‘YourTube’ the role of different diets in gastrostomy-fed children: Baseline findings from a prospective cohort study
Abstract
Aim
To assess the risks, benefits, and resource implications of home-blended food for children with gastrostomy tubes compared with a formula diet.
Method
This prospective cohort study of children (aged 0–18 years) collected baseline data on gastrointestinal symptoms, nutritional intake, anthropometric outcomes, parent and child quality of life, and resource use. A propensity score-weighted generalized linear mixed model was used to compare children receiving a home-blended versus formula diet.
Results
Baseline data were obtained for 180 children (2019–2021; 107 males, 73 females; mean age 9 years 7 months [SD 4 years 5 months]). Children receiving a home-blended diet (n = 104) had similar diagnoses and age but more lived in areas of lower deprivation and parental education was higher compared to the parents of children receiving a formula diet (n = 76). Children receiving home-blended diets had significantly better gastrointestinal scores than those receiving formula diets (B = 13.8, p < 0.001). The number of gut infections and tube blockages were similar between the two groups but with fewer stoma site infections in the group receiving home-blended food. Children receiving a home-blended diet had more fibre in their diet compared to children receiving a formula diet.
Interpretation
Home-blended diets should be seen as a safe option for children who are gastrostomy-fed unless clinically contraindicated. Equality of access to home-blended diets for children with gastrostomy should be assessed by local clinical teams.
What this paper adds
- Children with gastrostomy receiving a home-blended diet had fewer gastrointestinal symptoms compared to children receiving a formula diet.
- Children with gastrostomy receiving a home-blended diet had no more complications than children receiving a formula diet.
What this paper adds
- Children with gastrostomy receiving a home-blended diet had fewer gastrointestinal symptoms compared to children receiving a formula diet.
- Children with gastrostomy receiving a home-blended diet had no more complications than children receiving a formula diet.
Abbreviations
-
- DRV
-
- dietary reference value
-
- PedsQL
-
- Pediatric Quality of Life Inventory
In the 40 years since the first percutaneous gastrostomy placement in a child,1 increasing numbers of children living with complex health conditions are dependent on medical technologies to maintain their health, including gastrostomy feeding. For example, in 2019, there were 3143 children aged 14 to 19 years with a life-limiting condition and gastrostomy in England.2 Children requiring their nutrition via gastrostomy tubes have a wide range of underlying diagnoses, including neurodisability, inherited metabolic diseases, congenital cardiac conditions, cystic fibrosis, gastrointestinal diseases, and cancer. Limited research evidence3 and reports from clinicians suggest that the long-term use of gastrostomy feeds in children can result in complications, including progressive feed intolerance and gut failure.3 There are suggestions that a home-blended diet may reduce the risk of gut failure but there is currently no evidence to support this.
When this study was commissioned in the UK, the recommendation for children on enteral feeding was a commercially produced formula prescribed by the child's dietitian.4 However, there are more parents who are interested in, and choosing to feed, their children meals they have prepared themselves, which are then liquidized so they can be administered via gastrostomy (referred to as ‘home-blended foods’).5, 6
Surveys of paediatric dietitians in the UK5 and USA6 found that more than half of respondents would recommend the use of a home-blended diet (56% and 58% respectively) for those being fed via gastrostomy. In the UK, however, the recommendation was to use home-blended food as a supplement to formula feeds rather than its exclusive use. Both surveys highlighted the need for further evidence.
A recent systematic review identified seven primary research studies assessing the outcomes of children who are fed a home-blended diet via their gastrostomy. These were mostly small studies, with the largest sample size of 70 children; none of these studies were undertaken in the UK.7
During the period of conducting this study, many of the organizations who had previously raised concerns over the risks associated with feeding a child with gastrostomy a diet of home-blended foods, including the European Society for Paediatric Gastroenterology Hepatology and Nutrition and the British Dietetic Association, released new guidelines or position statements that are more supportive of the use of home-blended diets. However, even the most recent European Society for Paediatric Gastroenterology Hepatology and Nutrition position statement stated:
This study aimed to assess the risks, benefits, and resource implications for using home-blended food in children with gastrostomy tubes compared to currently recommended formula feeds. This article reports the baseline findings from this study.There is little evidence published to formally inform about the potential health benefits or risks of this practice and how to use it in the best way. This leaves health professionals caring for such patients in a relative vacuum regarding what to consider when providing a duty of care to patients and carers who wish to pursue this method of feeding. This article provides guidelines for safe and appropriate use of a home-blended diet, but more research is needed.8
METHOD
Study design
This prospective cohort study was carried out according to a published9 and registered protocol (ISRCTN13977361) and is reported according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines. Because of the impact of the COVID-19 pandemic, there was an enforced pause in recruitment from March to July 2020, a subsequent reduction in recruitment target numbers, and change to the data collection schedules.
