UK's Association of British Clinical Diabetologist's Diabetes Technology Network (ABCD-DTN): Best practice guide for hybrid closed-loop therapy
Abstract
This best practice guide is written with the aim of providing an overview of current hybrid closed-loop (HCL) systems in use within the United Kingdom's (UK) National Health Service (NHS) and to provide education and advice for their management on both an individual and clinical service level. The environment of diabetes technology, and particularly HCL systems, is rapidly evolving. The past decade has seen unprecedented advances in the development of HCL systems. These systems improve glycaemic outcomes and reduce the burden of treatment for people with type 1 diabetes (pwT1D). It is anticipated that access to these systems will increase in England as a result of updates in National Institute of Health and Care Excellence (NICE) guidance providing broader support for the use of real-time continuous glucose monitoring (CGM) for pwT1D. NICE is currently undertaking multiple-technology appraisal into HCL systems. Based on experience from centres involved in supporting advanced technologies as well as from the recent NHS England HCL pilot, this guide is intended to provide healthcare professionals with UK expert consensus on the best practice for initiation, optimisation and ongoing management of HCL therapy.
1 INTRODUCTION
Hybrid closed-loop (HCL) insulin therapy (sometimes referred to as an artificial pancreas) represents a form of automated insulin delivery (AID) system that consists of an insulin pump connected to a real-time continuous glucose monitor (rtCGM) (Figure 1). The rtCGM device measures interstitial glucose levels. An algorithm, which can increase or decrease the amount of insulin delivered based on the interstitial glucose level is housed in the insulin pump or in some cases an intermediary device such as a smart phone application. With current sensors, glucose readings are received by the algorithm at regular intervals and are used to constantly adjust insulin delivery, considering multiple factors (which may include current glucose levels, insulin on board and predicted glucose levels). All systems currently available have unique algorithms and use different user programmed factors in their decision making process, which are discussed later. Currently available systems are called ‘hybrid’ as they still require user input for tasks such as announcing carbohydrate intake and exercise to get optimal results.
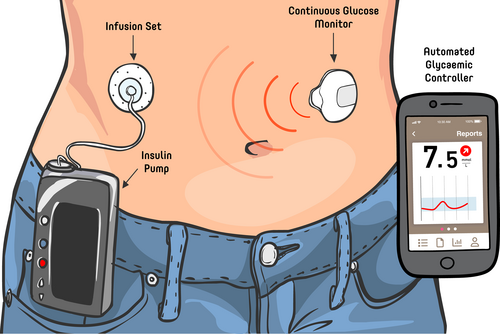
- Section 1: Supporting people living with type 1 diabetes (pwT1D) in understanding and choosing the right system for them.
- Section 2: Onboarding safely onto systems.
- Section 3: Important considerations during clinic review and optimising outcomes.
- Section 4: Structuring services to support HCL therapy users.
This guide will focus on commercial HCL systems available in the UK at the time of writing. Methods are detailed in Supplementary information. An overview of these systems has been covered elsewhere.1 This guide builds on previous work that has been undertaken in this area including the international consensus recommendations for the use of AID technologies in clinical practice.2 Many clinicians will also be aware of open-source or ‘do-it-yourself’ AID systems used by some pwT1D. Detailed guidance covering these systems is provided elsewhere.3
1.1 Why are HCL systems needed?
Type 1 diabetes mellitus (T1DM) is a lifelong condition associated with risks of multiple complications.4 Burden from treatment negatively impacts quality of life.5 In England, approximately one third of pwT1D successfully achieve the National Diabetes Audit target of HbA1c target of 58 mmol/mol.6 HCL systems have become increasingly available over recent years and have demonstrated improvements in glycaemic outcomes and reductions in diabetes distress in clinical studies and real-world use, including ABCD-DTN's audit of the NHS England closed-loop pilot.2, 3, 7-14
1.2 Indications for use
“It is the belief of DTN-UK that the majority of pwT1D will benefit from HCL therapy
AND
many people with other types of insulin requiring diabetes may also benefit.”
With the launch of the amended NICE T1DM guidelines,15 we hope many more people will be able to access HCL therapy. However, access to CSII and real-time CGM, as well as to teams with experience and skills in managing HCL therapy, may remain limiting factors. At the time of writing, NICE multiple-technology appraisal evaluating the cost-effectiveness of HCL therapy is undergoing consultation.16 Suggested indications for HCL are displayed in Table 1. HCL systems may not suitable for all people with diabetes17 (Table 2). HCL use will vary from country to country. Timing of HCL initiation after diagnosis should be considered. There is emerging evidence that starting CGM within the first year of diagnosis of T1DM may lead to sustained improved glycaemic outcomes compared with people who do not start CGM or who start CGM >3 years after diagnosis.18 Furthermore, the major impact of glucose control in the early years of diabetes on long-term outcomes is well established.19 Although evidence to support early initiation of HCL therapy is not yet available, extrapolating data from studies of early CGM initiation and early glycaemic control and long-term outcomes, HCL therapy may result in sustained improved glycaemic outcomes if initiated early. Further study in this area is warranted.
It is the belief of DTN-UK that the majority of pwT1D will benefit from HCL therapy. However, access to HCL therapy may vary depending on local funding pathways. We suggest that the following groups should be prioritised:
|
- a Not all devices are licensed in pregnancy. For people considering pregnancy, they should be offered advice regarding which pumps are licensed in pregnancy. For HCL systems not licensed in pregnancy, the option of continuing in closed loop or open loop should be evaluated at different stages of pregnancy. The option which can provide the individual with the best chance of successful diabetes management should be supported by the care team.
|
In addition to the above, there are a cohort of individuals with extremely elevated HbA1c levels who struggle with the daily management of diabetes that may have been considered unsafe in the past and in whom conventional pump therapy may have been contra-indicated due to the risk of diabetic ketoacidosis. As demonstrated in the NHS England Hybrid Closed-Loop (HCL) Pilot, these high-risk individuals may also stand to benefit from HCL if intensive input is available from the supporting diabetes team and risk mitigation strategies are employed.
2 SECTION 1—BASICS AND HCL SYSTEM SELECTION
2.1 Setting the right expectations
It is vital to set realistic expectations of the work required and the outcomes that can be achieved with HCL. Some pwT1D may have misconceptions that HCL is a ‘plug in and go’ system that does not require user involvement or have unrealistic outcomes with ‘flat lines’ on CGM. It is important to discuss the timing and delivery of mealtime insulin in order to reduce postprandial hyperglycaemia (in the case of delayed or omitted insulin) or postprandial hypoglycaemia (when the correct dose mealtime insulin is delivered late and the algorithm has already intervened to reduce the postprandial hyperglycaemia). It can take several weeks of HCL use to achieve optimal performance.
