Short-term efficacy and safety of total skin electron beam therapy in mycosis fungoides: Systematic review and meta-analysis
Abstract
Total skin electron beam therapy (TSEBT) is one of the mainstays of treatment for mycosis fungoides. The most common modalities are standard dose (30–36 Gy) and low dose (10–12 Gy). To review the literature on the efficacy and safety profiles of standard dose and low dose TSEBT. We searched electronic databases for studies that enrolled patients with Mycosis Fungoides and treated with TSEBT. We estimated the event rates associated with low dose and standard dose TSEBT. The Preferred Reporting Items for Systematic Reviews and Meta-analyses reporting guideline was followed. Main outcomes were complete response rate, partial response rate, mild and severe adverse events rate low dose TSEBT had a Complete Response Rate of 28% [0.19, 0.37], an Overall Response Rate of 85% [0.76, 0.93], a mild adverse events rate of 93% [0.82, 1.04] and a severe adverse events rate of 5% [−0.04; 0.14] Standard dose TSEBT had a Complete Response Rate of 57% [0.41; 0.73], the Overall Response Rate was 99% [0.97; 1.02], the mild adverse events rate was 100%, the severe adverse events rate was 7% [−0.01; 0.16]. Comparing standard dose TSEBT in the early versus advanced stages, advanced stages patients had a Risk Ratio = 0.77 in obtaining a Complete Response [0.64, 0.92](p = 0.0158). TSEBT is an associated with an excellent short term safety profile. Both schedules show high ORR, with standard dose TSEBT demonstrating highest CRR. Advanced stage of disease negatively influence the CRR.
1 INTRODUCTION
1.1 Rationale
Total skin electron beam therapy (TSEBT) is a skin-directed technique were linear accelerator generated electron beams are used to treat the whole skin surface to a limited depth. It is recommended as second line therapy in early stage Mycosis Fungoides (MF) and as first-line option for MF tumor stage and erythrodermic MF.1 The ‘standard’ treatment course consists in a total dose of 30–36 Gy over a period of 8–10 weeks, and it is associated with high Overall Response Rates (ORR) and Complete Response Rates (CRR) both in early and more advanced stages MF. However, it is associated with a non-negligible rate of acute and delayed toxicities. After a standard dose TSEBT (sd TSEBT) patients are usually retreated with a second course at lower doses, with only a few patients reported who have been treated with two standard dose courses.2 In order to mitigate this issue, several centers have moved to ‘low dose’ TSEBT (ld TSEBT), applying 10–12 Gy usually over 2–3 weeks time. Despite the first publications related to its use in the management of cutaneous lymphomas date back to 1961, all the available literature derives from single center, retrospective studies, or prospective case series limited in numbers of enrolled patients and derived from one to few centers only.
1.2 Objectives
The aim of this meta-analysis is to compare CRR and ORR of both ld and sd TSEBT in early stage and advanced stages MF. Secondary outcomes are to report pooled measurements of CRR, ORR, Toxicity rates in both early and advanced stages MF patients exposed to ld and sd TSEBT.
2 METHODS
2.1 Protocol and registration
The protocol was registered with PROSPERO under the registration number CRD42020221852.
2.1.1 Literature search and study selection
A systematic literature search was performed to answer the question ‘What is the effect of ld and sd TSEBT on Mycosis Fungoides?’ The conduct and reporting of this review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Systematic searches were conducted in MEDLINE, Embase and Web of Science from inception to 28th May 2020 using terms ‘radiotherapy’ ‘Total skin electron beam therapy’ and ‘Mycosis Fungoides’. Searches were performed by two researchers trained in systematic reviews (Vieri Grandi, Tommaso Grassi). References of included studies were reviewed for additional papers of relevance.
2.2 Inclusion and exclusion criteria
We included original articles evaluating ld or sd TSEBT in MF patients which reported Complete Responses and Overall responses. We excluded all works with less than 5 cases, with inadequate clinical details, and those published before 2011 and not following the standardized staging system and evaluation of outcomes.3 We also removed older publications from single centers, which published follow up cohorts in more recent papers.