Leeds West Research ethics committee and Health Research Authority approvals were obtained for this study (ref. 19/YH/0028).
Participants
Participants were children who received most or all of their nutrition via a gastrostomy tube (see Table 1 for the eligibility criteria).
| Eligible | Ineligible | |
|---|---|---|
| A | Child is at least 6 months old and under 19 years. | Infants up to 6 months and young people who are 19 years and older. |
| B | Child is gastrostomy feed-dependent. | Child has another type of feeding tube (e.g. nasogastric tube, jejunostomy). |
| C | Child receives most or all of nutrition via the gastrostomy. | |
| D | Child is living with parent(s), biological or adoptive. | Child is not living with a parent (e.g. in a residential setting or foster care). |
| E | Family is resident in England. | Family is not resident in England. |
Recruitment
Children and their parents were recruited via paediatric services at 30 NHS sites and one children's hospice from August 2019 to November 2021. Written consent was obtained from the parents and child (if appropriate), more information on the recruitment and consent processes are available in the protocol.9
Data collection
Data collection was informed by the qualitative component of this study.10 Data on clinical, feeding, and demographic outcomes were collected from parents and clinicians and, where possible, the child or young person. An Index of Multiple of Deprivation score was assigned based on the lower super-output area of residence, a small geographical area of approximately 1500 individuals.11
Data from parents were collected either online (using Qualtrics, Seattle, WA, USA) or on paper. Parents whose children received home-blended diets also provided dietary information via the online myfood24 (University of Leeds, Leeds, UK) tool,12, 13 with support from the research team if necessary. The myfood24 database includes data about tube feeding. Where appropriate, participating children and young people completed a short questionnaire to collect self-report quality of life. Up to three reminders via text or post were used at each time point.
Clinical information (e.g. diagnoses, medications, anthropometry) were collected from the child's paediatrician or dietitian. Dietitians also provided details about the formula feeds used by participating children (Table S1).
Link to routinely collected health care data (inpatient, outpatient, and emergency department) was undertaken by NHS Digital.14
Data quality and variable derivation
An assessment of data quality was undertaken; only questionnaires with at least one outcome measure completed were included in the analyses.
Primary outcome
As informed by the qualitative component of this study,10 gastrointestinal symptoms as measured by the Pediatric Quality of Life Inventory (PedsQL) Gastrointestinal Symptoms Scale was the primary outcome for this study. This scale has 58 items and 10 subdomains. Items are reverse-scored and linearly transformed to a 0 to 100 scale (0 = 100, 1 = 75, 2 = 50, 3 = 25, 4 = 0), with lower scores indicating more gastrointestinal symptoms. Scale scores were computed as the sum of the items divided by the number of items answered (this accounts for missing data). If more than 50% of the items in the scale were missing, the scale score was not computed.15
Secondary outcomes
Child quality of life
The short-form (12 items) self-report or proxy report from the parent or caregiver consisted of six domains (independence, physical, emotion, social exclusion, social inclusion, treatment). Dimension scores were calculated as means if at least one item for each dimension was available. The total score was calculated from the sum of the dimension scores when at least 10 items were available. Final scores were linearly transformed to a 0 to 100 scale with 100 indicating the highest quality of life.
Parental quality of life
Three measures were collected: (1) the EuroQol-5 Dimension Visual Analogue Scale; (2) the five-component scale of the five-level version of the EuroQol-5 Dimension;16 and (3) the 10-item Parenting Morale Index.17 The five-level version of the EuroQol-5 Dimension score was converted to a single score using a UK-specific value set.16 The Parenting Morale Index was calculated from the sum of the individual responses,17 taking into account the reverse scores,18 to give a score from 0 to 100.