Basic knowledge of insulin pump and CGM systems is an essential component of HCL therapy. The user must be able to use the pump in manual mode. Clear guidance should be offered on when to come out of closed-loop mode, for example potentially during periods of illnesses or in the hours after an infusion set failure. Furthermore, additional training is required for HCL-specific topics (see special situations; Table 8). Taking a pre-meal bolus remains important for achieving optimal glycaemia. To get the best results from a HCL system, the user still needs to bolus at least 15 min before meals if using rapid acting insulins such as Novorapid or Humalog. However, even in those who are not able to do this, or who are advised not to do so due to concomitant conditions such as gastroparesis or hyperemesis gravidarum etc, HCL can still support improved glucose levels.20 In this first phase, user effort and commitment are necessary to take onboard new information, establish new routines and learn to trust the system. The timescale for this process is highly variable and depends on individual differences. In the second phase, users often report reduced burden along with improved glycaemic outcomes. Some users find it easier to break down goals and expectations into realistic targets to support their journey onto HCL. Documenting and periodically re-visiting these with the diabetes team can highlight achievements and progression as well as areas where a user needs further support to achieve therapeutic goals.
2.2 Choosing the right HCL system—what do HCPs need to know about the system?
As more HCL systems become available, there is an increasing need to understand how these systems compare and implications for their practical use. Clinicians and educators may not need to understand subtle technical and algorithm differences, but they will need to understand essential system characteristics for supporting users of HCLs. The CARES paradigm was published in 2019 as a framework for clinicians to understand HCL systems21 (Tables 3 and 4). The CARES paradigm as applied to systems available in the UK is summarised in Table 5. In Table S1, The CARES paradigm for HCL systems expected to become available in the UK soon is outlined. To help discussion with pwT1D or their careers, other factors that need consideration are listed in Table 6.22
| C—How does each system Calculates insulin delivery? |
| A—Which parameters can be Adjusted? |
| R—When users should Revert to traditional insulin pump settings. |
| E—Critical Education points |
| S—Key aspects of the Sensor and Sharing capabilities of the system. |
- Note: Reproduced with and adapted with permission from Laurel Messer.21
 C: Calculate C: Calculate |
How does the algorithm calculate insulin delivery? Which components of insulin delivery are automated (e.g. basal suspensions, basal modulation, high glucose corrections, food boluses, etc.)? |
 A: Adjust A: Adjust |
|
 R: Revert R: Revert |
|
 E: Educate E: Educate |
|
 S: Sensor/Share S: Sensor/Share |
|
- Note: Reproduced with and adapted with permission from Laurel Messer.21
| HCL system | SmartGuard | Tandem Control IQ | CamAPS FX |
|---|---|---|---|
| Pump | Medtronic MiniMed™780G | Tandem T:Slim |
Dana RS/ Dana I Compatible YpsoMed pump |
| CGM | Medtronic Guardian sensor 3 and 4 | Dexcom G6 | Dexcom G6/Libre 3 |
| License |
|
|
|
| Bolus route | Via pump | Via pump | Via pump or android app |
| Calculate | |||
| Parameters for automated basal insulin delivery |
|
|
|
| Target glucose |
|
Range:
|
|
| Logic for insulin adjustments (simplified) |
|
|
|
| Basal adjustments |
|
|
|
| Adjust | |||
| Adjustable parameters |
|
|
|
| Overrides | Temp Target (fixed at 8.3 mmol/L) |
|
|
| Revert | |||
| Revert to manual mode |
|
|
|
| Manual mode |
|
|
|
| Education | |||
| HCP education |
|
|
|
| User education |
|
|
|
| Share | |||
| User interface |
|
|
|
| Smartphone integration |
|
|
|
| Remote follower function |
|
|
|
| Data platform |
|
|
|
- a Expected to be available soon via Glooko.
| Accessibility/Availability/Evidence |
What systems are available? What systems can meet funding requirements? What systems are licensed for user's situation? What evidence is there from RCT and RWE on outcomes with this system? |
HCL features |
UKCA Mark indications (age, pregnancy considerations, minimum and maximum doses) User variable/settings Glucose targets Special modes and auto-mode suspensions Insulin compatibility (rapid/ultra-rapid insulin) |
| CSII features |
HCL algorithm compatible? Device size Device interface Ability to update software Ease of priming and cannula insertion Cannula options Insulin reservoir size Battery type (rechargeable/replaceable) Smartphone connectivity |
Other aspects |
App and share function Automatic cloud uploads Alarms |
| CGM features |
Sensor duration Sensor accuracy Sensor calibrations Ease of insertion |
We recommend the use of independent resources such as the ABCD DTN-UK website (https://abcd.care/dtn-education/expert-views-on-devices), that has a range of materials available to help support decision making by pwT1D or carers.23, 24 Supporting the user to choose a system that is best suited to their requirements is important for user satisfaction. Published data on outcomes and user satisfaction can provide valuable reassurance when choosing devices.25-27
2.3 HCL concepts for pwT1D
For open-loop insulin pump therapy, HCPs and pwT1D are familiar with the concepts of bolus (i.e. insulin that is typically delivered for meals or as a correction) and basal insulin (i.e. insulin that is constantly delivered in the background). In HCL systems, the concepts of user-initiated boluses and algorithm-modulated insulin are more appropriate.2 User-initiated boluses are instigated by the pwT1D in advance of meals or as correction doses. Algorithm-modulated insulin delivery is instigated by the HCL system in response to glucose level data. As a rough indication, about 40% of insulin is usually user-initiated bolus depending on the individual's carbohydrate intake.2 A lower proportion of user-initiated insulin delivery may indicate that boluses are being missed.2
2.4 Calculate
- How does the system determine insulin delivery? Some systems rely on body weight and/or previous total daily dose of insulin (TDD), while others rely on optimised pre-programmed basal rates. Some systems provide automated microboluses while other systems adjust the basal rate.
- What glucose target values or target ranges can be specified? These provide the algorithm with a specified value or range to aim for in its calculations of insulin doses.
- How does the system adjust insulin and offer corrections?
