUR 2,530; 2020: EUR 1,736; p < 0.001), respectively.
3.3 Insurer perspective
Analysing the health insurance perspective, cost development from 2000 to 2020 deviated based on risk stratification (Figure 2c). More precisely, insurers invoice amounted to EUR 752 in 2000, EUR 572 in 2010 and EUR 1,161 in 2020 for low-risk RCC. The initial drop followed by a surge might be explained by reverse adjustments in reimbursement rates implemented by the SHI (Table 1). On average, the SHI lowered tariffs rates by −34% from 2000 to 2010 to then raise rates by +2% from 2010 to 2020. At the same time, reimbursement rates of the PHI were not altered since their introduction in 1996, resulting in a 2.2× (2000), 3.4× (2010) and 3.3× (2020) higher burden for the private in contrast to the statutory insurance. Regarding medium-risk RCC, insurers were billed less over the examined period (2000: EUR 1,621; 2020: EUR 1,291). However, follow-up costs for high-risk RCC surged from 2000 to 2010 to then drop below 2000 levels in 2020 (2000: EUR 1,621; 2010: EUR 2,135; 2020: EUR 1,459).
3.4 Societal perspective
The introduction of advanced imaging (CT scans of the chest and abdomen) for at-risk RCC patients resulted in fewer time consuming physician-patient visits which translated into a reduced economic burden for high-risk (2000: EUR 5,538; 2020: EUR 4,299) and medium-risk (2000: EUR 5,538; 2020: EUR 3,367) RCC patients. In contrast, the introduction of advanced imaging for low-risk RCC patients led to an incline of follow-up expenditure (2000: EUR 2,563; 2020: EUR 3,377) as the overall recommended follow-up period was increased from 5 to 10 years. However, the socio-economic impact of follow-up consultations and diagnostics was unequally allocated to the three stakeholders (Figure 3a). Whilst patients had to bear 27% of all follow-up costs in 2000, their relative share dropped to 22% by 2020. Meanwhile, healthcare providers were able to maintain their share of 43% from 2000 to 2020. Consequently, there was a shift of the economic burden from patients to insurers, bearing 35% of follow-up costs in 2020.
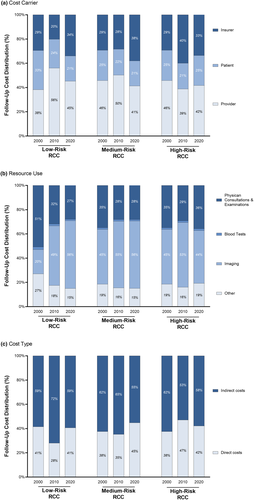
Resource use significantly diverges between low-, medium- and high-risk RCC due to varying relapse rates that translate to different follow-up recommendations (Figure 3b). In 2000, doctor visits and examinations consumed the majority (40%) of resources, whereas advanced imaging only accounted for 37%. In contrast, a more frequent use of advanced imaging (52%) in combination with less physician consultations and examinations (31%) led to a significant redistribution of the socio-economic burden until 2020. Independent of risk-stratification, travel expenditure induced at least 15% of the entire economic burden. Direct and indirect medical costs accounted for 43% and 57% of follow-up expenditure, respectively (Figure 3c).
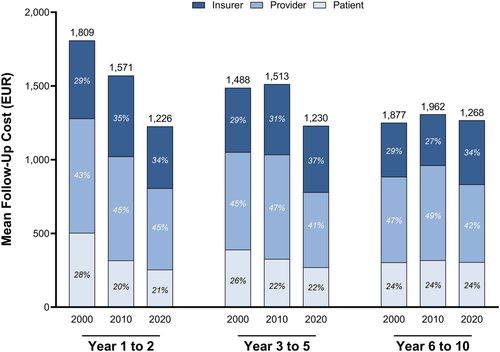
Moreover, independent of cancer types, costs were unequally distributed over the follow-up period of 10 years after treatment (Figure 4). In 2020, follow-up costs were calculated at EUR 1,226 for year 1–2, EUR 1,230 for year 3–5 and EUR 1,268 for year 6–10. Regarding follow-up years 1–2, the burden was reallocated from patients to insurers, who shouldered 34% of all resource consumption by 2020. Patients were relieved by 7% of the overall decreased follow-up costs in 2020 during year 1–2. During follow-up years 3–10, the economic burden was transferred from providers to insurers.
According to the sensitivity analysis (Table S4), our results widely depend on the assumption of a 100% adherence to EAU guideline recommendations. However, daily clinical practice of patients' and physicians' non-adherence to guidelines might reveal a decrease in physician-patient consultations and examinations, which ultimately entail a decrease in the socio-economic burden. A variation of the discount rate by ±2.5% proceeds in maximum ±12% deviations in follow-up costs.