Sleep disturbance
Both child (parent-reported) and parental sleep were reported using the Patient-Reported Outcomes Measurement Information System Sleep Disturbance short-form 4-item scale. Each question has five response options ranging in value from one to five. The total raw score was computed from the sum of the values of each response and then transformed into T-score rescales using item response theory-based T-score conversion tables.19 The T-score rescales the raw score into a standardized score with a mean of 50 and an SD of 10 (e.g. a person with a T-score of 40 is 1 SD below the mean).
Anthropometric information
Height and weight were collected at each data collection point from the parents and clinicians. The most up-to-date data were used and both data types were collected from the same source. The body mass index SD score, using the package childsds v0.8.0, was calculated and considered child age and sex and 1990 UK-specific reference values.20 The mid-arm upper circumference was also obtained from the clinicians or from the parents using an instruction video and standard tape measures.
Nutritional intake
For home-blended feeds, dietary intake (total calories, and macronutrient and micronutrient content) was reported by parents using myfood24,21 an online dietary analysis software. For formula feeds, information about the type and amount of formula was collected in the parent questionnaire. The commercial supplier nutritional information (obtained via dietitians) was used to calculate the dietary intake.
Analyses compared the calories (total kilocalories [kcal], kcal per kg, and percentage of energy from macronutrients considering the dietary reference values [DRVs]), macronutrients (protein, carbohydrate, fat, and Association of Official Analytical Chemists fibre), and micronutrients (vitamin B12, vitamin D, folate, calcium, iron, manganese, zinc). We calculated whether the child met the DRV (based on government dietary recommendations considering the child's age and sex22) by calculating a percentage of the nutritional intake over the DRV. We excluded incomplete data or input errors.
Resource use
The use of health and social care services (e.g. appointments with paediatric and dietetic teams; Accident & Emergency visits, medications), and the financial and time resource use for the previous 12 months were collected via parental report.23 Clinicians also provided the medications prescribed for the children. The unit costs of formula food and supplement were mainly obtained from the British National Formulary.24
Statistical analyses
All statistical analyses were undertaken using R (R Foundation for Statistical Computing, Vienna, Austria), two-sided tests, and an alpha of 5%. Descriptive statistics for all clinical, demographic, and outcome information used means and SDs for continuous data and counts and percentages for categorical data. When appropriate, group comparisons used Student's t-tests and χ2 tests. Box plots were used to represent data on the secondary outcomes. Summaries were provided overall and by the two groups of interest: those who were 100% formula-fed and those with any amount of home-blended feeds.
Propensity scores were used to balance the sample for the demographic baseline data25, 26 using the Index of Multiple Deprivation score and calculated using the package WeightIt v0.13.1. The propensity score weights were applied in a generalized linear mixed model using the PedsQL total score measured at baseline as the outcome, that is, group, age, sex, and diagnosis as fixed effects, and recruitment site as the random effect. Assumptions were checked using graphical and generalized linear mixed model inspection of Akaike information criterion values. Inferential analyses were not performed on secondary outcomes because of the large amount of outcome data collected and concerns over multiple testing.
Patient and public involvement
The parents of gastrostomy-fed children have been involved in this study via a study-specific parent advisory panel; they named the study, informed the selection of study outcomes and data collection tools, helped prepare and test the study documentation, including the data questionnaires, and advised on recruitment and retention strategies.
RESULTS
Two hundred and forty-two families consented to the study. Thirty families dropped out or were not contactable between consent and the baseline assessment mainly due to the COVID-19 pandemic. A total of 187 families started the baseline questionnaire but seven did not complete any outcome data; therefore, the final sample consisted of 180 children (104 receiving a home-blended diet, 76 receiving formula) (Figure S1).