2.5 Adjust
To support users safely, HCPs need to have a full understanding of the settings that can be adjusted on a given HCL system and their impact. For each HCL system, only specific settings will affect insulin delivery while in closed loop. These will vary depending on the system and are summarised in Table 5. For example, glucose targets can be adjusted in CamAPS FX across a range of 4.4–11 mmol/L at different times; Medtronic MiniMed™ 780G has three target glucose options (5.5, 6.1 and 6.7 mmol/L, unless temporary targets (fixed setting of 8.3 mmol/L) are enabled); while the Tandem Control-IQ system offers a target range of 6.25–8.9 mmol/L (unless sleep or exercise activities are enabled). The aggressiveness of how the system reacts to raised glucose levels can be modified by changing the active insulin time on the Medtronic MiniMed™ 780G between 2 and 8 h, on the CamAPS FX system by using either the ‘boost’ or ‘ease-off’ function and on the Tandem Control-IQ system by weakening or strengthening the correction factor. Both the Medtronic and the Tandem systems have exercise activities that raise the glucose target or target range while CamAPS Fx uses the ‘ease-off’ function. Users can adjust programmed basal profiles which will alter AID in Control-IQ, but not in other systems. Insulin to carbohydrate ratio can be altered in all systems.
2.6 Revert
Certain situations, discussed below in Section 3: Important considerations and clinic review, may require the user to come out of closed loop and use their system in manual mode or revert to injections. There are also situations where the system may ‘kick out’ the user from closed-loop mode. Reasons for system-initiated exits from closed-loop mode will differ by device (Table 5). Once automated modes are turned off, ‘open-loop’ or ‘manual’ pump use will continue. Data from the CGM will continue to be available, provided issues with the sensor did not cause the closed-loop exit. In certain systems, such as Medtronic MiniMed™ 780G, predictive low glucose suspend that stops insulin delivery if hypoglycaemia is predicted, can still be utilised in manual mode if desired. At present, users of the Tandem Control-IQ HCL system or CamAPS cannot revert to a predictive low glucose suspend system in manual mode (important to note that Tandem Control-IQ does not revert to Tandem Basal-IQ).
2.7 Educate
Clinical experience has highlighted that user education regarding HCL systems is a key factor in determining success. All manufacturers provide system-specific educational resources, including online training platforms for users (Table 5). Education needs to ensure users understand the basics of how the system works, what settings can be adjusted and how, how to manage special situations including hypoglycaemia, exercise, sick days, alcohol use and suspected infusion site failure. Users must also be empowered with contact details for clinical and technical support and signposted to additional support (e.g. peer support forums).
2.8 Sensor/Share
Differences among sensors, such as calibration requirements, customisation of alerts and duration of wear, are important for the user experience and also maintaining full functionality of a closed-loop system (Table 5). All systems now offer the ability to share glucose data to cloud-based systems remotely and offer access to designated people (i.e. ‘followers’ who could be carers or relatives, as well as clinics). Some systems also allow insulin data to be shared automatically (Table 5). This has benefits in reducing the user requirement and burden to manually upload device data for HCP advice and appointments.
2.9 Peer support
Many users find shared experiences of other pwT1D helpful. Users' own experiences can give HCL candidates a realistic impression of what it is like to live with diabetes technology. Experience sharing can take place via one-to-one support/coaching, websites, social media, videos or peer support groups. DTN-UK strongly believes that every T1DM service should have a peer support group; successful examples include that from Derby where a Facebook group (now with over 780 members) was set up by a couple of enthusiastic service users with support from clinic leads. Groups like this drive good care, and keep the service accountable and up to date. Principles of good peer support for pwT1D are outlined elsewhere.28
3 SECTION 2—ONBOARDING
During the initial few weeks of HCL user onboarding, HCPs will need to provide intensive support in terms of education, training and ongoing evaluation. Our experience suggests that after this period, follow-up requirements tend to be reduced compared to pre-HCL care. Onboarding needs to be tailored to the individual and system requirements (e.g. face to face vs. remote, group vs. one-to-one). Careful consideration should be given to pwT1D who have had T1D for many years, those unfamiliar with technologies and those with a high HbA1c. Rapid improvements in glycaemic management, particularly in those with pre-existing retinopathy and longstanding history of HbA1c >86 mmol/mol, may potentially be associated with a transient worsening of retinopathy. In addition, in these individuals the potential risks of temporary neuropathic pain, insulin oedema or worsening albuminuria should be considered. For these people, we recommend starting with higher glucose targets and then gradually bringing them down over 3 months. This may involve a period of open-loop pump and CGM use and/or a higher target glucose level or range (e.g. staying in exercise activity or using temporary targets) may be appropriate. Depending on the status of pre-existing retinopathy, early follow-up may be recommended.
3.1 Remote starts
The DTN-UK has produced a standard operating procedure for starting insulin pump therapy remotely, with checklists and pathways.29 For remote starts involving industry partners, HCPs should carry out a pre-assessment of the pwT1D, complete consent forms, collect contact details and agree the predetermined pump and HCL settings for each individual attending the group. These should be provided to the industry representative who will be delivering the technical training. A technical checklist needs to be completed confirming that the HCP is happy for the pwT1D to proceed with onboarding of HCL (Table S2). Due to the complexity of the topics covered, users may wish to have family or friends present at initiation. This should be facilitated where possible to provide the individual with additional support.
It is important to confirm that the pwT1D feels comfortable to use the device following training. This allows any training issues or concerns to be raised which could be followed up outside of the group training. The HCP team should provide support on a regular basis (see Section 4: post-initiation follow-up) until confidence and therapy aims have been achieved. This model promotes equitable access to diabetes technologies while supporting and releasing the clinical team to provide more intensive face-to-face support for those requiring individualised support.
3.2 Moving from MDI to HCL
Existing service pathways can be used when moving from MDI to pump therapy. It is recommended that the user receives training for open-loop pump therapy and uses this until competence is achieved on that particular pump and CGM system prior to switching to HCL. The prospective HCL user needs to have these basic insulin pump and CGM skills, before entering closed loop. The person needs to be able to operate the insulin pump in open loop and understand the use of CGM technologies. Many centres suggest a few days in open loop before transitioning to HCL, although the decisions around the speed of transition and learning needs should be individualised. On average, we anticipate this may take 7–14 days.