2.3 Data collection process
After de-duplication, abstracts and titles were screened independently by two researchers (Vieri Grandi, Gabriele Simontacchi). Full-text articles of selected titles/abstracts including texts, tables, and figures were reviewed against a priori defined inclusion and exclusion criteria, again independently by two different researchers (Vieri Grandi, Gabriele Simontacchi). Discrepancies were resolved by discussion with the senior author (Nicola Pimpinelli). Data extraction and quality assessment were performed by one researcher and checked by another against the original article.
2.4 Summary measures
Data were pooled using a random-effects model calculated via the Mantel–Haenszel method or the inverse variance method and adjusted via the Hartung-Knapp-Sidik-Jonkman method.4 Tau2 estimator was calculated used the method by Sidik and Jonkman.5 Risk ratios or Standardized Mean Differences (SMD) were used as effect size. To allow a better prediction of treatment effects in different settings, we also included prediction intervals in all analyses.6
2.5 Risk of bias across studies
To evaluate potential risk of bias across studies we used the Higgin's & Thompson's I2 statistic.7 I2 values of <25%, 50%, and >75% represent low, moderate, and high heterogeneity, respectively. If there was substantial heterogeneity (= or > than 50%) we performed an Influence analysis.8 Specific analyses considering confounding factors were not possible because raw data were not available. All p-values were 2-sided, and statistical significance was set at p < 0.05. To investigate publication bias, we visually inspected funnel plots and conducted Egger's test of the intercept.9 If Egger's test proved to be significative, we did apply Duval and Tweedie's trim-and-fill method.10 All statistical analysis was conducted with Rstudio.11-13
3 RESULTS
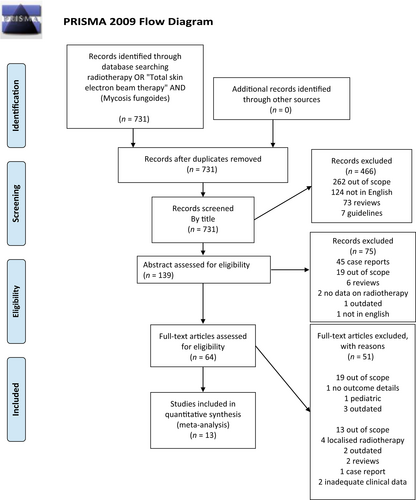
Search criteria and selection procedures of this study are depicted in Figure 1. We found 11 case series and 1 comparative observational non-randomized study fitting all our selection criteria. A 8 of these were retrospective, 4 were prospective. A total of 7 studies had a cohort of patients treated with ld TSEBT, 4 studies focused on sd TSEBT, one study included both. The study by Goujon et al.14 did fulfill the MF/SS standardized staging system and evaluation of outcomes despite being published before the official publication of the consensus paper,3 and therefore we included it in the analysis. The characteristics of the studies included in the final analysis are summarized in Table 1 alongside patients demographic details.15-26
| Authors (Year) | Study design | Regimen | Dose (Gy) | Number of patients | Median age (min–max) | Early stage MF | Erythrodermic MF/ SS | Tumor stage MF | M, F, M:F ratio | Median follow up in months (min–max) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ioannis et al (2020) | Prospective single center observational | Low dose | 12 | 14 | 60 (42–74) | 5 | 2 | 6 | 7, 7, 1:1 | 39 (3–54) |
| Song et al (2020) | Prospective single center observational | Low dose | 12 | 25 | 70 (37–89) | 13 | 8 | 4 | 20, 5, 4:1 | 16 (4–54) |
| Elsayad et al (2019) | Retrospective single center case series | Low dose | 12 | 48 | 68 (35–88) | 31 | 4 | 13 | 29, 19, 1.5:1 | 13 (1–69) |