Demographic and clinical characteristics of the cohort
Table 2 shows the clinical and demographic characteristics of the cohort, overall and according to the two groups of interest. The mean age of the cohort was 9 years 7 months (SD 4 years 5 months); there were more males (60.5%) than females and most were from a White British background (child ethnicity: 84.9%; parent ethnicity: 86.4%). No significant differences were observed between groups regarding age, sex, or ethnicity. The group receiving a home-blended diet had significantly more parents with higher education qualifications (65.7% vs 34.7%, χ2 [3] = 18.0, p < 0.001) and a higher frequency of families living in the least deprived areas (25.0% vs 9.2%) compared to the formula-fed group, but this difference was not significant (χ2 [4] = 8.8, p = 0.065).
| Receiving a home-blended diet (n = 104) | Formula-fed (n = 76) | Total (n = 180) | p a | |
|---|---|---|---|---|
| Age, years | ||||
| Mean (SD) | 9 years 2 months (4 years 5 months) | 10 years 2 months (4 years 5 months) | 9 years 7 months (4 years 5 months) | 0.132 |
| Sex | ||||
| Male | 64 (62.7%) | 43 (57.3%) | 107 (60.5%) | 0.467 |
| Missing | 2 | 1 | 3 | |
| Index of Multiple Deprivation | ||||
| 1 – most deprived | 15 (14.4%) | 17 (22.4%) | 32 (17.8%) | 0.065 |
| 2 | 18 (17.3%) | 18 (23.7%) | 36 (20.0%) | |
| 3 | 18 (17.3%) | 16 (21.1%) | 34 (18.9%) | |
| 4 | 27 (26.0%) | 18 (23.7%) | 45 (25.0%) | |
| 5 – least deprived | 26 (25.0%) | 7 (9.2%) | 33 (18.3%) | |
| Parental educational | ||||
| School leaving qualifications | 13 (12.7%) | 20 (26.7%) | 33 (18.6%) | <0.001 |
| Further education | 21 (20.6%) | 25 (33.3%) | 46 (26.0%) | |
| Higher education | 67 (65.7%) | 26 (34.7%) | 93 (52.5%) | |
| Other/no educational qualifications | 1 (1.0%) | 4 (5.3%) | 5 (2.8%) | |
| Missing | 2 | 1 | 3 | |
| Child ethnicity | ||||
| White British | 71 (88.8%) | 53 (80.3%) | 124 (84.9%) | 0.156 |
| Other | 9 (11.2%) | 13 (19.7%) | 22 (15.1%) | |
| Missing | 24 | 10 | 34 | |
| Child diagnostic group | ||||
| Neurological (e.g. cerebral palsy) | 43 (41.7%) | 25 (32.9%) | 68 (38.0%) | 0.650 |
| Genetic (e.g. DiGeorge syndrome, CHARGE syndrome) | 41 (39.8%) | 33 (43.4%) | 74 (41.3%) | |
| Congenital (e.g. teratology of Fallot, exomphalos) | 11 (10.7%) | 10 (13.2%) | 21 (11.7%) | |
| Other (e.g. brain tumour) | 8 (7.8%) | 8 (10.5%) | 16 (8.9%) | |
| Missing | 1 | 0 | 1 | |
| Gastrostomy type | ||||
| Button (MINI or Mic-Key) | 89 (86.4%) | 64 (84.2%) | 153 (85.5%) | 0.263 |
| Percutaneous endoscopic gastrostomy | 12 (11.7%) | 7 (9.2%) | 19 (10.6%) | |
| Other | 2 (1.9%) | 5 (6.6%) | 7 (3.9%) | |
| Missing | 1 | 0 | 1 | |
| Gastrostomy duration a | ||||
| Mean (SD) | 5 years 7 months (3 years 11 months) | 7 years 2 months (4 years 8 months) | 6 years 4 months (4 years 4 months) | 0.022 |
| Range | 0–15 years | 0–18 years | 0–18 years | |
| Missing | 5 | 1 | 6 | |
| Fundoplication b | ||||
| No | 72 (69.2%) | 41 (54.7%) | 113 (63.1%) | 0.046 |
| Yes | 32 (30.8%) | 34 (45.3%) | 66 (36.9%) | |
| Missing | 0 | 1 | 1 | |
- a p-values calculated using χ2 tests for categorical variables and Student's t-tests for numerical data. bClinicians' data were prioritized. Abbreviation: CHARGE, coloboma, heart defects, atresia choanae (also known as choanal atresia), growth restriction, genital abnormalities, and ear abnormalities.
