Continuous glucose monitoring in older adults with diabetes: Data from the diabetes prospective follow-up (DPV) registry
Prior presentation
Parts of this work were presented at the 57th annual conference of the German Diabetes Association (DDG), May 17th–May 20th 2023, Berlin.
Abstract
Aims
To analyse predictors for continuous glucose monitoring (CGM) use in people with diabetes aged ≥60 years using insulin therapy and to assess the rates of CGM use during recent years (2019–2021).
Research Design and Methods
Prospective study including 6849 individuals with diabetes and insulin therapy (type 2 diabetes: n = 5320; type 1 diabetes: n = 1529) aged ≥60 years. Data from 129 treatment centres were retrieved from the Diabetes Prospective Follow-up Registry (DPV) in March 2023.
Results
Sensor use in individuals aged ≥60 years has increased in type 1 (2019: 28%, 2020: 39%, 2021: 45%) and type 2 diabetes (2019: 10%, 2020: 16%, 2021: 18%). Predictors for sensor use in older individuals with type 1 diabetes are younger age and CSII use (p < 0.001). Predictors in older individuals with type 2 diabetes are younger age, longer diabetes duration, higher BMI and CSII use (p < 0.001).
Conclusions
CGM has become more common in older adults with diabetes and will presumably increase further. Age is a predictor for sensor use in older adults with diabetes. Age-related physical barriers and insufficient usability of devices, lack of interest in technologies, but possibly also effects of prejudice on the grounds of age may contribute to this finding.
What's new?
- CGM use in older adults has shown to improve glycaemic control and other outcomes in people with type 1 and type 2 diabetes.
- CGM use has increased in older adults aged ≥60 years. Age is a predictor for CGM use in older adults.
- Age-related physical barriers and insufficient usability of devices, lack of interest in technologies, but possibly also effects of prejudice on the grounds of age may contribute to this finding.
- There is a need for guidelines for delivering care in clinical practice and devices that are usable for the older population.
1 INTRODUCTION
The use of continuous glucose monitoring (CGM) systems can improve glycaemic control and other outcomes in people with type 1 diabetes1 and type 2 diabetes.2 A growing number of real-world studies support the use of CGM in type 1 and type 2 diabetes independent of the treatment regimen.3, 4 However, older adults aged ≥60 years are often underrepresented or not included in clinical studies. With increasing numbers of elderly individuals with diabetes there is a need for more data from national and regional sources on this population subset.5, 6
Older adults carry a high burden of diabetes: increased risk of hypoglycaemia and hypoglycaemia unawareness can have detrimental effects in this vulnerable population7, 8 pointing towards potential benefits of CGM use.9, 10 Reducing the risk of hypoglycaemia is a major treatment goal for older adults with diabetes.11 A small number of clinical studies specifically in older populations have pointed out that CGM may reduce hypoglycaemia in people with type 112, 13 and type 2 diabetes,14 and can lead to greater reductions in HbA1c compared with a non-CGM control group.15 A recent review on benefits and challenges of CGM in older adults concludes that while CGM use has shown positive effects on diabetes-related and patient-reported outcomes in several trials, its use in older adults is still largely based upon anecdotal and extrapolated data due to a small number of clinical trials including or focusing on this population.9
Determinants of sensor use in older adults have not yet been studied and despite the evidence suggesting improvements in glycaemic control and patient satisfaction, CGM use in older adults has been neglected in guidelines and recommendations in the past, particularly in type 2 diabetes.9 More recently, the attention on diabetes technology use in older adults has increased and recommendations on CGM use have been published by several professional diabetes organizations. The American Diabetes Association (ADA) just recently published guidelines that recommend CGM use for older adults with type 1 diabetes to detect and reduce hypoglycaemia, and for those with cognitive and/or physical impairments whose diabetes self-management is performed by a caregiver.16 The ADA does not specifically recommend CGM for older adults with type 2 diabetes and insulin therapy. The German Diabetes Association (DDG) does not yet provide recommendations for CGM use in older adults. In 2019, an expert panel on CGM use published guidance on target percentages for time in range to address the specific needs of diabetes populations such as older adults and to facilitate safe and effective therapeutic decision-making.17 Even though there is a lack of evidence for time in range in older individuals, numerous previous studies have emphasized the elevated risk for hypoglycaemia in this population. Thus, the expert panel recommends a lower time in range (70–180 mg/dL [3.9–10.0 mmol/L]) target of 50% of readings (compared with 70%) and a time below range (<70 mg/dL [<3.9 mmol/L]) of <1% of readings (compared with <4%) to reduce risk for hypoglycaemia.17
Even though the American Association of Clinical Endocrinology Clinical Practice Guidelines on the use of diabetes technologies suggests that CGM may improve quality of life and glycaemic control in older adults18 and rates of CGM use are generally growing,19-22 sensor use in the older population might still be initiated more cautiously compared with younger age groups.23, 24 Further, it remains unclear if the relatively low CGM uptake in older adulthood reported in the T1D Exchange Registry24 is comparable to CGM uptake elsewhere.
