‘We do need to keep some human touch’—Patient and clinician experiences of ovarian cancer follow-up and the potential for an electronic patient-reported outcome pathway: A qualitative interview study
Present address: Leanne Shearsmith and Molly Megson, Academic Unit of Palliative Care, Leeds Institute of Health Sciences, University of Leeds, Leeds, UK
Funding information: Yorkshire Cancer Research, Grant/Award Number: L392
Abstract
Objective
This study aimed to explore experiences of follow-up after treatment and views on an electronic patient-reported outcome (ePRO) pathway among ovarian cancer patients and clinicians.
Methods
Semi-structured qualitative interviews were conducted with clinicians and patients previously treated for ovarian cancer. Interviews explored experiences of the current follow-up pathway, patients' needs and views on an ePRO pathway enabling patients to report symptoms online rather than attend clinic-based appointments. Transcripts were analysed using framework analysis.
Results
Sixteen patients and 10 clinicians participated from four hospitals in England. Four key themes were identified: transition into follow-up, key features of effective follow-up, issues in follow-up and views of ePRO. Both patients and clinicians saw benefits of an ePRO pathway alongside continued access to specialist support and discussed various practicalities (e.g., frequency, introduction and communication). Technology concerns and feelings of abandonment were highlighted as barriers. The proposed impact on clinical and individual patient outcomes was discussed.
Conclusion
Patient and clinician views on follow-up and an ePRO pathway informed key recommendations on the development/introduction of ePRO follow-up. Technology use in healthcare will continue to grow and may offer solutions to facilitate responsive and tailored care. Further research should explore the safety, experiences and acceptability of ePRO follow-up.
1 BACKGROUND
Over 7000 ovarian cancers are diagnosed every year in the United Kingdom, and this is projected to rise further by 2035 (Cancer Research UK, 2020). The 2018 ‘One Size Doesn't Fit all’ report estimates that two million people are living with and beyond cancer in the United Kingdom, estimated to rise to 3.4 million by 2030 (MacMillan Cancer Support, 2018, using data extrapolated from Maddams et al., 2012). Therefore, clinical services have mounting pressure to support individuals on routine follow-up with ongoing toxicity, emotional needs and symptom-monitoring (Davies & Batehup, 2011).
Ovarian cancer usually presents in advanced stages (Cancer Research UK, 2020; Marcus et al., 2014), and relapses are common. Standard follow-up involves clinic-based appointments for 5 years (3-monthly for 2 years, gradually reducing to 12-monthly), alongside serum biomarker testing (CA125), physical examination and imaging (Kargo et al., 2019). These surveillance methods have been informed by retrospective studies and expert opinion (Salani et al., 2017) and may not impact on survival (Marcus et al., 2014). In a randomised control trial (RCT) post first-line treatment, Rustin et al. (2010) illustrated no benefit of ‘early’ (<28 days of CA125 rise) second-line treatment versus waiting for symptomatic relapse, and quality of life (QoL) was lower in the ‘early’ group.
Some patients delay help-seeking for symptoms until their scheduled appointment (Olaitan et al., 2001), and appointments can heighten anxiety by reactivating memories of diagnosis/treatment (Kew et al., 2009). However, scheduled appointments allow disease monitoring, management of late effects and reassurance (Bradley et al., 1999), which are needed given the long-term physical and emotional issues (MacMillan Cancer Support, 2017). Hence, tailored interventions to support follow-up patients are important (Marcus et al., 2014).
Alternative follow-up methods are increasing (Høeg et al., 2019); qualitative studies of nurse-led telephone follow-up (Beaver et al., 2017; Cox & Faithfull, 2015; Lydon et al., 2009; Williamson et al., 2018) have illustrated positive experiences among endometrial and ovarian cancer patients receiving psychosocial support alongside blood tests. A small UK-based RCT by Morrison et al. (2018) explored nurse-led telephone follow-up among 24 gynaecological patients recruited within 3-month post-treatment. Positive changes in QoL, and £27 per patient lower provider costs, were observed in the intervention group at 6 months.
UK clinical practice surveys indicate that alternative follow-up availability has increased from 2012 to 2019 (Coleman & Newton, 2020; Leeson et al., 2013), including telephone follow-up (25% to 32%) and patient-initiated follow-up (PIFU, 32% to 42%), defined as ‘the patient is not followed up in secondary care but seen only if the patient requests or initiates a contact’ (Leeson et al., 2013, p. 2). PIFU in ovarian cancer was reported by 26% of centres, but recommendations highlight that not all diagnoses and stages are suitable for PIFU (Newton et al., 2020). Furthermore, despite reduced hospital-based visits in PIFU, overall appointment frequency was similar in an RCT as patients attended more primary care appointments (Jeppesen et al., 2018).
Further large-scale RCTs are needed to explore the outcomes (safety, QoL, cost and psychological) of different follow-up methods (Clarke et al., 2014; Høeg et al., 2019). The National Health Service (NHS) long-term plan emphasises the importance of personalised care planning, stratified follow-up and using digital technology (NHS England, 2019). Use of virtual care models for cancer survivors is growing to address increasing service need (Pham et al., 2020). Nama et al. (2013) suggested gynaecological follow-up could use electronic or paper-pencil patient-reported outcomes (PRO), facilitating a patient's own assessment of their health (U.S. Department of Health and Human Services Food and Drug Administration, 2009). PRO monitoring could prompt face-to-face reviews when symptoms are reported, and PRO reporting has been shown to improve patient-provider communication, resulting in better symptom control and QoL (Greenhalgh & Meadows, 1999; Velikova et al., 2004). Furthermore, online, electronic PRO (ePRO) could provide additional support (Dickinson et al., 2014; National Information Board, 2014) that may be absent in a PIFU model. ePRO follow-up in lung cancer resulted in increased survival and earlier relapse detection (Denis et al., 2017), and there are emerging examples of other oncology services providing ePRO follow-up (Lindner et al., 2020; Qaderi et al., 2021).
The feasibility and patient value of ePRO reporting during cancer treatment has been shown (Absolom et al., 2019; Absolom et al., 2021), especially alongside clinician engagement (Warrington et al., 2019). An ePRO system may enable patient's symptoms/needs to be monitored remotely, integrated into electronic patient records to inform care and provide rapid access to clinicians. However, to ensure clinical relevance and patient acceptability, patient and clinician views are needed to shape how this could be embedded within current practice. This study aimed to explore general experiences of follow-up among ovarian cancer patients and clinicians, and their views on a gynae-oncology ePRO follow-up pathway.