There was no significant difference in diagnoses (χ2 [3] = 1.6, p = 0.650) and gastrostomy type (χ2 [2] = 2.7, p = 0.263). Genetic conditions (41.3%) were the most frequent diagnosis in both groups (38%), followed by neurological diagnoses. Most children had a button gastrostomy (MINI or Mic-Key = 85.5%). Fundoplication was significantly more common among formula-fed children compared to children receiving a home-blended diet (45.3% vs 30.8%, χ2 [1] = 4.0, p = 0.046) as well as a higher gastrostomy duration in years (7 years 2 months vs 5 years 7 months, t[143] = −2.32, p < 0.022).
Primary outcome
Table 3 shows group comparisons at baseline for the PedsQL Gastrointestinal Symptoms Scale. The group receiving a home-blended diet had significantly higher scores (fewer symptoms) on the PedsQL total score (t[156] = 5.62, p < 0.001) and for the subdimension scores of stomach pain (t[147] = 4.40, p < 0.001), food and drink limits (t[165] = 4.03, p < 0.001), wind and bloating (t[164] = 5.05, p < 0.001), constipation (t[152] = 5.68, p < 0.001), blood in bowel movement (t[90] = 3.16, p = 0.002), and diarrhoea (t[125] = 3.61, p < 0.001). There were no significant differences in the subdomain scores of stomach discomfort, trouble swallowing, heartburn and reflux, and nausea and vomiting.
| Receiving a home-blended diet (n = 104) | Formula-fed (n = 76) | Total (n = 180) | |
|---|---|---|---|
| PedsQL – gastrointestinal symptoms (high values = fewer symptoms) | |||
| Mean (SD) | 68.1 (15.5) | 55.0 (15.0) | 62.7 (16.6) |
| Missing | 3 | 4 | 7 |
| Stomach pain | |||
| Mean (SD) | 68.4 (21.7) | 52.8 (24.2) | 61.9 (24.0) |
| Missing | 2 | 2 | 4 |
| Stomach discomfort | |||
| Mean (SD) | 73.6 (23.1) | 70.3 (26.6) | 72.2 (24.6) |
| Missing | 6 | 8 | 14 |
| Food and drink limits | |||
| Mean (SD) | 49.4 (36.6) | 28.5 (30.7) | 40.5 (35.6) |
| Missing | 7 | 4 | 11 |
| Trouble swallowing | |||
| Mean (SD) | 44.0 (32.6) | 39.4 (32.2) | 42.1 (32.4) |
| Missing | 11 | 8 | 19 |
| Heartburn and reflux | |||
| Mean (SD) | 62.3 (26.0) | 63.4 (26.8) | 62.8 (26.3) |
| Missing | 4 | 3 | 7 |
| Nausea and vomiting | |||
| Mean (SD) | 64.4 (27.4) | 63.3 (26.6) | 63.9 (27.0) |
| Missing | 8 | 5 | 13 |
| Wind and bloating | |||
| Mean (SD) | 61.1 (23.8) | 43.3 (22.5) | 53.5 (24.8) |
| Missing | 4 | 1 | 5 |
| Constipation | |||
| Mean (SD) | 74.9 (21.4) | 56.1 (21.0) | 66.9 (23.1) |
| Missing | 8 | 5 | 13 |
| Blood in bowel movement | |||
| Mean (SD) | 97.5 (7.5) | 90.2 (18.9) | 94.4 (14.0) |
| Missing | 3 | 2 | 5 |
| Diarrhoea | |||
| Mean (SD) | 81.3 (22.4) | 66.4 (28.4) | 74.9 (26.1) |
| Missing | 11 | 7 | 18 |
- Abbreviation: PedsQL, Pediatric Quality of Life Inventory.
Table 4 shows that the symptom burden of retching and gagging (items that were added on the advice of parents but that were not part of the PedsQL) was lower in the group receiving a home-blended diet (t[134] = 2.43, p = 0.017).
| Receiving a home-blended diet (n = 104) | Formula-fed (n = 76) | Total (n = 180) | |
|---|---|---|---|
| Retching and gagging | |||
| Mean (SD) | 70.5 (27.8) | 58.8 (33.2) | 65.6 (30.6) |
| Missing | 6 | 5 | 11 |
In the generalized linear mixed model, children on a home-blended diet had significantly higher PedsQL gastrointestinal scores compared to those on a formula diet and therefore lower symptom burden (mean difference = 13.8, 95% confidence interval = 9.6–18.1, p < 0.001) (see Table S1).