The aim of this study was to assess the use of continuous glucose monitoring (CGM) in people with type 1 diabetes and type 2 diabetes aged ≥60 years over the years 2019 to 2021 in Germany, and to describe predictors for CGM use in this population subgroup.
2 RESEARCH DESIGN AND METHODS
2.1 Data collection
Data were extracted from the multicentre DPV Registry. The DPV initiative prospectively collects data on diabetes care and outcomes in 488 specialized diabetes centres in Germany, Austria, Luxembourg, and Switzerland. DPV participating centres reflect all levels of specialized diabetes care. Data are pseudonymized and subsequently transmitted twice a year to Ulm University for central analyses and benchmarking. The electronic health record contains demographic data, information on diabetes type and onset, data on metabolic control and treatment regimen, and information on co-morbidities. The DPV initiative was approved by the University's Medical Faculty Ethics Committee and by the local review boards of the participating treatment centres and outpatient clinics (for more details see https://www.d-p-v.eu). Data from individuals with type 1 or type 2 diabetes on insulin therapy aged ≥60 years were retrieved from 129 DPV centres in March 2023 and comprised the treatment years 2019–2021. Data from older individuals with types 2 diabetes on other treatment regimens were retrieved but not included in the analyses due to the very low rates of CGM use (≤5%) in this population subset.
2.2 Measures
Demographic data included age, sex, and body mass index (BMI). Clinical data comprised diabetes duration, insulin dosage (I.E. × kg-1 bodyweight), glycated haemoglobin (HbA1c), mathematically standardized to the Diabetes Control and Complications Trial reference range (21–43 mmol/mol/4.05%–6.05%) using the multiple of the mean method,25 diabetic long-term complications, severe hypoglycaemia (defined as an event requiring the assistance of another person to actively administer carbohydrates, glucagon, or other resuscitative actions26), hospitalization, CSII use, and CGM use (both real-time and intermittently scanned CGM) (Table 1). Demographics are presented as means and standard deviations (SD) for continuous variables and as proportions (%) and standard deviations (SD) for dichotomous variables. Trends of CGM use are presented in percentage (%) and in absolute numbers (Figures 1, 2). Event rates for hypoglycaemic events and diabetic ketoacidosis were modelled in a negative binomial regression model and are reported as event per person/year. Time in range is the amount of time spent in a specific glucose range (in %) as measured by the sensor (low: <70 mg/dL; in range: 70–<180 mg/dL; high: ≥180 mg/dL).
| Typ-1 diabetes | Typ-2 diabetes | |||
|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | |
| Age (years) | 1529 | 69 (7) | 5320 | 74 (8) |
| Women (%) | 1529 | 48 | 5320 | 46 |
| Diabetes duration (years) | 1529 | 31 (17) | 5320 | 18 (10) |
| BMI (kg/m2) (%) | 1518 | 26.8 (4.9) | 5215 | 31.4 (6.4) |
| BMI ≤18 (kg/m2) (%) | 1529 | 0.9 (9.2) | 5215 | 0.3 (5.5) |
| HbA1c (%) | 1502 | 7.4 (1.0) | 5165 | 7.5 (1.4) |
| HbA1c (mmol/mol) | 1502 | 57.8 (12.5) | 5165 | 58.8 (8.1) |
| Insulin dose (IU/kg/day) | 1198 | 0.6 (0.4) | 4548 | 0.6 (0.4) |
| Insulin pump use (%) | 1529 | 25 (43) | 5320 | 1 (10) |
| Myocardial infarction (%) | 1529 | 8 (27) | 5320 | 9 (29) |
| Stroke (%) | 1529 | 7 (26) | 5320 | 9 (28) |
| Diabetic foot (%) | 1529 | 7 (26) | 5320 | 21 (40) |
| Microalbuminuria (%) | 1054 | 30 (46) | 3092 | 42 (49) |
| Retinopathy (%) | 933 | 25 (43) | 2729 | 10 (30) |
| Frailty (%) | 1529 | 0.1 (3.6) | 5320 | 0.1 (3.6) |
- Abbreviations: BMI, body mass index; IU, insulin units; SD, standard deviation.