| Dault et al (2019) | Retrospective single center case series | Low dose | 12 | 13 | 70 (56–89) | 6 | 9 | 1 | 11, 5, 2.2:1 | 21 (2–79) |
| Dault et al (2019) | Retrospective single center case series | Standard dose | 32 | 13 | 61 (49–77) | 7 | 5 | 1 | 8, 5, 1.6:1 | 21 (2–79) |
| Tavernier et al (2019) | Retrospective single center case series | Low dose | 12 | 12 | 66 (39–88) | 8 | 1 | 3 | 8, 4, 2:1 | 24 (1–36) |
| Piotrowski et al (2018) | Retrospective single center case series | Standard dose | 32 | 40 | 60 (40–84) | 20 | 6 | 14 | 32, 8, 4:1 | 60 (1–120) |
| Morris et al (2017) | Retrospective single center case series | Low dose | 12 | 103 | 68 (26–91) | 54 | 12 | 37 | 76, 27, 2.8:1 | 20 (3–53) |
| Kamstrup et al (2015) | Prospective single center observational | Low dose | 10 | 21 | 66 (47–92) | 12 | 4 | 5 | 17, 4, 4.2:1 | 16 (4–40) |
| Hoppe et al (2015) | Prospective single center observational | Low dose | 12 | 33 | 63 (19–83) | 24 | 2 | 7 | 18, 15, 1.2:1 | NA |
| Morris et al (2013) | Retrospective single center case series | Standard dose | 30 | 41 | 64 (NA) | 17 | 3 | 21 | 30, 11, 2.7:1 | 18 (NA–NA) |
| Hinds et al (2013) | Retrospective single center case series | Standard dose | 36 | 77 | 65 (30–87) | 57 | 0 | 20 | 47,30, 1.6:1 | NA |
| Navi et al (2011) | Retrospective single center case series | Standard dose | 36 | 180 | 57 (19–86) | 103 | 0 | 77 | 110, 70, 1.6:1 | 77 (6–434) |
| Goujon et al (2009) | Retrospective single center case series | Standard dose | 34 | 68 | 54 (NA) | 54 | 0 | 0 | 38, 32, 1:1 | 78 (19–348) |
These studies included 616 patients, of which 67% were males. Average age for patients treated with ld TSEBT versus sd was 67 (19–92) and 61 (19–87) years, respectively. The median sample size was 23 (12–103) for the ld TSEBT group and 41 (13–180) for the sd TSEBT. The median follow-up was 20 months (1–79) for ld TSEBT group and 40 (1–434) for sd TSEBT.
In the ld TSEBT cohort, there were 153 early stages MF and 118 advanced stages MF (1.3:1). In the sd TSEBT cohort, there were 204 early stage MF patients, and 147 advanced stages MF (1.4:1). Finally in the ld TSEBT group there were 186 men and 86 women (2.1:1) while in the sd TSEBT cohort there were 227 and 124 (1.8:1).
3.1 Efficacy of ld and sd TSEBT on the whole cohort
Ld TSEBT data on the whole cohort is depicted in Figure 2 (CRR, ORR, as a and b, respectively). Overall, ld TSEBT had a CRR of 28% [0.19; 0.37], Prediction Intervals (PI) 0.00–0.56 (8 studies, 265 patients, I2 = 0) and an ORR of 88% [0.76; 0.93], PI 0.69–1.00 (8 studies, 265 patients, I2 = 0).
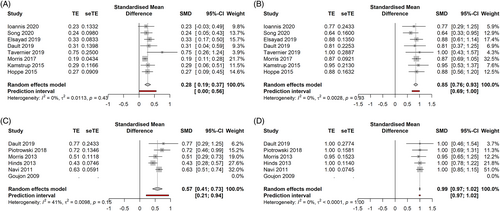
Sd TSEBT data on the whole cohort is depicted in Figure 3 (CRR, ORR as a and b, respectively). CRR was 57% [0.41; 0.73], PI 0.21–0.94 (5 studies, 351 patients, I2 = 41%), ORR was 99% [0.97; 1.02], PI 0.97–1.02 (5 studies, 351 patients, I2 = 0%),
3.2 Toxicity
Ld TSEBT had a mild adverse events' rate of 93% [0.82; 1.04], PI 0.74–1.12(4 studies, 190 patients, I2 = 0), a severe adverse events' rate of 5% [−0.04; 0.14] (7 studies, 256 patients, I2 = 21%). Sd TSEBT had a mild adverse events rate equal to 100% (2 studies, 220 patients, I2 = not applicable), the severe adverse events rate was 7% [−0.01; 0.16] (3 studies, 261 patients, I2 = not applicable). There were no cases of death related to TSEBT.