2 METHODS
2.1 Design
A pragmatic qualitative descriptive approach (Bradshaw et al., 2017) was undertaken as part of a larger mixed-methods project, whereby semi-structured interviews (conducted between September 2016 and March 2017, at four hospitals) explored patient and clinician views of current follow-up, and the prospect of an ePRO pathway. Ethical approval was granted by a Health Research Authority Research Ethics Committee. One experienced qualitative researcher (research fellow, PhD), trained two research assistants to conduct the interviews. All worked in a research group exploring PRO use in oncology practice, were unknown to the patients, but one was known to some clinicians due to previous research.
2.2 Participants
Patients were eligible if they had completed ovarian cancer treatment within the last 3 years, understood/spoke English and could give informed consent. They were purposively sampled across age, time since treatment, and first- versus second-line treatment. Clinicians were eligible if they provided follow-up care to ovarian patients.
2.3 Procedure
Eligible patients were identified and approached face-to-face by their clinical team, who passed the patient's details onto the research team if they were interested. Clinicians were approached by the research team directly by email. All participants received a study information sheet and gave written or audio-recorded informed consent. Interviews took place at a time/place preferred by the participant (hospital and telephone), were digitally audio-recorded and transcribed verbatim. Pseudonyms were used to distinguish participants, and any identifying features were removed from the transcripts to ensure confidentiality. Data collection ceased in both samples when no new issues were emerging.
Interview schedules were developed with input from a patient representative. Staff interviews explored their role/workload, current follow-up pathway, key symptoms, reporting process and information/support provision. Patient interviews explored information provision, symptoms and reporting, and confidence in monitoring symptoms. All interviews explored views on an ePRO follow-up pathway (see Table S1).
2.4 Data analysis
NVIVO 12 was used to store and organise the data. Transcripts were analysed using the framework approach (Ritchie & Spencer, 1994) following five stages: familiarisation, developing a thematic framework, indexing, charting, mapping and interpretation. The data were pragmatically explored in 2017 to inform the ongoing project, but in-depth analysis took place in 2020. One researcher carried out the five stages on all transcripts, with second coding independently undertaken by two other researchers (six patient/four clinician transcripts). There were no disagreements, but this process identified vocabulary differences (resolved through discussion) and the further articulation of one subtheme. Separate codebooks were initially developed (patient and clinician), but once similarities were established, combined datasets were explored to provide a more complete picture of follow-up and inform the development of an ePRO pilot.
3 RESULTS
Twenty-six interviews were conducted with 27 participants (one joint interview with two clinical nurse specialists, CNSs), and mean duration was 38 min (patients 15–45 and clinicians 35–73). All 10 clinicians approached participated, including consultants (n = 4), CNSs (n = 5) and a registrar. Five had worked in the area for 10+ years, whereas two had 1–5 years, and three 6–10 years of experience.
Twenty-four patients were approached. Four declined (16.7%, 4/24), two were not contactable, and for one, the interview could not be arranged. Seventeen took part (70.8%), but a recording error excluded one interview. Table 1 illustrates the patient characteristics, who ranged in age from 23 to 80 years (median 67).
| Study ID | Age | Months since treatment end at interview | Experience of treatment at interview (first line or first & second) |
|---|---|---|---|
| PT1 | 60 | 12 | First & second |
| PT2 | 49 | 12 | First line |
| PT3 | 59 | 35 | First line |
| PT4 | 68 | 11 | First line |
| PT5 | 80 | 8 | First & second |
| PT6 | 51 | 7 | First line |
| PT7 | 70 | 10 | First line |
| PT9 | 78 | 2 | First & second |
| PT10 | 23 | 8 | First line |
| PT11 | 69 | 8 | First line |
| PT12 | 52 | 8 | First line |
| PT13 | 69 | 19 | First line |
| PT14 | 55 | 12 | First line |
| PT15 | 75 | 6 | First line |
| PT16 | 66 | 23 | First line |
| PT17 | 79 | 36 | First line |
| Median 67 years; interquartile range 54.25–71.25 | Median 10.5 months; interquartile range 8–14.92 | First line = 13 (81.25%) | |
| First & second line = 3 (18.75%) |
Figure 1 depicts the four interconnected themes: transition into follow-up, key features of effective follow-up, issues in follow-up and views on ePRO. These themes will be discussed in turn, referring to subthemes and example extracts (Tables 2–5 provide further extracts).
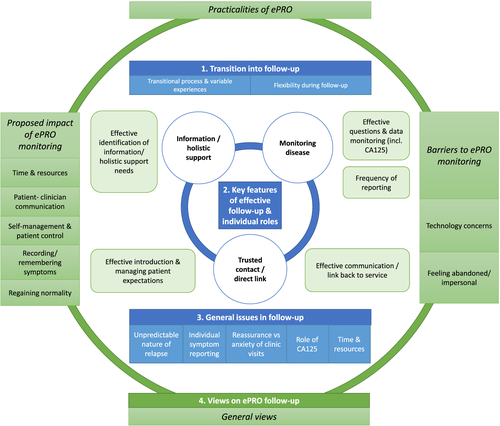
3.1 Theme 1: Transition into follow-up
This theme (Table 2) reflects on patient and staff agreement that entering follow-up is a transitional process that is adaptable to the patient's psychological needs and disease status. Some patients adjust very quickly, wanting to move on; others feel adrift in the abrupt change to less frequent visits, and some never settle and simply wait for the cancer to return.
| Transition into/during follow-up | |
|---|---|
| Transition into follow-up & variable experiences | 1. ‘… suddenly they are flying free and alone and that transition is something which can be quite worrying for some of them because every little symptom beforehand they could talk to the next chemotherapy nurse or somebody “oh I've got a twinge there, I've got a headache there, is that something to worry about?” and that's not the case anymore. So putting their own symptoms in the right little space in the back of their mind can be challenging’ (Consultant 4) |
| 2. ‘You know, you have a really full diary with various tests and things. And then all of a sudden it just seems to stop and there does not seem to be anything there anymore. But then I think you soon forget that. But if you back up work then you forget about it all completely really.’ (PT2) | |
| 3. ‘Personally I felt as if I'd lost a lifeline’ (PT7) | |
| Flexibility during follow-up | 4. ‘So people who are to struggle a little bit with the idea of 3 month appointment, either overtly or just subjectively from your judgement/assessment of how the conversations going, you might do a 6 week or a 2 month appointment to kind of ease them out of that kind of, you know, institutionalisation.’ (Consultant 1) |
| 5. ‘Some patients … perhaps do not have the support at home or you are worried that they might not report some of the symptoms that they have got then you might sort of want to follow up a little more frequently.’ (CNS1) | |
| 6. ‘It's difficult to explain really, it's really hard to explain to you because it depends on knowing your patient. Because I know one of my patients will ring up and just her name and I will think oh, she never rings, I need to ring her. So it's about knowing your patients as well, knowing where they are in their pathway, knowing what makes, how they tick. It's not, there aren't any black and white guidelines, and it's about knowing what you do, knowing your disease and knowing your patient.’ (CNS5) | |
The complexity of clinical pathways is highlighted, which are protocolised but flexible. Patients are seen at 3-monthly intervals, gradually increasing over time (4-monthly, 6-monthly and annual), which some patients find reassuring ‘it makes you feel like it's quite positive because [Doctor] is quite happy to leave it at 4 months rather than 3 months.’ (PT14). At 5 years, they may be discharged, but this is unusual as many patients relapse and others want continued reviews. Therefore, flexibility is evident for multiple reasons, including patient preference, patient characteristics, ongoing toxicity and clinical factors (e.g., residual disease and CA125).