Secondary outcomes
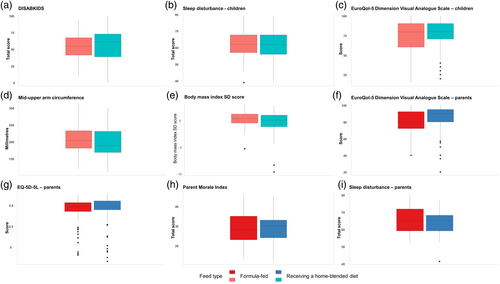
Figure 1 summarizes the secondary outcomes of interest. The sleep and quality of life of both children and their parents were similar between the two groups. The anthropometric measures showed similar body mass index spread of data, although there were some extreme values in both groups. The median mid-upper arm circumference was slightly lower in the group fed a home-blended diet, but the distribution in both groups was similar.
Nutritional intake
Nutritional intake could not be calculated for 24 (13.3%) children because of missing or extreme values (Table 5). Formula types are shown in Table S1. The nutritional intake was variable for both macronutrients and micronutrients. Comparing the two groups, children receiving a home-blended diet had a higher total protein (t[155] = 2.48, p = 0.014), fat (t[136] = 2.49, p = 0.014), and fibre (t[138] = 5.81, p < 0.001) intake. They also showed a higher kcal per body weight (t[147] = 3.99, p < 0.001), kcal (% DRV) (t[147] = 2.67, p = 0.008), and macronutrient intake per body weight (carbohydrate: t[144] = 3.07, p = 0.003; protein: t[121] = 5.41, p < 0.001; fat: t[143] = 4.13, p < 0.001). For micronutrient intake, mean intake was higher than 100% of the daily required intake for all micronutrients in the group receiving a formula diet and all apart from vitamin D in those receiving a home-blended diet.
| Receiving a home-blended diet (n = 104) | Formula-fed (n = 76) | Total (n = 180) | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Macronutrients | |||
| kcal per kg | 61.3 (33.3) | 44.0 (21.3) | 53.5 (29.7) |
| Missing | 18 | 6 | 24 |
| Total kcal | 1231.2 (573.2) | 1114.2 (443.9) | 1177.9 (520.0) |
| Percentage kcal from protein | 14.3 (4.6) | 12.8 (3.5) | 13.7 (4.2) |
| Percentage kcal from carbohydrate | 45.5 (11.0) | 47.9 (3.0) | 46.6 (8.5) |
| Percentage kcal from fat | 40.2 (12.2) | 38.0 (4.6) | 39.2 (9.6) |
| Missing | 18 | 6 | 24 |
| Total amount | |||
| Carbohydrate, g | 139.3 (72.6) | 134.8 (56.9) | 137.2 (65.7) |
| Protein, g | 44.0 (22.9) | 35.9 (18.9) | 40.3 (21.5) |
| Fat, g | 55.6 (31.0) | 46.3 (17.2) | 51.4 (26.0) |
| Fibre, g | 14.1 (10.7) | 6.3 (6.0) | 10.6 (9.6) |
| Missing | 18 | 5 | 23 |
| Grams per kg | |||
| Carbohydrate | 7.0 (4.3) | 5.3 (2.6) | 6.2 (3.7) |
| Protein | 2.2 (1.3) | 1.3 (0.6) | 1.8 (1.1) |
| Fat | 2.8 (1.7) | 1.9 (1.0) | 2.4 (1.5) |
| Missing | 18 | 6 | 24 |
| kcal (% DRV) | 76.5 (40.7) | 61.9 (27.1) | 69.9 (35.8) |
| Missing | 18 | 5 | 23 |
| Micronutrients (% DRV) | |||
| Vitamin B12b | 285 (223) | 253 (133) | 270 (187) |
| Folate (% DRI) | 155 (94) | 242 (107) | 193 (110) |
| Vitamin D (% DRI) | 55 (51) | 120.4 (56.4) | 85 (63) |
| Calcium (% DRI) | 126 (71) | 144.6 (100.7) | 134 (86) |
| Iron (% DRI) | 120 (73) | 136 (56) | 127 (66) |
| Manganese (% DRI) | 136 (108) | 120 (90) | 129 (100) |
| Zinc (% DRI) | 124 (79) | 212 (147) | 164 (123) |
| Missing | 18 | 5 | 23 |
- a Supplements not included. bMissing (n = 26). Additional missing because of inconsistent data (e.g. extreme values). Abbreviations: DRI, diet reference intake; DRV, diet reference value.