Most common G1-G2 adverse events were partial or total reversible alopecia, radiation dermatitis, xerosis, nail toxicity, limb oedema.
Data on alopecia shows that the mean incidence rate in ld TSEBT is 44% [0.05; 0.82](4 studies, 175 patients, I2 = 71%) and in 100% of cases with sd TSEBT (3 studies, 289 patients, I2 = 0).
Radiation dermatitis occurred in 28% of patients treated with ld TSEBT [0; 0.64] (4 studies, 175 patients, I2 = 89%) and in 83% of patients treated with sd TSEBT [0.1; 1.56] (3 studies, 289 patients, I2 = 88%).
Nail toxicity occurred in 16% of ld TSEBT patients (4 studies, 4 studies, 175 patients. I2 = 67%) and in 72% of the sd TSEBT cohort (3 studies, 289 patients, I2 = 98%).
Xerosis occurred in around 11% of patients treated with ld TSEBT (2 studies, 54 patients, I2 = 72%) and in 100% of patients treated with sd TSEBT (2 studies, 248 patients, I2 = 0%).
Limb edema was observed in 11% of patients on ld TSEBT (4 studies, 171 patients, I2 = 37%), while it was reported in 21% of patients treated with sd TSEBT (2 studies, 109 patients, I2 = 0%).
Rarer mild side effects included fatigue, pruritus, ocular irritation, pain in extremities, scalp pain, actinic keratosis, anorexia, cheilitis, hypomagnesemia, oral ulcers, vesicular rash, hyperpigmentation, hypohidrosis, gynecomastia, skin infection, skin pain, bullous dermatitis.
Notably, the mean number of G1-G2 AEs per patient treated with ld TSEBT was 1.5. The mean number of concurrent G1-G2 AEs per patient treated with sd TSEBT was 3.5.
3.3 Efficacy of ld TSEBT in early stage MF
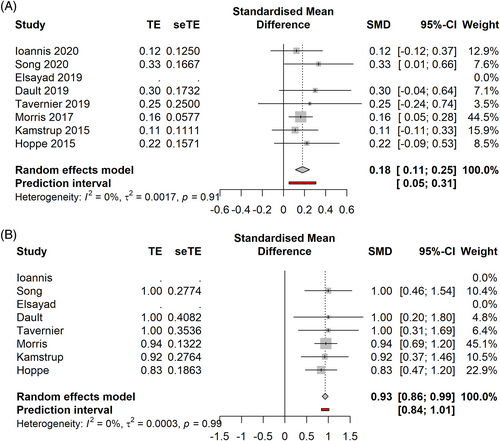
In the early stage cohort (Figure 4A,B) ld TSEBT showed a CRR of 28% [0.10; 0.49], PI -0.26–0.85 (7 studies, 122 patients, I2 = 17%), and an ORR of 93% [0.86; 0.99] PI 0.84–1.01 (6 studies, 117 patients, I2 = 0%).