3.2 Theme 2: Key features of effective follow-up and individual roles
Three key features of effective follow-up (Table 3) were noted: monitoring disease, trusted contact/direct link and information/holistic support. This theme also highlights how the patient, CNS and doctor have defined roles during follow-up. The doctor's role is to monitor disease, the CNS helps patients navigate the cancer experience holistically, while the patients' role is to self-monitor and make contact if/when they are concerned.
| Key features of effective follow-up and individual roles | |
|---|---|
| Trusted contact/direct link | 1. ‘I do know that I can speak to the clinic, I do know that I can still speak to the Macmillan nurses even though I'm not necessarily needing them, I can speak to them and they'll have the knowledge as well, if it was really really urgent then I really would have to contact my GP, but like I say you cannot get an appointment, so I think the clinic would be the best thing because, you know, that gives me more confidence that somebody's actually listening.’ (PT6) |
| 2. ‘I know if I need them, there's always somebody there, yeah. Researcher - There's always someone on the other end of the line. And that is the main thing for me, you know.’ (PT4) | |
| 3. ‘we know our patients are going to relapse, we know the high majority of them are going to run into trouble and we know they are going to come back. So we just keep them, so they just ring us and we slot them back into a clinic.’ (CNS5) | |
| Monitoring disease (symptoms & bloods) | 4. ‘Now that very very rarely happens because usually our patients are the ones who notice they are not quite as well and they come back to clinic earlier. It's very rare that we diagnose a relapse or a recurrence at a consultation.’ (CNS5) |
| 5. ‘I was just at the hospital on Friday, sorry, last Thursday for my second three month follow-up and then (the consultant) said have you got any symptoms, I said oh no, no and then I walked out and thought I wonder what symptoms I could be having, I did not okay yes, so you know, I did not know whether it would be headaches or pains or temperatures or so I do not really know what symptoms I am looking for’ (PT12) | |
| 6. ‘Well, I had symptoms similar to before my diagnosis, that's when I rang [CNS} and she got me in to the clinic and then they sent me for the CT scan, that was April last year and that was the last CT scan I had, but everything was okay, there was no sign of … I mean I'm not thinking about it all the time but anyone will say to you once you have had cancer and you get a pain somewhere, you know, you think “oh, that's something to do with the cancer”, yeah.’ (PT13) | |
| 7. ‘on my first 3-monthly appointment I did not have a blood test so I think that did not help and they said that I did not need a blood test, they did not feel I needed one. Then obviously i'd had no pains at all so I thought oh I will not have one but then I think I should have done now. So I had one this time and then everything came back fine so I'm okay now. But I think that's why, because I did not have that evidence.’ (PT10) | |
| Information and holistic needs | 8. ‘We do not do very much in the way of education about what symptoms people might look out for because we kind of screen purposefully. But people know that they can contact us in-between clinic visits if they are concerned about any symptoms that they might have. And they might do that by contacting the CNS who might kind of triage as to whether they think those are significant symptoms or not and if needs be bring them back to clinic sooner, or direct to GP if they think it's not related.’ (Consultant 1) |
| 9. ‘I think just having like leaflets and things to hand. You know, because obviously you are going to get niggles and pains because basically your body has changed from the chemo and stuff. I just think having that to hand that you can just look at any time. Because the internet, you google, you put it in the internet and they literally pronounce you dead you know what I mean. You cannot, looking at symptoms on the internet, to looking at ones on a leaflet, it's completely different.’ (PT10) | |
| 10. ‘I think everybody sort of dips in and has a look, frightens themselves to death [laughs] … There's a lot of good stuff out there but there's also a lot of, you know, bad stuff that you should not really look at.’ (PT2) | |
| 11. ‘if I've needed any information then I have done it at the, I've asked at the clinic’ (PT6) | |
| 12. ‘if I'm honest we tend to refer them on an ad-hoc basis when they raise that need to us because they have not had that opportunity to do that proper holistic assessment.’ (CNS5) | |
| 13. ‘We will talk to them about those sorts of things [symtoms of relapse]. We do have some nice cards that we need to get developed and printed to give to patients because it's there, we have done it, we have done the work on it … but to me the ideal would be when they come for that 6 week scan to have another appointment that runs alongside that for holistic end of treatment assessment.’ (CNS5) | |
3.2.1 Monitoring disease
Disease is monitored through regular appointments, symptoms and blood tests. Staff highlighted that routine appointments provide an opportunity to check in with patients but ‘usually our patients are the ones who notice they're not quite as well and they come back to clinic earlier. It's very rare that we diagnose a relapse or a recurrence at a consultation’ (CNS5). Therefore, patients' self-monitoring was crucial, and the clinical appraisal/interpretation then triaged and planned necessary actions. However, some patients were unsure what symptoms to monitor: ‘I don't really know what symptoms I am looking for’ (PT12). Other patients knew the symptoms, but did not trust their own symptom appraisal, hence relying on the blood test.
3.2.2 Trusted contact/direct link
The importance of having a trusted contact and direct link back to the service was emphasised, which was usually facilitated through contacting the CNS. However, some patients would contact their GP, the cancer ward or consultant's secretary. The CNS triaged symptoms, offered advice, discussed issues with the consultant, booked investigations and/or clinic-based appointments where necessary.
3.2.3 Information and holistic support
Information provision was continuous during the whole pathway, but written information (common at diagnosis) was less frequent when entering or on follow-up. Post-treatment most staff verbally highlighted the symptoms to monitor, but some staff felt education on relapse symptoms was insufficient ‘We don't do very much in the way of education about what symptoms people might look out for because we kind of screen purposefully’ (Consultant 1). Patients agreed that general verbal information was given (i.e., if unwell to ring). Patients highlighted the need for reliable, trustworthy information from their clinicians, as they tended to avoid internet-based information (quotes 9–11, Table 3).