3.3 Moving from CSII or open-loop to HCL
If users are transitioning from another pump system (pump renewal), it is recommended to use their current pump settings if glycaemic management is deemed reasonable and safe. We recommend using the DTN-UK guide to pump settings to re-calculate basal rates, insulin to carbohydrate ratios and insulin correction factors prior to starting HCL.30 Users who are new to CGM may have the option to go straight into closed loop with the sensor start, if the system being used can operate based on TDD/weight alone (some systems require a few days with sensor and pump before HCL can be initiated; Medtronic MiniMed™ 780G). Some users may switch to a different CGM system in order to use the HCL they have chosen and will need time to adapt. Therefore, a period of learning and experience on open loop if using a new pump device/CGM system is valuable.
3.4 System-specific considerations
3.4.1 SmartGuard/Medtronic MiniMed™ 780G
This system requires the user to use the pump alone for at least 48 h before the user can enter closed loop (called SmartGuard™). The SmartGuard target can be set at 5.5, 6.1 or 6.7 mmol/L based on the user's preference, with an active insulin time between 2 and 8 h. A shorter active insulin time makes the system more reactive while a longer active insulin time makes the system less reactive. This may be more suitable when there are concerns about rapidly lowering blood glucose levels. Higher targets of 6.1 or 6.7 mmol/L can be used for people with high baseline HbA1c, unstable retinopathy or fear of hypoglycaemia. Suggested settings are listed in Table 7. Basal rates should be set appropriately (based on about 40% of TDD) as these will be the default settings if the patient comes out of closed loop for any reason. In those with HbA1c >86 mmol/mol for a prolonged period at baseline, an exercise mode that has a target of 8.3 mmol/L (needs to be reactivated every 24 h) and turns off automatic microboluses can be used for a couple of weeks. This allows the pwT1D to become accustomed to lower glucose levels before reducing the blood glucose target on SmartGuard.
| HbA1c (mmol/mol) | SmartGuard Target (mmol/l) | AIT (h) |
|---|---|---|
| (a) | ||
| >86 | 6.7 | 3 |
| 69–86 | 6.1 | 2.5 |
| <69 | 5.5 | 2 |
| HbA1c (mmol/mol) | Target (mmol/L) |
|---|---|
| (b) | |
| >75 | 7.0 |
| 58–75 | 6.5 |
| <58 | 5.8 (default) |
3.5 Tandem control IQ
This system does not require a run-in period: the user can switch on closed-loop mode by providing an up to date weight (kilograms) and TDD (average of the previous 7 days). This system uses the programmed basal settings as a baseline and so for this system it is particularly important to optimise the settings in open loop before moving to closed loop. We recommend re-calculating the settings based on the DTN guidance. Close support is needed in the first few weeks to optimise settings. Exercise activity can be used for >2 week period for people with high baseline HbA1c, unstable retinopathy or fear of hypoglycaemia. Up to two sleep activity (glucose target range 6.25–6.7 mmoL/L) schedules can be pre-programmed to turn on and off at pre-specified times (e.g. one schedule for weekdays and one for weekends). In sleep activity the system will use a different target range but does not give automatic boluses.
3.6 CamAPS Fx
This system does not require a run-in period. The user can switch on the closed-loop mode by providing an up to date weight (kilogram) and TDD (average of the previous 7 days). The currently used pump settings are entered as back up but only the carbohydrate ratio is used by the bolus calculator in closed loop. The correction factor and bolus calculator target only impact if the user chooses to give additional manual corrections. For Dana pumps, the extended bolus feature must be switched on for the system to initiate closed-loop mode. The personal glucose target for this system can be set between 4.4 and 11.0 mmol/L, with a default glucose target of 5.8 mmol/L. Higher personalised glucose targets can be used for people with high baseline HbA1c, unstable retinopathy or fear of hypoglycaemia (Table 7A). The system has ‘ease-off’ and ‘boost’ modes which modify the responsiveness of the algorithm. This is the only system that is licensed for use in pregnancy. An important factor for a person to consider is the ability to manage insulin delivery using an app or an android phone.
Examples of suggested onboarding pathways for new and upgrading users, with suggested follow-up support per HCL system, are detailed in Figures S1–S6. Each HCL system is different so the importance of HCL system-specific training groups and follow-up are important. Individuals who are starting the same HCL system may be trained together in a group, but group starts are not appropriate if individuals are starting on different systems.
4 SECTION 3: IMPORTANT CONSIDERATIONS AND CLINIC REVIEW
4.1 Important considerations
Important considerations for using HCL systems are summarised in Table 8. Table 9 outlines when to consider discontinuation of closed loop. Three very important aspects based on our experiences are detailed further below.
| Safety strategies to avoid problematic hyperglycaemia and DKA | ||
|---|---|---|
| Timely infusion set changes and site rotation | This remains the corner stone for safe and successful insulin pump therapy. In addition to lumpy sites (lipohypertrophy), reduced insulin infusion by a cannula being kept in longer than the recommended can negatively affect insulin absorption, and in some cases lead to cannula blockages | |
| ‘If in doubt change it out!’ | With HCL, users should be advised that sustained glucose >15 mmol/L for >2 h should be considered as suspected cannula failure | |
| Basal rates for open loop | Often TDD requirements can change after commencing HCL. For all HCL users, manual settings must be adjusted at every subsequent review, ensuring these remain up to date in the event of a sensor failure or other reasons requiring the pwT1D to revert to open loop | |
| Sick day guidance | HCL users should be provided with sick day rules and advised on how to manage unexplained hyperglycaemia as part of their training and at every clinic visit (Figure S7) | |
| Back-up insulin pens | Users must carry, or have access to, an alternative means of rapid or ultra-rapid-acting insulin delivery (pen or syringe) at all times. They should also have access to long-acting insulin and know the dose to take in the event of a pump failure. This is particularly important if they are travelling away from home | |
| DKA avoidance toolkit | In addition to sick day guidance and back-up insulin pens, further aspects in the DKA avoidance toolkit should revised (Table S3) | |
| Hypoglycaemia treatment and avoidance | ||
| Hypoglycaemia prevention/treatment | Glucose level/trend arrow | Rapid acting carbohydrate (g) |
| 4–6 mmol/L ↓ | 4–5 | |
| 4–6 mmol/L ↓↓ | 8–10 | |
| <3.9 mmol/L | 8–10 | |
| <3.9 mmol/L AND meal bolus <2 h OR exercise OR alcohol | 15 | |
| If glucose level <3.9 mmoL/L wait 15 min before re-treating to avoid over-replacement leading to fluctuating blood glucose levels | ||