- Note: Dichotomous variables presented as proportions (%); continuous variables presented as mean and standard deviation.
2.3 Statistical analyses
The percentages of people with type 1 or type 2 diabetes aged ≥60 years using CGM over the most recent treatment years (2019–2021) were retrieved based on age groups: 60–69, 70–79, 80–89 and ≥ 90 years, and types of therapy: CGM, CSII, and sensor-augmented pump therapy (SAP).
Separate logistic regression analyses were performed for type 1 and type 2 diabetes to test if CSII (yes/no), age group (60–69, 70–79, 80–89, and ≥ 90 years), sex (men/women), and diabetes duration (60–69, 70–79, 80–89, and ≥ 90 years) were significant predictors for CGM use. Additionally, odds ratios (OR) were calculated. The logistic regression models for comparisons between groups (age groups; insulin pump use) were adjusted for sex, diabetes duration and BMI. Due to the large number of subjects, a p-value <0.01 was considered significant. The SAS 9.4 statistical software package (build TS1M7, SAS Institute Inc., Cary, NC, USA) was used on a Windows Server 2019 mainframe for data analysis.
3 RESULTS
3.1 Participant characteristics
Data from 6849 individuals with diabetes (type 1 diabetes: n = 1529; type 2 diabetes: n = 5320) aged ≥60 years on insulin therapy using CGM were included. Clinical characteristics of the study population aggregated over the treatment years 2019–2021 are presented in Table 1. In type 1 diabetes, the estimated mean of severe hypoglycaemic event per person/year was 0.17 (95% CI, 0.14–0.21) and 0.05 (95% CI, 0.04–0.07) for hypoglycaemic event per person/year resulting in coma. Estimated mean of diabetic ketoacidosis was 0.01 (95% CI, <0.01–0.01) events per person/year. In type 2 diabetes, the estimated mean of severe hypoglycaemic event per person/year was 0.05 (95% CI, 0.04–0.06) and 0.02 (95% CI, 0.02–0.03) for hypoglycaemia resulting in coma. Estimated mean of diabetic ketoacidosis was 0.007 events per person/year. In individuals with type 1 diabetes, estimated mean sensor use was 196 days per person/year (95% CI, 179–215 days per person-year). In individuals with type 2 diabetes, estimated mean sensor use was 88 days per person-year (95% CI, 84–93 days per person-year).
3.2 Predictors of CGM use
3.2.1 Type 1 diabetes
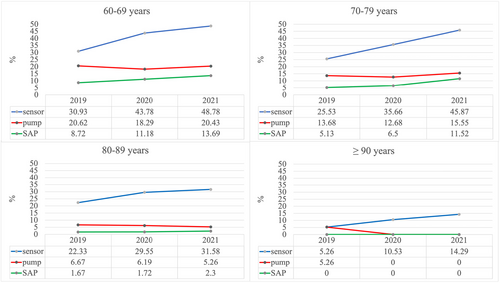
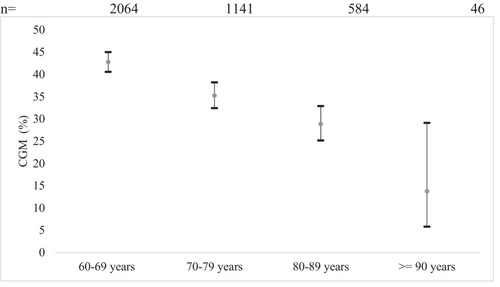
The results of the logistic regression model for sensor use in type 1 diabetes indicated that age (p < 0.001) and CSII use (p < 0.001) were significant predictors for sensor usage in older people with type 1 diabetes. Between age group comparisons are presented in Figure 3. All comparisons between the youngest age group (60–69 years) and the older age groups were significant (p < 0.001). All other age group comparisons were not significant. Regression models showed a 3.8 times higher OR (95% CI, 1.81–13.3) for CGM use in the youngest age group (60–69 years, n = 2064) and a 3.0 times higher OR (95% CI, 0.9–10.4) in the age group 70–79 years (n = 1141) (80–89 year, n = 584: OR, 2.4; 95% CI, 0.7–8.5) compared with the oldest age group ≥90 years (n = 46).