CNS staff discussed their role to provide holistic support (e.g., emotional, financial, work and family) but highlighted this was ad hoc (quote 12), and they strived to enhance this support in future service developments (e.g., holistic end-of-treatment assessment and information about relapse signs).
3.3 Theme 3: General issues in follow-up
A range of issues were raised in follow-up about the disease, service or individual patient needs (see Table 4). Throughout this, uncertainty is emphasised (e.g., nature of relapse and CA125), and how the follow-up process strives to manage this.
| General issues in follow-up | |
|---|---|
| Unpredictable nature of relapse | 1. ‘If your purpose of the screening element of follow up is early detection of recurrent cancer and intermittent, randomly timed follow up appointment, there's no particular sense why that's an effective way to do that when cancer can come back at any time … So it is not logical that a 3-monthly versus a 4-monthly versus a 6-monthly versus a 2-weekly is a better way of picking up recurrence than not.’ (Consultant 1) |
| 2. ‘I often feel our patients go into follow up not really knowing what to expect and what not to expect and we do get quite a few patients that get very emotionally distressed and hit rock bottom. And I think sometimes, again, that's because we have not been able to do the correct end of pathway … and that's very scary for them going back out into that world because they know that sometime soon it's going to come back but, yet well we are saying we'll see you in 3 months we'll see you in 6 months. Then in their heads it's like, well, but I could relapse in a months' time. It is a very difficult time living alongside a cancer diagnosis, I think any cancer diagnosis must be very very difficult because it changes your life, but I think with things like, or if I say purely with our ovarian patients, it's that not knowing when and where that's going to come back.’ (CNS5) | |
| Symptom reporting | 3. ‘But sometimes they come to clinic and they have got symptoms but they are mild, they are potentially waited, so they can be seen in clinic. Well yeah actually I have noticed in the last couple of weeks in getting a bit more tired and you know I've lost a bit of appetite. But there's certainly the significant ones that are dramatic symptoms they do phone us do not they?’ (CNS3) |
| 4. ‘You know sometimes you, the patients might have symptoms but they know they are coming to the doctors in a couple of weeks and have their blood tested the day before so they do not say anything.’ (Consultant 4) | |
| 5. ‘No, it depends what it is really. I mean obviously you do not ring them straight away, it's only if you are really worried about something. You sort of take some tablets or depending on what it was. It's just, if I have any pain in my sides or lower stomach where I've had my operation, then I probably would contact the hospital, contact [CNS], and just have a chat with her about it.’ (PT13 | |
| Pros and cons of clinic visits | 6. ‘Knowing that you have got regular check-ups, it's a bit of a comfort blanket really.’ (PT2) ‘Some of them completely blank everything out until 2 days before their appointment when they have a blood test … Some patients get really uptight, so 2 weeks before their appointment they start panicking, and then you talk to them and everything alright and then they are okay again until that next time. Some patients just take it in their stride and find it helpful and some patients cling onto that as if this is you know when you get the sort of “mini all clear” which is of course not an all clear but sort of that reassurance that everything seems to be steady at the moment and they. So sometimes it generates anxiety, sometimes it can relieve anxiety, it think it is very individual …’ (Consultant 4) |
| Role of CA125 | 7. ‘we have got 1 or 2 ladies who do, they do live for that blood test.’ (CNS4) |
| 8. ‘Well the main thing when I go to clinic is because when you have had ovarian cancer there's this marker CA125 is it, so that is what I look at when I go to clinic, I go for my bloods doing before I go into clinic so that they have got the results back.’ (PT13) | |
| 9. ‘I did ask them to do the blood tests each time I go, not for them to tell me that I'm okay, but for me to sort of say I'm okay, things are moving along nicely, all my aches and pains, I'm getting used to what is now normal again, but then you know, they would have a look at my blood results and say you are saying you are okay and we can confirm that you are, and that from my point of view that just made me feel so much better.’ (PT6) | |
| 10. ‘We're trying to move away from the CA125 … we explain to them that you know, we do not treat just on a rising CA125—we treat when they have symptoms and the rationale and the reason for that and the evidence … At the moment it's patient choice, yeah. And some patients are like “no, I need to have my CA125 done” and that's fair enough, we'll do it for that and other patients are like “actually, I do not want to know because it worries me”. Because if it has gone up, and it can go up for so many other reasons that's the other thing you know any other inflammation area it can be raised.’ (CNS1) | |
| Time & resources | 11. ‘But then you go in and they say to you everything's fine, you think “oh I've sat there for an hour and everything's fine”. I mean you come out feeling happy, obviously, but it seems a bad use of time, their time, as well as my time is not it.’ (PT13) |
| 12. ‘… they travel quite a bit, they wait quite a bit and then if everything's alright then they might have a, they have a very short appointment and so the satisfaction with that is probably not necessarily so high … But it's not necessarily the most economical way of dealing with it if they are well and especially if they are well educated and confident how to manage symptoms and how to look out for symptoms.’ (Consultant 4) | |
Staff highlighted the unpredictable nature of ovarian cancer relapse and how ‘9 times out of 10 the 3-monthly follow-up has changed because they have symptoms in-between’ (CNS1). Some staff and patients suggested symptom reporting for mild symptoms may be delayed if they have an upcoming routine appointment (quote 5, Table 4). Staff discussed the need for flexibility, how patients differ in their follow-up needs, and attending clinics may provide reassurance but also trigger anxiety. Many patients were very reliant/confident in the CA125 blood test. In contrast, clinicians highlighted the poor sensitivity/specificity of CA125 testing and how fluctuations due to other factors (e.g., chest infection) can trigger cancer-related anxiety. Finally, limited time/resources were highlighted, staff emphasising ‘we do … an awful lot of responsive work, just reactive to whatever the situation brings through the door’ (CNS5), while patients described long waiting times for short appointments.
3.4 Theme 4: Views on ePRO follow-up (Table 5)
3.4.1 General views
Staff anticipated the system would suit some, but not all patients. They emphasised the importance of not losing all contact: ‘we do need to keep some human touch. So whether that is a human being that rings them back about their results but I just feel like … maybe it's me not liking to lose that contact’ (CNS5). Various positive and negative factors were discussed as influencing suitability, including travelling distance from hospital, work/caring responsibilities, technology skills, language/translation barriers, and clinical criteria (e.g., non CA125 secreting patients). Anxiety levels could both positively and/or negatively influence (e.g., anxiety may prompt wanting to see a clinician face-to-face, or prompt avoidance of attending hospital). Staff also emphasised that patients would need to be relied upon to adhere to systematic self-reporting, but that ‘At the end of treatment you would know those patients you want to bring back and those that you would be able to offer the online service’ (CNS1).