| Avoidance/Prevention | Consider higher targets/temporary targets or modes which may reduce algorithm insulin delivery (Temp Targets—SmartGuard, Ease off—CamAPS FX; exercise mode—Control IQ) activated 60–120 min before periods of activity where hypoglycaemia is more likely to occur even if not classically considered to be exercise (e.g. routine changes, travel to work, shopping, short walks) | |
| Being aware of effect of active insulin | Active insulin on board (e.g. from a prior bolus) may have a larger impact in situations where hypoglycaemia is more likely to occur (e.g. activity). In this situation carbohydrate intake may be needed to avoid hypoglycaemia | |
| Special situations | ||
| Ultra-fast acting insulin | The only system currently licensed for use with ultra-fast acting insulin is CAM APS FX. Real-world experience suggest that these insulins can be used in all currently available HCL systems.30, 31 Any off-license use would need to be discussed with the individual user. We would recommend close follow-up and review of settings following any switch to ultra-fast acting insulin; this would include review of insulin action time for Medtronic systems | |
| Surgery | There is limited data to support the use of HCL throughout surgery. We would defer to the pre-existing DTN-UK guidelines for CSII use in hospitalised patients.32 For those undergoing short procedures where pump therapy may be continued in this guideline, it may be appropriate to continue HCL if the user is likely to be able to manage this independently with the alternative option being a switch to ‘manual mode’ | |
| Radiological imaging |
Pump: The pump must be disconnected and infusion set removed for MRI imaging. The pump (and telephone if CamAPS Fx) and infusions sets should not taken into the MRI room. For CT scans, removal of the pump is recommended by the manufacturers prior to imaging—however, some authors suggest that covering it with a lead shield may be satisfactory. The pump does not need to be removed for plain x-rays unless its position interferes with imaging the relevant area28 CGM: There is currently no CGM that is approved for exposure to MRI, CT or plain X-rays. Studies suggest that exposure to X-ray or CT may have limited impact on the MARD. In line with the JBDS guidelines, we recommend that all CGMs should be removed during MRIs.33 We recommend that users resume in ‘manual mode’ post-scan or, if a replacement sensor is available, immediately resume HCL once this is inserted and activated. Please see the DTN-UK guidelines32 and JBDS guidelines for managing insulin pump therapy/CGM in hospitalised patients28, 33 |
|
| Hospital admission | For individuals admitted to hospital using HCL, we recommend referring to the DTN-UK insulin pump guidelines32 and recently published JBDS-IP guidelines.28, 33 Sensors should not routinely be used in lieu of capillary blood glucose monitoring in an inpatient environment. Where the DTN-UK insulin pump guideline would advise pump therapy can be continued safely, individuals may be supported to continue HCL with input from the diabetes team. We would advise against the use of HCL in individuals hospitalised for severe hypoglycaemia, diabetic ketoacidosis or hyperglycaemic hyperosmotic state until these issues have resolved | |
| Pre-pregnancy/Pregnancy | HCL systems are used in pregnancy and pre-pregnancy optimisation. At present only one system is licensed (CamAPS FX). Detailed guidance for the use of HCL in pregnancy is beyond the scope of this guideline and is covered elsewhere.34 It may be appropriate to continue a commercial HCL system that is unlicensed in pregnancy if benefits outweigh risks, especially in situations where underlying glycaemic levels on manual pump therapy are suboptimal | |
| Steroid use | Steroid use is complex with the problems predominantly relating to the marked changes in insulin requirements both on commencement and sudden cessation. Tailor management to the individual taking into account the dose of steroids, intended duration, any plan for prolonged steroid weaning and the HCL system in question. Short very high-dose steroid treatments: Consider manual mode for Medtronic MiniMed™ 780G and CamAPSFX systems and automated mode with modified basal program (depending on steroid dose) for Control IQ. CamAPS-FX boost function could be considered with modified guidance for corrective insulin dosing. Systems with inbuilt learning such as Medtronic MiniMed™ 780G and CamAPS-FX (without the boost function on) may struggle to adjust quickly to the changes in insulin requirements both at the start and end of steroid therapy. For individuals receiving long-term steroids or a planned weaning dose: Continuation of HCL is advised with close monitoring by the supporting diabetes team. The system may require initial adjustments (e.g. lower targets CamAPSFX or modified basal program for Control IQ) Stopping steroid: use of temporary targets for 24–48 hours with Medtronic Minimed 780G and CamAPSFX after the cessation of steroids maybe helpful to reduce the risk of hypoglycaemia. For Control IQ, revert to usual pre-steroid basal program | |
| Pump vacation | Ensure that each individual has guidance on switching to open-loop or MDI therapy | |
| Alarm fatigue | Work with the pwT1D to increase the proportion of alarms that are clinically actionable and reduce nuisance alarms which contribute to alarm fatigue | |
| Ghost or Phantom Carbs | Ghost or Phantom or Fake carbs which can be inputted to trick the HCL system can impact on the ability of the HCL system to respond correctly to a given situation resulting in decreased overall performance of the system and increased glucose variability | |
| Missed or delayed mealtime bolus | If a mealtime bolus is missed or delayed, we suggest giving half the bolus, 30–60 min after the meal has started. If a mealtime bolus is delayed by >60 min from the start of the meal, we recommend not giving the mealtime bolus but a user-initiated correction instead (if the blood glucose is within the recommended range no insulin delivery may be advised) | |
| Long-term discontinuation of HCL | |
|---|---|
| When to consider discontinuing automation on a long-term basis? |
|
4.1.1 Hypoglycaemia treatment
HCL systems are designed to suspend insulin delivery before glucose levels drop below the target glucose level or range, thus minimising the risk of hypoglycaemia. This usually avoids the need for user intervention. Sometimes, if there is too much bolus or active insulin, or if the pwT1D has undertaken exercise, suspension of insulin delivery alone is insufficient and the individual may experience hypoglycaemia unless they take additional carbohydrate in response to low glucose alerts. When treating or trying to avoid hypoglycaemia, smaller amounts of carbohydrate are generally needed due to the reduction or suspension of insulin delivery prior to the onset of hypoglycaemia.31 Half of a person's usual treatment is a good starting point (e.g. 4–8 g, such as one to two jelly babies). Standard hypoglycaemia treatment (15 g of carbohydrate) is likely to lead to rebound hyperglycaemia, in turn triggering additional algorithm driven insulin delivery and the potential for further hypoglycaemia.