3.4 Efficacy of ld TSEBT in advanced stage MF
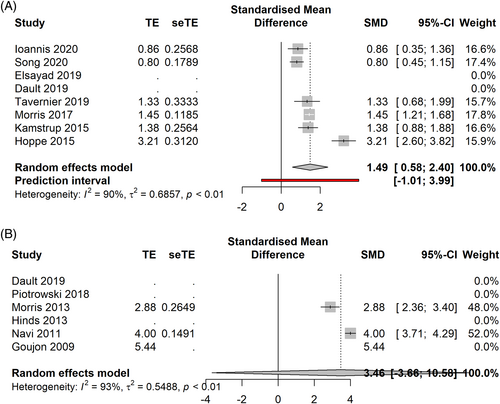
In the advanced stage cohort (Figure 5A,B), ld TSEBT showed a CRR of 18% [0.11; 0.25] PI 0.05–0.31 (7 studies, 101 patients, I2 = 0%), an ORR of 75% [0.58; 0.91], PI 0.46–10.4 (6 studies, 93 patients, I2 = 0%). In the ORR analysis, a potential publication bias was identified. After application of the Duval and Tweedie's trim-and-fill method, ORR was calculated as 68% [0.49; 0.87], PI 0.22–1.14.
3.5 Efficacy of sd TSEBT in early stage MF
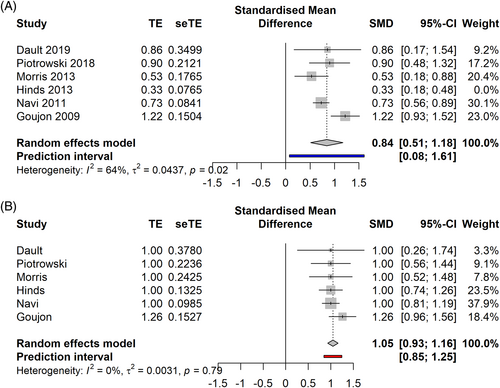
In the early stages (Figure 6A,B), sd TSEBT demonstrated a CRR of 61% [0.31; 0.91], PI −0.08–1.30 (5 studies, 204 patients, I2 = 75%), an ORR of 100% [1.00; 1.00] PI 1.00–1.00 (5 studies, 204 patients, I2 = 0%). After influence analysis, Hinds study was removed, leading to a CRR of 72% [0.54; 0.94] (4 studies, 127 patients, I2 = 0%), PI 0.21–1.23.
3.6 Efficacy of sd TSEBT in advanced stage MF
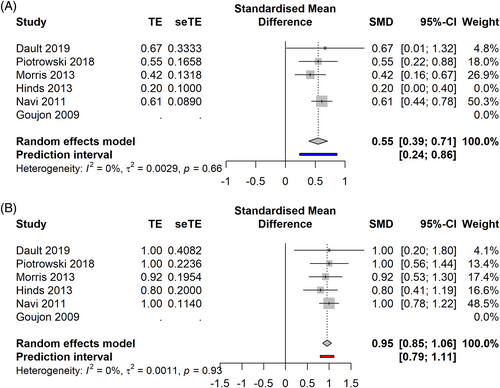
In the advanced stages (Figure 7A,B), sd TSEBT demonstrated a CRR of 45% [0.22; 0.69] PI −0.08–0.98 (5 studies, 351 patients, I2 = 61%), an ORR of 95% [0.85; 1.06] 0.79–1.11 (5 studies, 351 patients, I2 = 0%). After performing the influence analysis, Hinds' paper was removed, showing a CRR of 55% [0.39–0.71], PI 0.24–0.86 (4 studies, 274 patients, I2 = 0%).
3.7 Comparison between ld TSEBT efficacy in early versus advanced stage MF
Comparing ld TSEBT in early versus advanced stages (Figure 8A) we observed that advanced stages patients had a Risk Ratio of 0.70 to achieve CR compared to early stages [0.33, 1.49] PI 0.13–3.91 (p = 0.29) (7 studies, 223 patients, I2 = 0%).