Comparison of sensor use in individuals using CSII vs. not using CSII (CSII: yes/no) was significant (p < 0.001). OR for CGM use was 0.4 (95% CI, 0.3–0.4) in people without CSII compared with people using CSII. Estimated means for CGM use in people with type 1 diabetes and CSII (n = 623) were 54% and 34% in people without CSII (n = 3212). There were no significant differences in sensor use with regard to diabetes duration, BMI or sex.
3.2.2 Type 2 diabetes
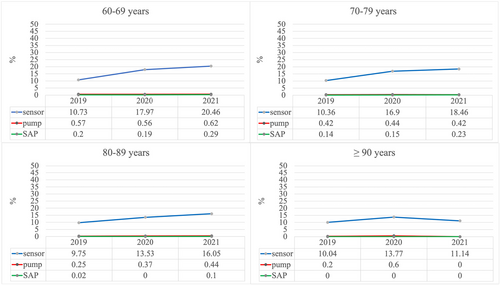
In people with type 2 diabetes, age group, diabetes duration, CSII use, and BMI were significant predictors for sensor use (p < 0.001).
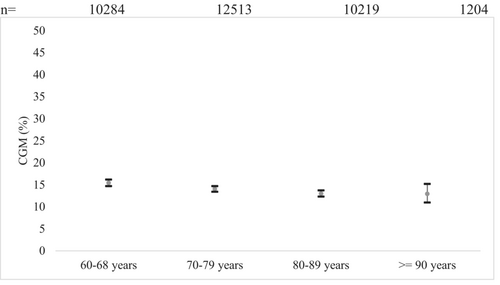
Between age group comparisons are presented in Figure 4. Comparison between age group 60–69 years and age groups 70–79 years (OR 1.1, 95% CI, 1.0–1.2) and 80–89 years (OR 1.2, 95% CI, 1.1–1.4) were significant.
Comparisons between diabetes duration groups were all significant. CGM use was associated with longer diabetes duration. OR for diabetes duration <10 years compared with 10–30 years was 0.8 (95% CI, 0.8–0.9) and 0.7 (95% CI, 0.6–0.8) compared with a diabetes duration of >30 years. Individuals with a diabetes duration of 10–30 years had a 0.8 lower OR (95% CI, 0.7–0.9) to use CGM compared with individuals with a diabetes duration of >30 years. Estimated means for CGM use were 12% in people with a diabetes duration <10 years, 14% in people with a diabetes duration of 10–30 years, and 17% for diabetes durations >30 years.
Comparison between insulin pump uses (yes/no) was significant (p < 0.001). OR for CGM use was 0.4 (95% CI, 0.2–0.5) in people without CSII compared to people with CSII. Estimated means for CGM use in people with type 2 diabetes insulin pump therapy (n = 163) were 32% and 14% in people without insulin pump therapy (n = 34,057).
Comparisons between the lowest BMI group (BMI < 25 kg/m2) and the higher BMI groups were significant (p < 0.001). CGM use was associated with higher BMI. OR for CGM use in individuals with BMI <25 kg/m2 (n = 5557) compared to BMI 25 < 30 kg/m2 (n = 10,205) was 0.8 (95% CI, 0.8–1.0) and 0.8 (95% CI, 0.7–0.9) compared with individuals with a BMI > 30 kg/m2 (n = 15,156). Comparisons between the higher BMI groups showed an OR of 0.9 (95% CI, 0.9–1.0) for individuals with a BMI 25 < 30 kg/m2 compared with a higher BMI.