4.1.2 Unexplained hyperglycaemia/avoidance of diabetic ketoacidosis
Hyperglycaemia will cause an increase in algorithm-modulated insulin delivery. If glucose levels are sustained above 15 mmoL/L for more than 2 h canula failure should be considered (Figure 2). High amounts of insulin on board in such situations may reduce further corrective insulin advice and delivery. Undetected cannula failure can lead to diabetic ketoacidosis within a matter of hours. Any episodes of unexplained hyperglycaemia should be managed by changing the infusion set, checking for ketones, considering a manual correction by pen or syringe and reverting to open loop for at least 4 h after the last pen injection (Figure S7).

4.1.3 Exercise
Management of exercise needs to be tailored according to the type of activity and the duration and intensity of the activity. It needs to consider an individual's prior experience, previous strategies and the HCL system being used. General guidance is summarised below (Tables 10 and 11). The main learning points for the users are to set the activity mode at least 1 or 2 h prior to exercise, understand that bolus insulin prior to exercise may need to be reduced and strongly advise against carbohydrate loading before exercise. An observed rise in glucose will cause an increase in algorithm driven insulin, increasing the risk of hypoglycaemia during the activity. We advocate a gradual, consistent intake of carbohydrate adjusted according to sensor glucose readings. Carbohydrate intake can be estimated at 0.5 g/kg body weight/h. This can be taken in small amounts every 15–20 min (e.g. 10 g every 20 min for person weighing 60 kg), with intake delayed if sensor glucose is rising.
| HCL System | Standard Algorithm Target | Activity Terminology | Activity target | Notes |
|---|---|---|---|---|
| SmartGuard | 5.5, 6.1 or 6.7 mmol/L | Temp target | 8.3 mmoL/L | Set for 0–24 h; will automatically deactivate when time period is up |
| Control-IQ (Tandem) | between 6.2 and 8.9 mmoL/L | Exercise activity | 7.8–8.9 mmoL/L |
Tap to start; must tap to deactivate. Consider the addition of an exercise basal programme |
| CamAPS Fx | 4.4–11.0 mmoL/L | Ease-off | Less aggressive algorithm. Personalised glucose target can also be adjusted | Set for 0–24 h; will automatically deactivate when time period is up (however, if personal glucose target used instead of ease off then this needs to be manually adjusted back to prior level) |
| Type of exercise | Before exercise | During exercise | After exercise | Overnight |
|---|---|---|---|---|
| Aerobic | Use temporary targets for 1–2 hs prior to exercise | Reduce basal rate using ‘exercise target’ or remove pump/suspend insulin delivery at start of exercise | Reduce basal rate with ‘exercise target’ 0–6 h after |
Consider ‘Exercise target’ overnight (up to 6 h) as necessary And/Or uncovered bedtime snack containing both CHO and protein |
| Aerobic and anaerobic | Reduce bolus amount by 0%–25% in 1–3 h prior (up to 75% if prolonged exercise is anticipated) |
Less than 5.0 mmol/L: 10–30 g CHO 5.0–6.9 mmol/L: 10 g CHO (aerobic) |
Reduce bolus 0%–25% at post-exercise meal | |
| Carbohydrates as needed | ||||
| Anaerobic | May not need insulin adjustments | May not need insulin adjustments | Cancel exercise target if used | |
- Note: Modified, adapted and reproduced with permission from Laurel Messer,38 based on.37, 39-41 Minimising the amount of exercise at times of peak bolus insulin can improve the functioning of the HCL at times of exercise. If disconnecting the pump during exercise, it is important to suspend insulin delivery so the HCL system does not account for insulin that was not in fact delivered to the pwT1D.
4.1.4 Co-morbidities
When commencing an individual on HCL therapy, initial education and follow-up plans should take co-morbidities such as gastroparesis, psychology, educational level, comfort with technology and peer/family support into account. The importance of a shared MDT with a common ethos cannot be over emphasised in developing individualised onboarding and follow-up strategies where a person has multiple co-morbidities. The diabetes MDT should ensure that there is appropriate ongoing support and individualised educational planning. In certain cases, in particular where there are concerns about cognitive impairment, it would be useful to have a carer present at training to support the individual on their HCL journey. Certain individuals commencing HCL therapy, may need bespoke individualised training outside of a group start.
4.2 Checklist for reviewing in clinic
A checklist for reviewing metrics in clinic is provided in Table S4. This may provide a useful step-by-step approach to review patients in clinic. Top tips for pwT1D are provided in Table S5.22
5 SECTION 4: SERVICE REQUIREMENTS
Essential and desirable requirements for an adult HCL service based on the consensus of the working group are outlined in Table 12.
| Workforce (staff) requirements | |
|
E |
|
E |
|
E |
|
E |
|
E |
|
|
|
E |
|
D |
|
E |
|
E |
|
|
|
E |
|
E |
|
E |
|
E |
|
E |
|
D |
|
E |
|
D |
|
E |
|
|
|
E |
|
D |
|
D |
|
E |
|
D |
|
E |
|
|
|
E |
|
E |
|
D |
|
D |
|
D |
|
|
|
D |
|
E |
- a Topics include exercise, carb-counting refreshers, blousing, HCL sick day rules.
5.1 Workforce requirements
As with to all T1DM services, the cornerstone of the HCL service is the multidisciplinary team (MDT) (Table 13).
| Multidisciplinary team |
| Administrator/Pathway co-ordinatora |
| Consultant diabetologist |
| Diabetes specialist nurseb |
| Diabetes specialist dietitianb |
| Diabetes technologistc |
| Diabetes psychologistd |
| Patient and public involvement |
- a A dedicated administrator is an essential component of the MDT to order and re-order devices, CGMs and consumables, in addition to co-ordinating pump starts, upgrades and appropriate clinic reviews. In the future, we envisage centralised administration for ordering pumps, CGMs and consumables for the NHS to optimise efficiency. We cannot overemphasise the importance of an administrator so that the DSN/dietitian is not the first people in line to complete the order process for diabetes technologies.
- b A diabetes educator role (trained in structured education and pump therapy with intensive insulin management skills) can be fulfilled by either a diabetes specialist nurse or dietician or both. The nurse and dietitian should ideally be trained so that both can function as diabetes educators and are competent to see pump patients independently.
- c A diabetes technologist is part of the wider type 1 diabetes MDT and is in a position to assist people with downloading their data and can provide education to people on cannula insertion, reservoir changes, etc.
- d Access to clinical psychology services with interest and experience of diabetes related issues, ideally integrated with the MDT.