Seven out of 16 patients emphasised the benefits and were enthusiastic about an ePRO pathway, but some emphasising the importance of parallel CA125 testing, annual face-to-face contact, and/or only starting ePRO after 12 months. Two patients specifically expressed preferring a PIFU pathway with no routine appointments (or ePRO) but blood test plus open-access if any concerns. In contrast, one patient was very unsure, and 6/16 felt ePRO follow-up was not for them; despite recognising the benefits for others, they were resistant to any change to their follow-up. Some indicated they may consider it later or saw the value if used in-between clinic-based appointments.
| 4.1 General views on ePRO follow-up | |
|---|---|
| General views | 1. ‘if when a patients diagnosed that is the follow up model and they are told that, it just becomes the follow up model because they do not know no different. Patients that are currently on follow up, there are definitely a group of those patients that would jump at that. Some of our patients are working so they do not want to take time off because not all companies allow you time for hospital visits and they have to spend annual leave. A lot of them get very anxious … whereby if they were not coming to see us and they could just fill in a little thing at home in their own time and do a blood test, I think a lot of patients would like that. I think eventually, you will always get a group of patients who change is a difficult thing.’ (CNS5) |
| 2. ‘they'll be some people who prefer to see a doctor, some people who would prefer to be seen face-to-face and there would be some people would prefer not to come to clinic and sit in clinic for 3 hours waiting for somebody to tell them that they are okay, which they already knew.’ (Consultant 1) | |
| 3. ‘And there are patients that come in to clinic and say “yeah, yeah I'm fine” but you can look at them and say “you are not, you are clearly not” because they look so unwell. So those sorts of patients, you would need to use your clinical judgement before you put them on that sort of thing. I think it would only be a minority, there would only be a few of those patients that you would want to actually see … You get to know your patients, yeah. At the end of treatment you would know those patients you want to bring back and those that you would be able to offer the online service to.’ (CNS1) | |
| 4. ‘… when I went on Thursday it was 45 minutes [to get there] and because you know my bloods were fine and I was fine I was only in the surgery for two minutes, and then another 45 minutes to home so this sounds like a brilliant idea! … There would be a series of questions that have I answered - have I got, do I feel and so on, if I had any concerns then I can ring somebody and speak to them straight away and if there was anything that came back that would be dealt straight away. So no I have no concerns about it and I think it would be brilliant idea’ (PT12) | |
| 5. ‘As long as I get results of that CA125 level and I've got the option to ring somebody like [CNS] if I needed to go into clinic, then yeah, no it would not bother me not going in to see the oncologist.’ (PT13) | |
| 6. ‘I think for the first sort of maybe a year I would prefer to see somebody face to face, but after that I think I'd be quite happy to do it over the net.’ (PT3) | |
| 7. ‘personally I would not want to do it. Mainly because I'm not that good at typing things in so it would take me so long. But also I can see how it would benefit some people, I do not think it should be dismissed, but it would not be for me at all … I just would not want to do it but I'm not that good with the internet to rely on it or I would be deleting something that I should not have done and no, it would not be for me at all. But I can see how it would benefit people who are quite good with it and look on the internet a lot and deal with it.’ (PT7) | |
| Practicalities of ePRO | |
| Frequency of reporting | 8. ‘I think it should be similar to a clinic appointment because we are going to have to invest man power into looking into the questionnaires and if suddenly we are doing them twice as often, that's sort of defeating a lot of the object of doing this.’ (Consultant 3) |
| 9. ‘I think the gaps that we have at the moment which is the three month gap, for myself the three month is a nice gap, it's not too long but it's not too short, and anything in-between then I would put it on the online system.’ (PT6) | |
| Effective introduction & managing patient expectations | 10. ‘if patients know that's the way the follow up is and things, again they are more accepting, so it's targeting sort of new patients that are coming through and this is how it is.’ (CNS4) |
| 11. ‘I think as well it's got to be introduced from the very beginning so when you first see the patient, when you are talking about “when you go into follow up we will not be seeing you, we will be, this is how we do our follow up”’. (CNS2) | |
| 12. ‘something like “personal individualised follow up”. I do not know. But I think the terminology has to be right for the patients to accept it, most definitely.’ (CNS1) | |
| 13. ‘Yeah, we all term it remote monitoring, there's something slightly sinister about, you know like the big brother aspect, it's always in the news and stuff is not about, remote monitoring seems more akin to that’ (Consultant 2) | |
| 14. ‘I would have thought that the way that you would do it is you would start while they are on treatment, and getting, so you are already starting that process of them telling you how they are. I would have thought that would be the easiest thing because the fall back is they are being seen every so many weeks, so then it becomes second nature to them.’ (CNS2) | |
| 15. ‘we are not abandoning them by going down this route, it's just a different route of accessing our service.’ (CNS4) | |
| Effective communication/link back to service | 16. ‘I think what you have to do is reassure that patient that they still have complete access to the hospital, to the CNS team. We are primarily the ones that patients ring in and I think it's saying alongside this, you also can ring these girls at any time, or boys … this allows you to continue working, it allows you to access it at any time, I'm assuming they can access it and put symptoms in at any time, but it's there. You know, if you are not sure and you do not particularly want to ring up you have got this or if you want to ring up you still can ring up.’ (CNS5) |
| 17. ‘as long as there is the option to go and see the Oncologist or to get in touch with somebody.’ (PT13) | |
| 18. ‘I think you could probably do sort of a standard response, that you have looked at the symptoms that I've put and that the medical team find no cause for concern’ (PT6) | |
| Effective questions & data monitoring | 19. ‘I think the questions would have to be quite specific. When you are thinking of the parameters, you'd have to be asking “has it increased in frequency, has it increased in severity?” Does that make sense? … and at the same time you'd need a baseline, so for example I've had patients that've had abdominal pain since they have had their surgery, and that pain has never gone away and so that's their norm.’ (CNS1) |
| 20. ‘Yeah it's the change rather than the actual number. I mean the safest, if it cannot do that because it's quite a complicated thing to do … if it cannot do that the safest thing is to just have it at a level, because that's safer, it's better to over-call it than to under-call it … Yeah, because you can graph it cannot you as well, you can look at things over time. So it's better to pull it and over call it than miss. Because if it says this patient is scoring 3's and 4's and you go well they always score 3's and 4's, that's fine. But you might just phone them and make sure they are still alright if you are having a moment of paranoia. But yeah, it would be a disaster to have somebody at home with a major problem that you have missed.’ (Consultant 3) | |
| 21. ‘One level it could be computer generated algorithm led, so that the computer alerts you to … positive responses to symptom questions. If we are clever enough in how we do the symptom list, how you then fine tune them. What you do intuitively in a clinic is that you ask them a screening question and then they'll give you an answer and you'll probe as to whether you think that's a significant symptom or not. And whether we can construct a series of questions, a kind of tree of questions that allows you to do that or not remains … To stratify your level of concern. So you could just do it that, whatever the trigger is in terms of number of symptoms, frequency of symptoms, severity of symptoms, triggers and alerts, CA125 above a normal level.’ (Consultant 1) | |