3.3 Trends of CGM use in older adults 2019–2021
Sensor usage in older individuals with type 1 diabetes has increased over the years 2019–2021 (2019: n = 2466; 2020: n = 2122; 2021: n = 1869) through all age groups with higher increases in the younger age groups. The use of insulin pumps and sensor-augmented pump therapy has increased in the age groups 60–69 years and 70–79 years but did not considerably change in the population aged ≥80 years and remained rather low (Figure 1). Over all age groups, sensor use increased from 28% in 2019 to 45% in 2021 (2020: 39%). FGM (2019: 78%; 2020: 80%; 2021: 77%) was more common among sensor users than CGM (2019: 22%; 2020: 19%; 2021: 22%). Pump use changed from 17% in 2019 to 16% in 2021 (2020: 15%) and sensor-augmented pump use changed from 7% in 2019 to 11% in 2021 (2020: 8%). In a small number of cases, information on time in range was available (2019: n = 8; 2020: n = 54; 2021: n = 15). In 2019, the average proportion of time in range was 53% (low: 0.7%; high: 45%), in 2020, the average proportion of time in range was 71% (low: 1.2%; high: 26%) and in 2021, the respective number was 66% (low: 2%; high: 31%).
In older individuals with type 2 diabetes, the CGM use has increased through the years 2019–2021 (2019: n = 10%; 2020: n = 16%; 2021: n = 18%) in almost all age groups (Figure 2) but remains lower than 20% with an exception for the age group of 60–69 years in the year 2021 (20.6%). CSII and SAP were very rare (<2%) in this group (Figure 2). Individuals who used a sensor, predominantly used FGM (2019: 97%; 2020: 96%; 2021: 98%). Pump and sensor-augmented pump use between 2019 and 2021 was very rare in all age groups (<1%). In a small number of cases, information on time in range was available (2019: n = 13; 2020: n = 62; 2021: n = 74). In 2019, the average proportion of time in range was 60% (low: 0.3%; high: 39%), and in 2020, this increased to 66% (low: 1.1%; high: 31%) and in 2021, the average proportion of time in range was 71% (low: 0%; high: 28%).
4 DISCUSSION
Only few studies have yet been conducted on CGM use in older adults with diabetes. Scarce evidence has pointed out potential benefits of CGM use such as better glycaemic control and higher quality of life in this population subset, but reports have also discussed barriers for usage such as lack of rules for practical implementation and usability. This study aimed to analyse the predictors for CGM use in individuals with diabetes aged ≥60 years on insulin therapy and to assess the trend of CGM use over the most recent treatment years (2019–2021).
Mean HbA1c in our study population was comparable to data on older adults using CGM from the T1DX Registry.22 Our results show that sensor usage has increased over all age groups in the older population, but the percentage of CGM utilization decreased with increasing age. The same effect has been found in an analysis of the T1DX registry in the United States recently.24 However, the number of older people using CGM compared with younger cohorts are still much lower. According to data from the DPV and U.S. T1D Exchange registries, CGM use increased exponentially in all paediatric age groups (DPV: 2015: 4%; 2017: 44% and T1DX: 2013: 4%; 2015: 14%; 2017: 31%)22 and among young adults aged <25 years (DPV: 2017: 40%; 2020: 76% and T1DX: 2017: 25%; 2020: 49%).27 A recent publication by Auzanneau et al. (2023) using data from the DPV Registry showed that the use of diabetes technology decreased continuously and significantly with age (p for trend <0.001) in adults (analyses based on >13.000 individuals with type 1 diabetes aged 18–100 years).28 Contrary to that data from the T1D Exchange Registry showed lowest use of both CGM and CSII in individuals aged 18–25 years old compared with older participants.23 A possible explanation for these different findings might be the high cost and lack of reimbursement for diabetes technologies in the absence of health insurance in the US. In Germany, the Federal Joint Committee (Gemeinsamer Bundesausschuss, “GBA”) has made a reimbursement decision for CGM systems in June of 2016. Real-time CGM systems get reimbursed for persons with type 1 or type 2 diabetes who are treated with intensive insulin therapy, and in whom therapy goals are difficult to achieve. The reimbursement decision for real-time CGM in Germany did not include intermittent scanning CGM (iscCGM); however, some health insurers have since then started reimbursing iscCGM on a voluntary basis. Since then, CGM use has constantly and rapidly increased in Germany, however, lesser so in older adults compared with younger ones.
Lower CGM uptake in older adults might partly reflect barriers due to missing financial coverage of CGM in some countries.24 Other factors that might contribute to lower CGM use in older individuals could be age-related barriers such as lack of usability of devices for people with impaired fine motor skills, vision, or hearing. Diabetes technologies should be tailored to older adults' needs and motivations. CGM constantly provides information, which can be overwhelming and might lead to challenges in troubleshooting and decision-making, especially in older adults.29 Education on the use of diabetes technologies specifically targeting older age groups and/or their caregivers might be helpful to overcome some of the barriers mentioned and to enable more older adults to profit from potential positive effects of sensor use.