4 DISCUSSION
RCC remains a challenging uro-oncological cancer entity with a worldwide rising incidence (Padala et al., 2020). Due to its asymptomatic development, many RCC are found incidentally during advanced imaging procedures. The rate of incidental RCC has increased to 57% during 2005 to 2010 due to advanced imaging (Sand et al., 2013). Two thirds of these incidental findings were registered during follow-up for other comorbidities. Considering treatment advances, such as immune-therapies and targeted approaches, the overall number of patients requiring specialised follow-up is growing (Jemal et al., 2017). This ongoing development underlines the necessity to assess and evaluate the costs induced by RCC survivors. However, there are few studies conducted to quantify the socio-economic burden of RCC follow-up survivorship costs. Previous cost analysis literature evaluated post-nephrectomy follow-up schedules from three North American clinical practice guidelines (Thana et al., 2020), compared follow-up strategies for a distinct cancer stage (Dion et al., 2010), analysed novel drug-based treatment strategies (Chien et al., 2019), assessed follow-up costs with a Markov model (Nazha et al., 2019), estimated the impact of targeted therapies on healthcare costs (Redig et al., 2019; Soerensen et al., 2015) and conducted observational country-specific studies to monitor expenditure per patient (Mantovani et al., 2008). Existing studies estimate and compare follow-up costs for RCC in countries other than Germany. To the best of our knowledge, this is the first socio-economic study on follow-up costs for patients with RCC aimed at estimating direct and indirect medical costs over 10 years occurring in clinical practice in Germany.
Analysing the cost development by risk-stratification, follow-up costs were cut from 2000 to 2020 for medium- and high-risk RCC, whilst costs surged for low-risk RCC. This development might be explained by guideline changes regarding physician visits, examinations and routine imaging. Resource use for at-risk patients was shifted towards more advanced imaging, which in turn decreased the necessity for physician-patient consultations. Ultrasound was especially replaced by CT imagining. CT imaging was shown to be more sensitive in detecting small cancer lesions than ultrasound and is able to distinguish angiomyolipoma from cancer entities (van Oostenbrugge et al., 2018). However, CT imaging has a modest sensitivity to discriminate RCC subtypes. Meanwhile, radiologist might require more time to interpret advanced imaging due to difficulties in detection of RCC metastases, assessing treatment response during systemic therapy, and judge the imaging response of immuno-therapies that cannot easily be captured with conventional radiologic criteria (Diaz de Leon et al., 2019).
Magnetic resonance imaging (MRI) and Single Photon Emission Computed Tomography (SPECT) are alternative imaging modalities that are increasingly used in clinical practice. Multi-parametric MRI offers the possibility to differentiate angiomyolipoma from RCC and classify RCC subtypes. However, MRI faces the challenges of common reporting criteria, technical protocols and large cost expenditure to improve inter-observer reliability and thereby widespread clinical use (van Oostenbrugge et al., 2018). Other imaging options that are currently under development, such as nuclear SPECT immune-imaging using Girentuximab with high positive and negative predictive values, will further promote the adoption of imaging technologies in the detection of malignancies and metastasis of cancer patients (Muselaers et al., 2013).
Furthermore, costs were reallocated among stakeholders. Whilst patients paid for 27% of all follow-up costs in 2000, their share was limited to 22% in 2020. As a result, the economic burden was transferred from patients to health insurers, who covered around one third of follow-up costs in 2020. This shift and reduction in the socio-economic burden are anticipated to further increase in the future as advances in digital follow-ups might supplement and help to reduce routine visits and thereby decrease costs for low-risk RCC. This development could further decrease the burden for patients and providers. Moreover, it is expected that cost-intense advanced nuclear imaging technologies, such as PSMA PET/CT, could improve staging of RCC and monitoring of patients on tyrosine-kinase inhibitor therapy in metastatic RCC (Mittlmeier et al., 2020; Raveenthiran et al., 2019). Consequently, expensive sophisticated imaging could further increase the cost share for insurers, especially for high-risk RCC patients.
Our results demonstrate that follow-up costs were particularly reduced during follow-up years 5 to 10 and transferred to insurers. Studies reveal that financial aspects impact adherence to recommended follow-ups (Dillon et al., 2018; Iuga & McGuire, 2014). The limited economic burden for patients could therefore positively incentivise adherence to follow-ups.