| 22. ‘quite often it's the small symptoms that are the alerts and I think, I think quite often it's quite difficult to say “well if they ring up with this, this and this, that's high, we need to be doing something”. But quite often it's a niggle that's become more than a niggle that's been going on for quite a long time, it's, I do not know, I mean you have obviously, you have got it going somewhere else maybe, but it's how you determine where that level of concern is, but I presume that will come out of your pilot’ (CNS2) | |
| 23. ‘I like the safety net of having a blood test as well though. If I could sort of like go to my local hospital and if they sent out the bloods thing and you just went to your local hospital and they sent it off to them without you having to actually go, do you know what I mean? … So that, that blood test just, you know, keeps me comfortable.’ (PT3) | |
| 24. ‘There could be something about maybe looking at markers adds some reassurance to us both if we are not seeing them so much as actually that's a way of picking up. Researcher: Markers as in? CA125, whereas we are not at the minute, but that's probably balanced out by the fact that we see them’ (Consultant 2) | |
| 25. ‘Oh that'll be me (laughs) … Because there is nobody else, [consultant]'s not always here all the time’ (CNS1) | |
| 26. ‘if there's a cohort are going across to this rather than coming to clinic, its having a bit of time in clinic and just sort of discussing those who have highlighted some concerns and then just planning around you know what it is we need to doing.’ (Consultant 2) | |
| 27. ‘Think probably initially it would have to be looked at by a nurse or a doctor, initially, until we got the flow of it. But it may be that you could have a band 3 that could triage it with guidelines’ (CNS5) | |
| Effective identification of information/holistic needs | 28. ‘I do not know if I was seriously worried about it I would make an appointment to see the consultant straight away. Researcher: Okay, so more serious? Only if I was seriously worried about something. I suppose you know for those times where you think “oh gosh, is this something to worry about or not”. You know I would perhaps look then if I thought it was a reliable website like the one you are talking about.’ (PT15) |
| 29. ‘I think you would be putting advice on about you know “if you experience this symptom have you contacted your GP? Or your clinical nurse specialist”, you could point them in the direction of, you could have a list of symptoms and say “if you are experiencing these then you do need to ring up and make an appointment to be seen” … My overarching thing would be “if you are concerned, regardless of what's on what's said here, you must ring up and ask” because I've had patients that say “well it said that was normal, I've read my book” and they have not done anything about it, so I think you have got to be very careful because people can be very literal and if it says I might feel like this, then it says so I'm fine’ (CNS2) | |
| Barriers to ePRO monitoring | |
| Technology concerns (access/skills/trust) | 30. ‘I was just wondering whether or not, because a 50 year old who does it all day at work is a very different animal to a 75 year old who does not, who might do it a bit. So for that group whether or not when they get registered, [research nurse] and co and the research team whether or not they do their baseline there and then with them so they know what it's like’ (Consultant 3) |
| 31. ‘I just would not want to do it but I'm not that good with the internet to rely on it or I would be deleting something that I should not have done and no, it would not be for me at all.’ (PT07) | |
| Feelings of abandonment/impersonal | 32. ‘I think it just takes away that personal touch completely, it just seems to be the way that everything's moving you know, other erm, other industries as well. It's just, with something like that do we really want to go down that route? I do not know, if I'm honest with you.’ (PT02) |
| 33. ‘I think it is about knowing that there is a person at the end and that they get some feedback and that they might just need a couple of personal lines afterwards, so say “look [name] I've just looked at your blood tests and the scans and everything's alright. I've looked at all the information you have given us”.’ (Consultant 4) | |
| 34. ‘and sell it as, you know, this allows you to continue working, it allows you to access it at any time, I'm assuming they can access it and put symptoms in at any time, but it's there. You know, if you are not sure and you do not particularly want to ring up you have got this or if you want to ring up you still can ring up. I think it's about just giving them the positivity's of it, but delivering it as a fait accompli.’ (CNS5) | |
| Proposed impact of ePRO monitoring | |
| Time and resources | 35. ‘I think in the long term the strengths would be we would not have as many patients coming in to be reviewed and, therefore, we would have the time to do holistic end of treatment reviews and signpost and look at survivorship more. I think it would allow the clinics to be quieter giving more time to concentrate on the patients that absolutely need input here and now.’ (CNS5) |
| 36. ‘So a certain level of incremental change above their previous values as triggers to someone looking at that case and contacting the patient or what have you. Versus a person looking at the results of every screening intervention.’ (Consultant 1) | |
| Self-management/patient control | 37. ‘I guess it's saying that really, if at 6 o'clock after tea you want to do your questionnaire and stuff, it's not saying I have to be with us at half past 9 on a Tuesday, it's fitting in around all of them’ (Consultant 2) |
| 38. ‘I think it would be more efficient and more effective than the current seeing everybody at once, I think it would give patients better control, and feel they are in control of their own disease really.’ (CNS1) | |
| 39. ‘we have picked up things that are not right when patients will come in and say they are absolutely wonderful. I have patients that come to clinic whose family have rung me and said “Mums due to come to clinic a week on Tuesday. She's really not right but she'll tell you she's okay”. Now if you were on straight forward telephone thing, are those patients, I'm just suggesting, are those patients going to slip under the radar?’ (CNS2) | |
| Patient-clinician communication | 40. ‘it sounds good because it is nice to know that you could ask somebody and you'd get a reply quite quickly, you know. If it was something you did not want to bother your consultant about because it wasn't worth bothering them about. That would be very reassuring, you know, that does sound good.’ (PT15) |
| 41. ‘It's just using our service differently is not it? Instead of picking up that phone and saying [nurse] I've got this problem, it'll come to us electronically.’ (CNS4) | |
| Recording/remembering symptoms | 42. ‘I think that'd be really helpful, you know, if I put some symptoms down and say look I'm having a bit of a problem with this, and then I can go in later and say, actually those symptoms were only there for so many days, and I've not had them since, then that'd make me feel better knowing that I've cleared up that problem, it's fine, it's gone, and at that point in time I'm telling you that I'm fine. So if I could go in and add further comments then the previous comments are not relevant anymore, it's gone and I've not had those symptoms ever again, it just keeps up-to-date how I feel on an ongoing basis.’ (PT6) |
| 43. ‘I would not be reporting anything on the system. And I can understand where you are coming from, there again I do write everything down … if I had anything at all, I would ring straight to the hospital, the line that I've been given, the helpline … I would tell you if I wasn't so you know, they would know because this is getting me into the internet again which I do not want.’ (PT7) | |
| Regaining normality | 44. ‘So for myself the first 12 months I'd rather have that appointment, because let us face it it's only, what is it, four times in a year, so it's not a big upheaval, but after that I think it's more a case of you get back to your normal routine of going to work, which does not include going to hospital anymore, it becomes more normal. And I think the online one that would bring more normality to people's lives, being able to do that.’ (PT6) |
3.4.2 Practicalities of ePRO
Various practicalities were discussed, which map onto the key features of effective follow-up (Figure 1).