5.2 Training and competence
The MDT should have formal certified training on core competencies of HCL therapy (insulin pump therapy, CGM and HCL therapy; e.g. Glooko Academy or company specific training). Each HCL system has a different user interface and adjustable parameters. The team should have the knowledge to support the individual to make the appropriate choice of system for them. Time should be allocated to upskill on core competencies and new pumps, HCL systems and CGMs as they become available. Competency frameworks for diabetes technology can be a useful tool (see Table S6).
While at least one consultant, DSN and dietitian in the wider team should be proficient in HCL therapy, the wider team (including trainees) should also have awareness of the different devices and systems. As a minimum they should be trained in data review and optimisation, the management of specific issues such as set failures, pump failures and connectivity issues. Furthermore, they should be able to sign post to other services as appropriate. In our opinion, HCL is becoming the standard of care for pwT1D; it is prudent for teams to start planning to ensure that ALL staff who manage T1DM are upskilled in anticipation of an expanding need.
It is important that the HCL service should not depend on individual team members—pathways and processes need to be robust enough to operate safely in effectively in the context of unplanned/planned staff absences. A team ethos and shared vision and consistent messaging within the MDT and wider service has been shown in studies to improve outcomes.32 Clinical services should consider out of service resources such as expertise available within clinical networks and from industry partners.
5.2.1 Organisation and capacity
Organisation
The HCL service should be integrated within the wider T1DM service. Clinic models are outlined in Table 14.
| Clinic types | Description | Pros | Cons |
|---|---|---|---|
| Physical | Face-to-face consultation in the traditional clinical environment |
Face to face Download data Can see one or more members of the MDT—matched to clinical expertise |
COVID-19 Wait time Inconvenient |
| Virtual | Telephone or video consultation | Efficient COVID-19 |
Access to technology Lack of face-to-face contact Challenging if person is unable to access data or is not technically proficient on pump Equity |
| Educator data review |
Virtual or face to face Solely focussed on HCL data Review behaviour, settings |
Offers users insights to improve experiences and glycaemic outcomes | Requires time to review detailed data and address settings and behaviours |
| Rapid Access Clinic | Clinic to offer intensive short-term support/reviews to target a specific issue | Intensive support for short period | Limited capacity |
| Data triage | Agreed virtual review of a person's data via their data upload platform at prespecified time points—capacity to transition/organise physical or virtual review as required (triage those who need urgent input) |
Allows regular review of a large number of persons identifying issues earlier, for example, CamAPS A light touch appointment |
No direct person contact |
| Group sessions (virtual or face to face depending on the skill deficit)1 |
Group clinic to address common skill deficits identified in the physical or virtual clinic Per system start (core mass n = 5) System-based continuous learning sessions—open invite, explore more advanced session System agnostic refreshers, for example, sick day rules→DTN website |
Efficient use of resources to address common problems |
People do not feel comfortable discussing issues in a group setting Not feasible in a timely fashion if a small number of people are using the HCL system |
Clinic setup and structure
The most appropriate structure and models utilised depends on the size of the diabetes technology service, the HCP skillset and other local circumstances.
Many services have a mix of dedicated consultant led multidisciplinary diabetes technology clinics with different specific clinic types, for example, transition/young adult clinic, new referrals, pre-conception etc. The rise in use of diabetes technologies means many services have separated diabetes technology users from routine mixed MDI/technology clinics. This can help ensure that staffing and skill mix are aligned to the clinic type ensuring users of technology will be seen by an HCP who is appropriately trained in the devices that the person is using. However, dividing resources should not come at the detriment of those pwT1D who are not comfortable using or who do not use technology—all people should have equal opportunity to access the resources/services that are available.
Clinic template list size and setup
- 45 minutes for new patient appointments
- 30 minutes for review patient appointments
- 20 minutes for educator data review appointments
- 5 minutes for data triage reviews
The precise list size and template is dependent on models used for the MDT clinic. Some models will require extra capacity for ad-hoc reviews by more than one MDT member to deliver the desired efficiencies. The clinic list template must take into account time needed for post-clinic MDT meetings. The service should try and use their resources appropriately to match the right person to the right consultation. The team must retain mechanisms to communicate updates on patients within and outside the clinic. Establishing data triage reviews (as a screening process), in addition to the traditional new and review patient appointments, will assist with identifying people who may require less input at a given point in time, freeing up staff time for people who may benefit from short-term intensive follow-up.
While it is desirable for services to support multiple HCL options, a diabetes service should only offer HCL systems which they feel their team are able to safely support, considering the experience and expertise within the team. As the number of devices with different operating systems increases, it may not be possible for each diabetes centre to offer access to all HCL compatible pumps and CGMs directly. A local hub and spoke model for centres that cannot offer the full suite of funded pumps and CGMs is advised. In addition, to providing equity of access for all people with diabetes attending the ‘spoke’, the hub could provide education and training for HCPs. We recognise that ongoing support from industry will be required in upskilling HCPs and in onboarding pwT1D in an efficient manner.
5.3 Pathway to HCL therapy
5.3.1 Choosing and Starting HCL (consensus minimum 1 h with time for questions)
Enough time should be allowed to support a person to choose the most appropriate system for them taking in to account the differences covered earlier. This can be done via virtual or face-to-face group demonstrations with the ability to provide 1:1 consultation if required. The DTN-UK HCL video is an excellent resource (Figure 324, 33). A selection of e-learning platforms, videos and pump simulators may be beneficial.
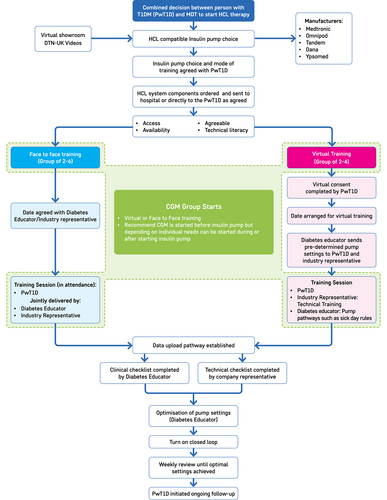
5.3.2 Onboarding process
The amount of time taken will depend on whether the pwT1D is staying on their current pump but initiating the previously unutilised closed-loop functionality, transitioning from one pump to a different pump which is HCL enabled, or completely new to pump therapy (Table 15).