Further, recommendations for implementation of CGM in older adults remain largely undefined, which might negatively affect HCPs confidence to implement diabetes technology in this population.9 Individuals using CSII might be more prone to technology or might be treated in a diabetes centre that has more experience in utilizing diabetes technologies in older adults. Previous publications have emphasized on the importance of human factors engineering and research on actual everyday use of diabetes technologies in different groups of users (e.g. older people) and have given recommendations on these topics.30, 31
The decision to use technology needs careful evaluation and constant re-evaluation and discussion of benefits, barriers, and preferences between the HCPs, the older person with diabetes, and potentially their caregivers.32 It should not be made based on ageist beliefs that contribute to the digital health divide. Ageism refers to the stereotypes (how we think), prejudice (how we feel), and discrimination (how we act) towards others or oneself based on age.33 Older people are often stereotyped regarding their abilities to use and learn how to use technology, thus ageism is often referred to as a barrier for the adoption of technology in this population. If an older person prefers to use diabetes technologies, it is important to assess their abilities and offer education for them and their support system.32 The group of older adults is very heterogenous with regard to their preferences and abilities for technology use, but also regarding their therapeutic goals, thus the use of diabetes technology is not ‘one size fits all’.
More research on CGM use in older adults is needed to improve prescription and uptake.9 An important but still understudied topic is the human factor. While the number of studies showing positive effects of CGM on glycaemic control is growing, determinants of CGM use in older adults remain largely undefined (e.g. cognitive abilities, frailty). The CGM use has the potential to improve quality of life – a major treatment goal not only in older adults, but more studies on patient-reported outcomes such as quality of life and well-being in older individuals are needed. Barriers for adopting diabetes technology such as usability of devices and digital literacy must be studied and addressed. Furthermore, data on the cost-effectiveness of CGM use in older adults are needed.9
A major strength of this study is the large number of individuals included in the analyses. However, reported CGM use frequency in our study needs to be generalized with caution. The DPV registry is population based for the paediatric cohort, but the DPV registry is less representative in older age groups. This is a potential source of bias, with probably lower use of CGM in treatment centres not participating in the DPV registry.
5 CONCLUSION
The present study showed a significant increase in CGM use in older individuals with diabetes in the DPV registry, but concurrently indicated much lower CGM uptake with increasing age. Our results emphasize the need for guidelines for delivering care in clinical practice and devices that are usable for the older population.
AUTHOR CONTRIBUTIONS
J.G., S.S., T.K. and R.W.H. designed the study. J.G. wrote the first draft of the manuscript. R.W.H. and S.S. analysed the study data. RWH is the coordinator of the DPV initiative. All authors reviewed and edited the manuscript.
ACKNOWLEDGEMENTS
The authors wish to thank all participating centres of the DPV initiative, especially the centres contributing data to this investigation, and their patients. A list is available at d-p-v.eu. Special thanks go to A. Hungele and R. Ranz for the support and development of the DPV documentation software (Institute of Epidemiology and Medical Biometry, CAQM, University of Ulm). A first version of the statistical analysis was provided by Katharina Strehle, Ulm. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
The DPV initiative is supported through the German Center for Diabetes Research (DZD, grant number 82DZD14E03), the German Diabetes Association (DDG), the Robert-Koch-Institute (RKI Berlin) and EU IMI projects (DIRECT, INNODIA, EHDEN, SOPHIA).
CONFLICT OF INTEREST statement
J.G. has received consulting and speaker honoraria from Novo Nordisk Pharma GmbH, Lilly Germany and MSD Sharp & Dohme GmbH. L.B. received speaker honoraria from Medtronic GmbH. T.K. received consulting and speaker honoraria from Novo Nordisk Pharma GmbH and Dexcom Germany. L.P. received speaker honoraria from Ypsomed GmbH Germany. LH is a consultant for several companies that are developing novel diagnostic and therapeutic options for diabetes treatment. He is a shareholder of the Profil Institut für Stoffwechselforschung GmbH, Neuss, Germany. RWH received no personal honoraria from pharmaceutical or diabetes-technology companies.