Safety outcomes
Table 6 summarizes the main safety outcomes of interest. Most of these outcomes were similar between the two groups apart from stoma site infections, which were fewer in those receiving home-blended diets (χ2 [1] = 14.0, p < 0.001). Although more children with a home-blended diet reported tube blockages, similar numbers of gastrostomy tube changes were required in both groups.
| Receiving a home-blended diet (n = 104) | Formula-only (n = 76) | Total (n = 180) | |
|---|---|---|---|
| Number of occurrences in the last 12 months (n [% of yes]) | |||
| Gut-intestinal infection | 9 (8.7%) | 13 (17.6%) | 22 (12.4%) |
| Missing | 1 | 2 | 3 |
| Stoma site infection | 6 (5.8%) | 19 (25.7%) | 25 (14.1%) |
| Missing | 1 | 2 | 3 |
| Tube blockage | 26 (25.2%) | 14 (18.4%) | 40 (22.3%) |
| Missing | 1 | 0 | 1 |
| Gastrostomy tube needed replacing | 76 (74.5%) | 55 (74.3%) | 131 (74.4%) |
| Missing | 2 | 2 | 4 |
| Pneumonia | 13 (12.6%) | 6 (7.9%) | 19 (10.6%) |
| Missing | 1 | 0 | 1 |
| Attended Accident & Emergency | 44 (43.6%) | 29 (38.2%) | 73 (41.2%) |
| Missing | 3 | 0 | 3 |
Resource use
There were no substantial differences in the use of health and social care resources between the two groups (Table 7). The mean cost of formula food was higher in the group fed a formula diet than the group fed a home-blended diet (GBP 17.5 vs GBP 5.8 per day). As a trade-off, families in the group receiving a home-blended diet spent an estimated GBP 294.0 in the previous 12 months on purchasing special equipment for blending and storing food at home. Parents of children receiving a home-blended diet also devoted more time to childcare than children fed a formula diet (201.7 vs 126.7 minutes per day respectively)
| Receiving a home-blended diet | Formula-fed | ||||
|---|---|---|---|---|---|
| Resource used | Unit | n | Mean (SD) | n | Mean (SD) |
| Health and social carea | n per year | 67 | 24.9 (24.1) | 54 | 20.4 (22.3) |
| Hospital stay | n per year | 100 | 5.0 (10.2) | 75 | 5.8 (12.4) |
| Accident & Emergency visit | n per year | 101 | 1.1 (1.9) | 76 | 1.4 (3.1) |
| Complicationsb | n per year | 99 | 3.4 (2.6) | 69 | 4.7 (5.4) |
| Complications requiring antibiotics or hospital care | n per year | 85 | 0.5 (1.1) | 61 | 1.7 (3.7) |
| Medications by parents | n per day | 96 | 2.9 (2.9) | 69 | 3.1 (2.8) |
| Medications by clinicians | n per day | 96 | 2.6 (2.4) | 75 | 2.4 (1.9) |
| Cost of formula food | GBP per day | 104 | 5.8 (9.8) | 76 | 17.5 (13.1) |
| Cost of blended food | We were unable to obtain the cost of blended food from the food dairy; a UK published source29 reported that a family of two adults and two children spent on average an expenditure of GBP 2.2 per day for an additional child. | ||||
| Cost of supplement | GBP per day | 104 | 0.5 (3.3) | 76 | 0.3 (1.7) |
| Cost of equipment | GBP per year | 63 | 294.0 (374.4) | 76 | Not collected |
| Time taken | Minutes per day | 96 | 201.7 (136.8) | 65 | 126.7 (173.6) |
- a Includes general practitioner, paediatrician, speech and language therapist, physiotherapist, community children nurse team, and dietitian visits. bIncludes gut intestinal infection, stoma site infection, tube blockage, gastrostomy tube replacement, and pneumonia. Abbreviation: GBP, pound sterling.