| Context | Process | Time (h) |
|---|---|---|
|
HCL start—Pump Naïve 8 h |
Pump start | 4 |
| Daily calls/troubleshooting | ~2 | |
| First Pump set change | 1 | |
| HCL switch/training | 1 | |
|
HCL start—Pump upgrade 4 h |
Pump upgrade | 3 |
| HCL switch/training | 1 | |
|
HCL start—Existing pump 1 h |
HCL switch/training | 1 |
5.3.3 Post-initiation follow-up—(Consensus 30 min per week for 4–6 weeks)
When a pwT1D transitions to HCL therapy, early personalised intensive contact is required to assess and optimize use of the HCL, guide behaviours, troubleshoot and manage expectations. Our experience to date, suggests that this can be the difference between successfully transitioning to HCL therapy with an excellent impact on quality of life or transitioning to HCL therapy with a smaller improvement in TIR and/or adverse impact on quality of life—with some people discontinuing HCL therapy. The optimal frequency of contact will differ for each person. The rate of reduction in HbA1c (increase in TIR) may need to be more gradual (i.e. higher target glucose) in those with higher HbA1c prior to HCL commencement—not only to reduce the risk of complications of rapid reduction in HbA1c but also to allow the user to build trust in the system and become more accustomed to having lower blood glucose levels than was previously the case. We recommend that adequate staffing is available to liaise with a pwT1D every week until the pwT1d no longer needs input. Our experience suggests that this can vary between 2 and 16 weeks with an average of four contacts required. It is essential to have individualised educational and follow-up plans and to reinforce the importance of taking a pre-meal bolus at follow-up.
5.3.4 Ongoing follow-up
The regularity of follow-ups should be individualised and decided with the pwT1D in line with their long-term goals (consider using clinic prioritisation tools see Table S8). For those meeting their targets who do not have increased care needs, an annual follow-up may be sufficient and for those who need extra support more frequent follow-up and virtual support will be required.
5.3.5 Urgent and out of hours support
Each person should receive a copy of their clinic letter with a weblink/QR codes to urgent and out of hours support. All pwT1D should have easy access to ‘emergency pathway’ or ‘sick day guidance’ information. People on HCL therapy should be provided with contact details for the technical support services offered by the supplier(s) of their system.
A diabetes educator (DSN or dietitian) with knowledge of the HCL systems offered by a centre should be available within working hours to address any issues that a user may be having. Optimally, out-of-hours support would be available to reduce the risk of emergency hospital admissions. If out-of-hours support is not feasible for a single centre, joint commissioning with other local centres could enable this.
The inpatient diabetes teams should have access to these educators in the event that a person on HCL therapy presents to hospital. The JBDS have published guidelines for people admitted to hospital on diabetes technologies.34
5.3.6 Structural requirements
- Appropriate facilities for group starts for diabetes technology, ideally with computer and audio–visual facilities for remote starts.
- Adequate facilities for multidisciplinary meetings that allow viewing of downloads on a large screen.
- Dedicated quiet clinic space with a computer (fit for purpose IT system), a minimum of two screens (one to view pump data/one for clinic letters, laboratory results etc) and webcam/telephone access.
5.3.7 Informatics and data requirements
Data upload
Centres offering HCL therapy must have access to the necessary software programmes to facilitate remote data upload from the insulin pump/CGM and fit-for-purpose IT infrastructure. To ensure equity of access, centres must offer data upload appointments for those who cannot do so remotely. Personal accounts for the data software associated with the users' devices should be established and appropriate training provided. Each diabetes team member should have their own login due to GDPR requirements. Where possible users should be encouraged to synchronise their data automatically. Where this is not possible, users should be invited to upload their data in advance of each clinical contact.
Education and information on software updates for pumps, CGMs and downloading platforms must be included as part of the HCL training programme. This is important to ensure that safety and technical issues are minimised given the ongoing rapid development of technologies.
Database/diabetes module
A bespoke diabetes technology database or diabetes module within an Electronic Health Record is recommended to help structure the appointment and collect the relevant information for clinical use and for audit.
6 CONCLUSIONS
Diabetes technology in particular HCL therapy is evolving at a rapid rate. This guidance is based on current HCP experience and consensus from experts across the UK and aims to provide HCPs an overview of HCL systems in use within the NHS. It is imperative that HCPs are trained and skilled in HCL therapy to enable pwT1D to get the best outcomes. Clinical services need to access technologies and implement them with dynamic pathways designed to support this with recognition for service impact, and staffing and training needs. We anticipate that the template provided in this guidance will be applicable to most HCL systems emerging on the UK market in the next few years.
ACKNOWLEDGEMENTS
We acknowledge and thank Laurel Messer and colleagues for allowing authors to adapt previous work, the diabetes team at Kings College Hospital NHS Foundation Trust for materials provided, ABCD DTN committee for editorial support and Michael Bonar, Creative Director, Leicester Diabetes Centre for support with designing figures and overall layout.
FUNDING INFORMATION
SHu is a recipient of the Medical Research Council Clinical Academic Partnership award (MR/W030004/1). The authors received no financial support for the research, authorship and/or publication of this article other than support from Leicester Diabetes Centre for formatting of the figures with no input on the factual content.
CONFLICT OF INTEREST STATEMENT
TPG: Personal fees from: Novo Nordisk, Sanofi Aventis, Dexcom, Astra Zeneca and Lilly. GG: Personal fees from: advisory work and talks: Abbott, Dexcom, Medtronic, Roche, Insulet. SHa: Speaker & Advisory Board fees from: Dexcom, Medtronic, Sanofi, Ypsomed. Director: ASK Diabetes Ltd. Receives consulting/training fees from: CamDiab Ltd. TC: Personal fees from: Abbott, Insulet, NovoNordisk, Lilly, Sanofi. MH: Personal fees from: Roche, Abbott, Insulet. Educational grant funding from Dexcom. FWG: Personal fees from: Abbott, Insulet, Novo Nordisk, Lilly for talks and advisory work. AL: Personal fees from: Abbott, Dexcom, Insulet, Lilly, Novo Nordisk, Sanofi. Institutional research support from: Abbott, Novo Nordisk. EGW: Personal fees from: Abbott, Dexcom, Eli Lilly, Embecta, Glooko, Insulet, Medtronic, Novo Nordisk, Roche, Sanofi Aventis, Ypsomed. PC: Personal fees from: Medtronic, Dexcom, Abbott, Insulet, Novo Nordisk, Sanofi, Lilly, Glooko, Mannkind. Research support from: Novo Nordisk, Abbott and Medtronic. SHu: No conflict of interest to declare.