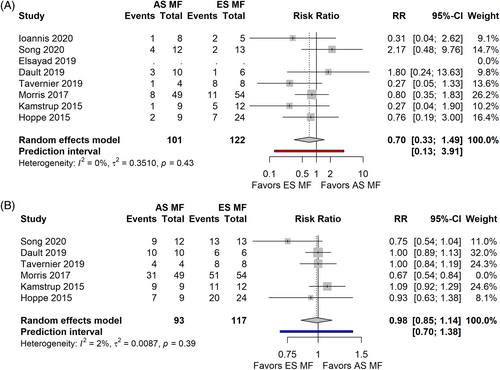
In terms of ORR (Figure 8B), the advanced stage cohort had a Risk Ratio of 0.91 [0.75; 1.11], PI 0.56–1.49 (p = 0.29) compared to the early stages (6 studies, 210 patients, I2 = 67%). After Influence analysis, Morris' paper was removed and calculation was performed with the remnant studies, showing a Risk Ratio of 0.98 [0.85–1.14], PI 0.70–1.38 (I2 = 2%).
3.8 Comparison between sd TSEBT efficacy in early versus advanced stage MF
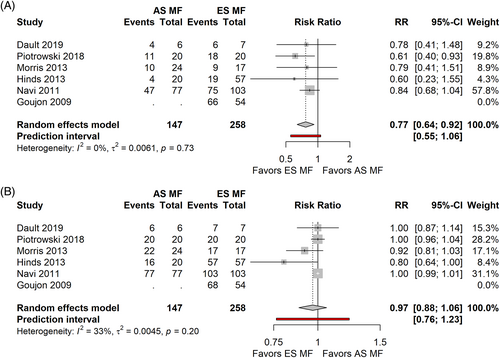
Comparing sd TSEBT in the early versus advanced stages advanced stages patients had a lower chance of obtaining a CR, with a Risk Ratio = 0.77, [0.64; 0.92] PI 0.55, 1.06 (p = 0.0158) (5 studies, 351 patients, I2 = 0) (Figure 9A), no significant differences in ORR (Figure 7B) (Risk Ratio = 0.97 [0.88; 1.06], PI 0.76–1.23, p = 0.366, 5 studies, 351 patients, I2 = 33%).
4 DISCUSSION
4.1 Summary of evidence
We performed a systematic literature review and meta-analysis on the short-term clinical effects and adverse events of ld and sd TSEBT in the management of early and advanced stage MF. All the evidences are derived from non-randomized, single center studies, often conducted with a retrospective design. Hence, although the results can be considered striking, the strength of the evidences fall in the range of low-to-medium quality. There are limited data on the safety profile of both ld TSEBT and sd TSEBT, but virtually all patients experience at least one adverse event. Moreover, patients treated with sd TSEBT experience a mean of 3 G1-G2 adverse events each. This might reflect the reported low tolerability and explains the reluctancy of patients to repeat over time another course of sd TSEBT.21 The available evidences show that in both schedules the risk of Severe Adverse Events (SAEs) is low although it is not clear if the risk is dose related or if there are some patient-related risk factors. Another possible explanation of the similar SAE rates of could be the fact that 4 out of 8 ld TSEBT studies are prospective while all sd TSEBT studies are retrospective; furthermore ld TSEBT studies are more recent than sd TSEBT. This could lead to a more accurate SAE reporting in ld TSEBT studies. It would be important, in future studies with TSEBT, to include safety outcomes to predict which patients are at higher risk of developing SAE. Available data show substantial effect in terms of CRR for ld TSEBT in the whole MF cohort. In the early stage MF subgroup analysis, pooled data showed an estimated CRR of 29%. In the advanced stages subgroup ld TSEBT led to a CRR of 18%.
The stage of disease does not seem to have an impact on the clinical benefit of ld TSEBT. In terms of ORR, this is easily explained by the extremely high rates in all clinical scenarios. In fact, even in the advanced stages, ld TSEBT allows to obtain a significant clinical response in no less than 68% of cases. Oppositely, CRR are limited both in the early stage and advanced stage MF cohorts (29% vs. 18%, respectively), again making difficult to highlight the influence of stage on the clinical response.