Frequency of reporting
Most staff and seven patients suggested ePRO intervals should be the same as clinic-based appointments (3-monthly), but with additional ad hoc access. Three patients suggested monthly intervals, three preferred exclusive ad hoc symptom-led use, and three did not specify as they were not keen.
Effective introduction and managing patient expectations
Staff discussed gradually introducing ePRO follow-up, as patients start asking about follow-up during treatment. Several staff felt that if ePRO follow-up is routinely offered acceptability may increase over time (quote 10/11, Table 5), and new patients may accept it more readily having not experienced face-to-face follow-up. Staff felt the clinical team should introduce ePRO follow-up. Various terms were discussed including ‘patient-initiated’, ‘patient-driven’, ‘self-directed’, ‘open-supported’, ‘personalised’, ‘individualised’ and ‘electronic monitoring’, but there was uncertainty in how to present it and ‘remote monitoring’ was viewed negatively because it implied patients were on their own.
A couple of staff suggested patients should start ePRO monitoring during treatment to increase familiarisation of the system, logging symptoms and communicating this way with their team. Importantly, most staff emphasised the need to manage patient's expectations, ensure they knew the clinical team were reviewing responses, and ‘there is still an open access. This is not, you're on remote and nobody wants to speak to you’ (CNS5).
Effective communication/link back to service
There was a unanimous view that patients should have direct access to the hospital/CNS as required. Patients emphasised needing communication that their blood test and/or symptoms had been reviewed, and an email, phonecall or letter confirming results. Both groups suggested if symptoms/concerns were raised a direct phonecall should be made to reassure and determine necessary actions.
Effective questions and data monitoring
Selecting the right questions to pinpoint key symptoms, similar to those asked during clinic-based consultations, was emphasised. Most patients emphasised that CA125 monitoring should continue, and staff working in services who did not currently routinely check CA125 emphasised it may have a place in an ePRO pathway.
Rigour in monitoring the ePRO data centred on safety concerns and ensuring nothing was missed. Computer-generated algorithms to alert clinicians when certain patient-reported symptoms were logged was discussed, but some staff highlighted the difficulty in setting thresholds as ‘often it's the small symptoms that are the alerts … it's a niggle that's become more than a niggle that's been going on for quite a long time’ (CNS2). Therefore change over time or comparing to baseline/patient's norm may be more important. Most staff and patients felt the CNS could monitor reports and triage issues with medical staff. Furthermore, staff emphasised the importance of having a scheduled time to review, keeping clinic slots available for any necessary urgent patient contact, and a virtual clinic/tracking to ensure any patients who failed to report when expected were still contacted.
Effective identification of information/holistic support needs
There was minimal discussion about the provision of online tailored self-management advice within the ePRO for mild symptoms or holistic needs. Some staff highlighted that ‘My overarching thing would be if you are concerned, regardless of what's … said here, you must ring up and ask’ (CNS2).
3.4.3 Barriers to ePRO monitoring
The main ePRO barriers centred on technology concerns, how the process may result in feelings of abandonment, and suggestions were given on how to alleviate these issues.
Interviewees recognised that an ePRO pathway necessitates computer skills and internet access. Three patients interviewed did not have a computer/internet and four expressed limited skills. It was emphasised that any electronic questionnaire/website should be easy to use, and training provided to increase user confidence.
Some unease was voiced about trusting technology, which links to how some patients may view the system as impersonal and may result in feeling abandoned: ‘it just takes away that personal touch completely, it just seems to be the way that everything's moving’ (PT02). Staff felt it was important patients could still directly contact and see them face-to-face if needed.
3.4.4 Proposed impact of ePRO monitoring
The most frequently stated impact was time saving and reallocation of resources to those who really needed the time and support, in particular for patients who were physically well, had work/caring responsibilities or who lived a long distance from the hospital. Staff were less clear of the benefits for their work—some feeling it would improve their efficiency or redirect their work, but uncertainty was voiced on the workload of reviewing/responding to ePRO data.
Staff mentioned that ePRO monitoring may increase patients' self-efficacy with symptom management: ‘a lot of patients feel helpless and powerless and this is something that they can do and take control of’ (Consultant 3), but one CNS was concerned about relapse being missed.
Staff and some patients felt the system enabled patients to communicate symptoms/concerns and gain advice/reassurance and could improve the recall of symptoms/concerns (quotes 40–42, Table 5). Finally, four staff and one patient stressed that attending hospital/clinic reignited difficult emotions in patients, and an ePRO pathway could help reduce this and regain more normality.
4 DISCUSSION
This study provides an in-depth insight into the experiences of patients on follow-up after ovarian cancer, and the potential for an ePRO pathway. Firstly, these interviews highlight how entering follow-up is a transitional process, and how three key features contribute towards effective follow-up (trusted contact/direct link, monitoring disease and information/holistic support), which are all connected flexibly to meet patients' follow-up needs. Secondly, views of ePRO follow-up were elicited. Remote methods of follow-up have been increasingly used during the COVID-19 pandemic as services had to reduce hospital footfall, leading to calls for the rapid introduction of ePRO monitoring (Marandino et al., 2020) and its use to facilitate provision of effective support for cancer survivors during/following the pandemic (Jones et al., 2020; Nekhlyudov et al., 2020). The current qualitative work was conducted before the pandemic, which may have triggered changes to ePRO views among patients and clinicians. The acceptability of telephone-based and remote appointments during the pandemic is now emerging (Dalby et al., 2021; Duncan et al., 2021; Hasson et al., 2021). While we cannot confirm exactly how the pandemic may have changed these views, the interview data highlight some important aspects that all clinical services should consider if introducing and planning remote follow-up services.