A cost analysis contrasting different follow-up schedules after nephrectomy for RCC in North America found that cost per patient and projected cost adjusted for attrition from predicted recurrences ranged from USD 497 to 2,746 (Thana et al., 2020). However, this study only included costs for testing and physician visits from a single data source over 5 years after initial treatment. In contrast, our calculation for the German healthcare system entails a 10-year time horizon and considers all direct and indirect medical expenses, which ultimately yields a significantly higher cost estimate.
With the rise of novel targeted- and immuno-therapy regimens, urologists and oncologist also need to learn how to capture, treat and monitor new side-effects associated with these treatments (Grimm et al., 2010). In addition, especially female and young RCC cancer survivors experience clinically relevant fear of cancer recurrence (Bergerot et al., 2020). These psychological comorbidities impose a significant impact on the health-related quality of life after treatment. Consequently, it is recommended to routinely assess psychological comorbidities during follow-up and decrease barriers for professional psycho-oncological support.
A structured disease management programme (DMP) for cancer survivors was recently proposed by several authors. The positive effects of DMP on patient outcomes and budget control were previously shown for chronically ill patients with diabetes, chronic obstructive pulmonary disease (COPD) and cardiovascular disease (Achelrod et al., 2016). DMP align all stakeholders' interests to efficiently improve the care for cancer survivors (Herald et al., 2012). DMP might further streamline treatment of a shared patient between providers, such as general practitioners (GPs), urologists and oncologists due to standardised procedures.
Current research on predictive biomarkers might enable individualised follow-up schedules adjusted to the growing number of survivors (Junker & Zeuschner, 2019). The introduction of biomarkers, the consideration of individual risk factors and the patient's request could lead to more personalised follow-up strategies (Alfano, Jefford, et al., 2019). Personalised follow-up regimes should enhance adherence and quality of life and reduce the social-economic follow-up burden.
However, the introduction of individualised follow-up pathways within the established general healthcare delivery framework requires the consideration of several barriers (Alfano, Mayer, et al., 2019). In addition, providers should promote self-management of cancer survivors' health with digital applications. Follow-up applications should be prescribed by physicians and reimbursed by insurers. In Germany, the Digital Healthcare Act entitles SHI patients for reimbursement for digital health applications (Dittrich et al., 2021). Follow-up through point-of-care self-management devices can be supplemented by virtual follow-ups through specialised nurses.
The dissemination of health applications might be fostered by pecuniary incentives to bypass barriers in cancer follow-up delivery (Alfano, Mayer, et al., 2019). Moreover, a holistic strategy involving long-term patient-centred survivorship trajectories developed between GPs, urologists and oncologists could incentivise adherence to follow-ups and thereby increase overall survival for patients (de Padova et al., 2011). First, GPs needed to be regularly trained to provide sophisticated survivorship follow-ups. Then, GPs can especially support specialised oncologists by following up on low-risk for relapse patients (e.g., after 5 years of recurrence free survival). When indicated, GPs can further refer survivors with abnormalities to oncologists or urologists. If these abnormalities cannot be solved by outpatient specialists, the survivor can be co-managed together with specialised cancer-specific centres. The whole process can be facilitated by an easily accessible electronic health record including cancer history, oncology treatment records and a survivorship care plan (Alfano, Mayer, et al., 2019).
4.1 Limitations
This study is prone to some limitations. First, follow-up costs are empirically determined and consequently not confirmed in clinical studies. Second, this health economic study does not permit conclusions about the efficacy and cost-effectiveness of advances in diagnostic options and improved EAU guidelines. Third, the underlying 100% ‘uptake’ of guideline recommendations might not model reality. Fourth, informal healthcare costs—direct and indirect expenses that occur during the voluntary care by non-professionals for persons in need—are not considered in the societal perspective. Fifth, our approach calculates follow-up costs for 10 years. However, overall survival depends on diagnosed stage of RCC. Research reveals that up to 30% of cases are diagnosed in advanced or metastatic stage (Jonasch et al., 2011). Despite the introduction of targeted therapies, the median overall survival for these patients does not exceed 24 months (Sørensen et al., 2014). As a result, our findings might overestimate 10-year costs due to preliminary death of high-risk patients. Finally, costs reported by the PSSRU were extracted from UK data. Their applicability in the German healthcare system is limited. Further research is necessary to examine the follow-up costs for non-RCC patients in and beyond Germany.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors were involved in the conception of the study. Data collection was performed by JCM and TM. Data analysis was performed by JCM, DTM and TM. JCM and TM prepared the first draft of the manuscript. All authors critically revised and edited multiple drafts of the manuscript. All authors read and approved the final version of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The input data that support the findings of this study are disclosed in the main text and the supporting information of this article. The cost estimation files of this study are available from the corresponding author upon reasonable request.