Sd TSEBT showed a clear clinical effect both in terms of CRR and ORR at all stages. In fact, albeit we could not directly compare results of cohorts treated with ld versus sd TSEBT, we observe a higher rate of CR when using sd-TSEBT in both early stages (61% vs. 28%) and advanced stages (45% vs. 18%). The potential impact of stage of disease on the clinical benefit of sd TSEBT in terms of CRR is clear. Our analysis highlighted a risk ratio of 0.77 in favor of early stages MF, which traduces in an estimated 23% increased chance to obtain a CR in the early stages compared to the advanced stages of MF with a single course of sd TSEBT.
Despite the limitedness of the available data, we can draw some conclusions. First, we confirmed that TSEBT is a safe technique in terms of acute toxicity profile. However, there is no available data on the long-term side effects, which we expect to be negligible, especially at lower doses. Moreover, reported side effects such as alopecia, skin atrophy, hyper- or hypo-pigmentation can be related to disease itself or to other treatments rather than being long-term side effects of TSEBT, making an accurate report extremely challenging. This meta-analysis confirms that TSEBT has the highest ORR among all available therapies for MF, both in early and advanced stages. Sd TSEBT is also associated with higher CRRs. It is questionable, though, if we should aim to obtain more CR at a cost of a higher toxicity and a lower chance for retreatments, given the usually indolent clinical course of this disease which is more similar to a chronic condition than to other types of lymphomas. Albeit might be reasonable to correlate CR with longer relapse free survivals, clear evidences are lacking. In ld TSEBT studies median RFS ranged between 3 and 7 months.17-19, 21, 22 In the three sd TSEBT studies with available data, it ranged between 6 and 12 months.14, 18, 24 Moreover, prolonged periods with no relapses or limited disease might not represent an adequate marker for improved overall survival. Since none of our available therapeutic options, except for allogenic stem cell transplant can be considered curative,1 future studies on TSEBT and other therapeutic options for MF should include patients' centered outcomes to help guide clinicians at the time of proposing the timing of treatments for patients both in early and advanced stage MF.
4.2 Limitations
The present meta-analysis has several limitations. First, the analysis does not adjust for potential confounders apart from early versus advanced MF stages. Significant differences may occur between early stages (for example patch only vs. predominantly plaque) and among advanced stages (tumor stage vs. erythroderma). Although there is some degree of standardization of the therapeutic schedule, heterogeneity in radiotherapy techniques may exist and might limit the validity of the results. Pooled analysis does not account for differences in patient compliance to therapy/modification of the protocol, as well as baseline differences in patient characteristics including age, sex, comorbidities, and duration of disease before diagnosis and number of previous treatment lines. Included studies were predominantly observational and retrospective in nature and thus susceptible to selection bias. Randomized clinical trials would be ideal to negate this bias, but we acknowledge the challenge of performing such studies in MF. Overall, the quality of evidence available was judged to vary from poor to moderate. Although we were able to include CR and OR rates, we could not include other important clinical outcomes, in particular Relapse Free Survival, Progression Free Survival, Duration of Response, Time to Next Treatment, due to lack of data from published studies.
4.3 Conclusions
This study provides an accurate analysis of the efficacy of ld and sd TSEBT in the management of MF, both in the early and advanced stages. Ld TSEBT is associated with lower CRRs but high ORR, while sd TSEBT is associated with high CRR (especially in early stage) and very high ORR. Almost every patient experience at least one G1-G2 Adverse event during or shortly after the treatment, and patients treated with sd-TSEBT experience multiple concurrent G1-G2 ae. SAE are rare in both ld and sd TSEBT. Stage of disease seems not to impact significantly the chances of obtain a CR, or to have a meaningful clinical response to ld TSEBT but patients with advanced stage MF might have a reduced chance of obtaining a CR compared to early stages when treated with sd TSEBT.
ACKNOWLEDGMENTS
All the authors were responsible for the concept and design of the study as well as interpretation of the data, writing of the article, reviewing, and final reviewing of this article. Vieri Grandi and Tommaso Grassi performed collection, collation, and analysis of the data. Vieri Grandi created all graphs.
Open Access Funding provided by Universita degli Studi di Firenze within the CRUI-CARE Agreement.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