This aside, the results highlight the complexities of providing follow-up care post-treatment, which appear relevant to all ovarian cancer patients and may be applicable to other cancers with high risk of relapse. Balancing the need to monitor for relapse, but within an uncertain timescale; patients can spend numerous hours waiting/attending outpatient appointments when they are well, which may be better utilised among those with specific symptom/support needs. Clearly, provision of rapid access to specialist care when symptoms/concerns are experienced is crucial, which currently happens by patients making contact between appointments. This study illustrates the potential for an ePRO pathway to provide the same key features (Figure 1) using a hybrid of online and telephone methods (plus face-to-face where necessary). The ‘proposed impact’ subtheme illustrates several positive outcomes of ePRO (e.g., increased autonomy, reduced hospital visits and economic benefits), which mirror previous literature on telehealth interventions (Cox et al., 2017; Cox & Faithfull, 2015; Lizée et al., 2019). Therefore, we propose an ePRO follow-up approach is explored in further research and clinical practice.
To date, gynae-oncology research has focused on telephone-based alternative follow-up, but PIFU is increasingly offered (Coleman & Newton, 2020), despite the British Gynaecological Cancer Society advising against PIFU in stage 1C-4 ovarian cancer (Newton et al., 2020) due to relapse risk. This qualitative work aimed to explore patient and clinician perspectives on ePRO monitoring, which would sit in-between PIFU and the clinic-based model, and may offer advantages over PIFU and/or telephone follow-up, especially the addition of online self-reported information and real-time algorithm-based alerts to prompt clinicians (Absolom et al., 2019). The CNS team highlighted the reactive nature of their work and how the regular ePRO data may help them consistently and promptly explore holistic needs and signpost to relevant services.
Importantly, the current work highlights several concerns and practicalities that require further consideration before the implementation/adoption of ePRO pathways. Views about ePRO follow-up were mixed among patients, and it may not be suitable for all patients. This finding is similar to other studies where patients are offered a choice (Lindner et al., 2020; Morrison et al., 2018; Qaderi et al., 2021). Feelings of abandonment were raised by some, which is common at treatment completion even within face-to-face follow-up (Lydon et al., 2009), and is cited in a narrative review of the impact of telehealth interventions (Cox et al., 2017). However, Cox et al. (2017) also present synthesised evidence that patients can feel reassured by the real-time connection that telehealth interventions provide. Therefore, alleviating any concerns and enhancing reassurance in the ePRO pathway design is important, which may be facilitated through the way it is introduced and the training provided to patients and staff (e.g., endorse open-access contact and prompt staff engagement to data/concerns).
It is noteworthy that the women interviewed had been on routine face-to-face follow-up for 2–36 months (median 10.5) and this past experience may have influenced their views. Staff suggested that new patients may be more open to alternative follow-up pathways, and provision of training/familiarisation of ePRO monitoring while on treatment may help. However, some patients emphasised their hesitancy of accepting ePRO follow-up during the first year post-treatment, so further work is needed to explore the optimal timeframe. Ultimately, forward-planning and future-proofing is important so that services using technology-based methods do not represent risks to patients health/relapse identification (NHS England & NHS Improvement, 2020). Furthermore, any ePRO pathway needs positive engagement from clinicians (patients need confidence their clinicians are reviewing the ePRO data), which leads to trust and patient engagement (Warrington et al., 2019). Therefore, there is a need to explore the most effective ways of utilising ePRO pathways to meet patient needs, and ease (not add to) clinician workload.
This interview study was undertaken in four hospitals in Northern England during 2016–2017, a large cancer centre and three district general hospitals, and findings were similar across these settings. However, the sample may not be representative of the UK ovarian population as a whole, with interviewees age varying between 23 and 80 years, but only four women (25%) were 75–80 years old, the age with the highest ovarian cancer incidence according to Cancer Research UK (2020). Therefore, the sample appears slightly skewed to younger, working age women who may be more computer literate. We did not routinely capture race, ethnicity, or educational background, the latter of which may have also influenced confidence with electronic methods. As already discussed, views may differ post-pandemic, and also between countries, geographical areas, patients' previous follow-up experiences and self-efficacy. Furthermore, the proposed ePRO pathway was anticipated to be begin shortly after treatment completion (<3 months), so views on its introduction 12 months+ of post-treatment were not explicitly explored. However, this work informs future development and feasibility of ePRO follow-up pathways, and Figure 2 presents the key recommendations.

Importantly, the views on the potential acceptability of ePRO follow-up may transfer to individuals receiving follow-up after treatment for other cancer diagnoses, as they mirror some of the findings in the literature where services have implemented remote methods (Qaderi et al., 2021). Alongside other development work (including patient involvement to guide the intervention design, as recommended by Cox et al., 2017) to develop the selection of symptom items (Shearsmith et al., 2020), these findings have informed an ePRO system and a pilot study is underway (patient-reporting symptoms/blood test 3-monthly, algorithm-based clinician alerts, CNS phonecall).
5 CONCLUSION
Our study suggests that ePRO monitoring in ovarian cancer follow-up has potential, its utility in the provision of care was endorsed by clinicians and some patients, and this work has informed recommendations for future work in this area. With ever-increasing patients living longer in our increasingly technologized societies, we must explore whether ePRO pathways can provide acceptable, responsive and tailored support to meet patient needs. Further research is needed to explore the transferability of these findings to larger samples of oncology patients, and the real-world acceptability, safety, and the practical implications of these methods.
ACKNOWLEDGEMENTS
We thank the patients and staff who participated, research nurses and clinicians who supported patient recruitment, members of the project steering group, and the wider Patient reported Outcomes research Group (POG), including Lewis Marston who conducted some of the interviews. This work was funded by Yorkshire Cancer Research (Award reference number L392). The charity had no influence on the data collection, interpretation or reporting.
CONFLICT OF INTERESTS
Fiona Kennedy reports a research grant from Breast Cancer Now. Galina Velikova reports honoraria from: Roche, Eisai, Novartis and Seattle Genetics, and research grants from Breast Cancer Now, EORTC, Yorkshire Cancer Research, Pfizer and IQVIA. Leanne Shearsmith, Marie Holmes, Oana Lindner, Rosemary Peacock and Molly Megson declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are not publicly available due to privacy/ethical restrictions, but the corresponding author may consider reasonable requests.