DISCUSSION
To date, this is the largest cohort study of children with gastrostomy living in England and their families. The analysis of the data collected at baseline showed that children who were fed a diet that included home-blended feeds had a lower burden of gastrointestinal symptoms, similar quality of life and sleep, and no indication of an increase in infections or requirement for more gastrostomy tube changes, compared to children who were fed formula only. Their nutritional intake was higher in terms of kcal per kg and fibre than children who were exclusively formula, but lower in vitamin D.
Lower burden of gastrointestinal symptoms in children who received a home-blended diet was reported in two smaller studies using the PedsQL Gastrointestinal Symptoms Scale from Australia27 and the USA;28 the comparable results offered in this larger study provide further evidence that a home-blended diet can be recommended as an option for children with gastrostomy, particularly if gastrointestinal symptoms are a key concern. Parents in our qualitative study also reported improved gastrointestinal symptoms with a home-blended diet, and described other consequences they associated with better gastrointestinal symptom control, such as improved well-being and participation.10 Quality of life was similar in children and parents in both groups in this study, which is interesting given the difference in gastrointestinal symptoms between the two groups. This may reflect the absence of relevant and validated tools to measure quality of life in children with neurodisabilities.29 Sociodemographic differences between the families of children who were using home-blended feeds and those who were only using formula suggest that parents with higher levels of education and lower levels of deprivation are more likely to feed their child with gastrostomy a home-blended diet. Such potential inequalities in access require further exploration; the analysis of the 12-month and 18-month data may demonstrate whether these differences are maintained, but factors such as the price of the blender (approximately GBP 500) may be a barrier for some families.
The risks of feeding a child a home-blended diet raised by professional organizations include nutritional inadequacy, microbial contamination, and blockage of the gastrostomy tube.30 The findings from this study show that there is no evidence of nutritional inadequacy, apart from vitamin D. Vitamin D deficiency is widespread in the general child population in the UK and can be addressed by appropriate supplementation.31 While obtaining accurate anthropometric measures and their interpretation in this group of children can be challenging,32 we found no difference between the groups who were formula-fed or received a home-blended diet in standardized body mass index score or mid-upper arm circumference. The finding of the current study that children who received home-blended feeds had a higher fibre intake than children receiving a formula diet was also found by Hron et al.28
There was no evidence of the sequelae of microbial contamination as there was no increased risk of gut infections and overall fewer stoma site infections among children receiving home-blended food. Although more parents of children with a home-blended diet reported tube blockages, this did not result in more gastrostomy tube changes in this group. Overall, this study did not find any difference in safety outcomes for the group receiving a home-blended diet.
Strengths and limitations
This is the largest study of outcomes for children who are fed a home-blended diet via a gastrostomy tube and the first prospective study to assess safety outcomes. Our outcomes of interest were informed by a primary qualitative study to ensure that we were collecting data of importance to children, parents, and health care professionals; we had extensive patient and public involvement throughout this study. The COVID-19 pandemic required us to maximize the use of parental report for data collection, for example, mid-upper arm circumference.
We are presenting baseline data; because this was an observational study, issues of study design, such as unmeasured confounding, may still be an issue. The children in this cohort had been on their home-blended diets for different periods of time. There is a lack of appropriate tools to measure outcomes, for example, to measure quality of life in children with neurodisabilities.29 Absence of good reference data for nutritional and anthropometric data for children with disabilities further hinders the interpretation of nutritional adequacy.
Conclusions
Children who were fed home-blended diets had a lower burden of gastrointestinal symptoms and no increased risk of gastrointestinal or stoma site infections or requirement for more changes of gastrostomy tube than those who were formula fed. These baseline findings show that home-blended diets for children who are gastrostomy-fed should be seen as a safe alternative to formula feeding for some children; equality of access to home-blended diets for children with gastrostomy should be assessed by local clinical teams.
ACKNOWLEDGEMENTS
This study was funded by the National Institute for Health Research Health Technology Assessment programme (ref. 17/76/06). This was a commissioned call; the funder identified the topic area for this study but had no role in the study conduct, data analyses, or conclusions of this study.
We thank all the children and parents who participated in this study and the health care professionals who recruited to this study. We also thank the members of our parent advisory board and the clinical and academic experts on our study steering committee.
J. Cade is the Director of Dietary Assessment Ltd, which supports myfood24. The other authors have no conflicts of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
Data may be available on request due to privacy/ethical restrictions. The data that support the findings of this study may be available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




