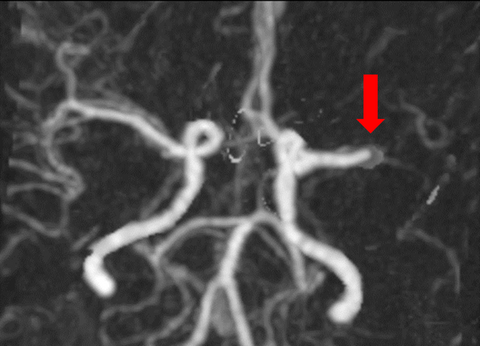
Urgent computed tomography angiography in paediatric stroke
Abstract
Aim
To improve delivery of acute therapies for acute ischaemic stroke (AIS).
Method
We identified factors influencing the speed of diagnosis and delivery of acute therapies in a prospective cohort of 21 children with suspected AIS (eight with AIS, 13 stroke mimics) and explored them in a retrospective cohort with confirmed AIS.
Results
Approximately half of the prospective and total AIS cohorts presented with acute, sustained hemiparesis, and were diagnosed relatively quickly. AIS was suspected and diagnosed more slowly in the half presenting with symptoms other than sustained hemiparesis. Thirty-one out of 51 patients with AIS (19 females, 32 males, mean age 8 years 6 months, SD 5 years 4 months) had arterial abnormalities identified by computed tomography angiography (CTA) or magnetic resonance angiography (MRA): 11 with large vessel occlusion, six with dissection, five with moyamoya disease, nine with other arteriopathies. Among these patients, those initially imaged with CTA were diagnosed more quickly than those with initial magnetic resonance imaging/angiography, which facilitated thrombectomy and thrombolytic therapy. Twenty out of 51 had AIS without arterial abnormalities on CTA or MRA: eight with lenticulostriate vasculopathy and 12 with other small-vessel AIS. Among these patients, 80% were ineligible for thrombolysis for reasons beyond delay to diagnosis, and all showed good outcomes with supportive treatments alone.
Interpretation
Clinical features at presentation influence rapidity with which childhood AIS is suspected and diagnosed. Readily available CTA can direct thrombectomy in patients with large vessel occlusion and thrombolysis in most, but not all, eligible patients.
What this paper adds
- Children with acute ischaemic stroke (AIS) commonly present with symptoms other than sustained hemiparesis.
- Stroke is more slowly recognized in these patients, which limits potential therapies.
- Computed tomography angiography (CTA) accurately identifies AIS with large vessel occlusion, enabling timely endovascular thrombectomy.
- CTA is sufficient to direct thrombolytic therapy in most eligible children.
- Most childhood AIS without arterial abnormalities identified by CTA had good outcomes.
What this paper adds
- Children with acute ischaemic stroke (AIS) commonly present with symptoms other than sustained hemiparesis.
- Stroke is more slowly recognized in these patients, which limits potential therapies.
- Computed tomography angiography (CTA) accurately identifies AIS with large vessel occlusion, enabling timely endovascular thrombectomy.
- CTA is sufficient to direct thrombolytic therapy in most eligible children.
- Most childhood AIS without arterial abnormalities identified by CTA had good outcomes.
Clinical features at presentation influence rapidity with which childhood stroke is suspected and diagnosed. Children presenting with dominant symptoms other than hemiparesis are diagnosed slowly, which reduces treatment options. Readily available, urgent computed tomography angiography provides sufficient data to facilitate thrombectomy in children with large vessel occlusion and to direct thrombolysis for most, but not all, eligible patients.
This original article is commented on by Tatishvili on pages 14–15 of this issue.
Abbreviations
-
- AIS
-
- Acute ischaemic stroke
-
- CTA
-
- Computed tomography angiography
-
- DSA
-
- Digital subtraction angiography
-
- EVT
-
- Endovascular thrombectomy
-
- LVO
-
- Large vessel occlusion
-
- MRA
-
- Magnetic resonance angiography
-
- NCCT
-
- Non-contrast computed tomography of brain
-
- PedNIHSS
-
- Pediatric NIH Stroke Scale
-
- SCH
-
- Sydney Children's Hospital
-
- tPA
-
- Tissue plasminogen activator
Acute therapy with intravenous tissue plasminogen activator (tPA) and endovascular thrombectomy (EVT) improves outcome in adults with acute ischaemic stroke (AIS). Application of these time-limited therapies to children with AIS, however, is often constrained by delays to diagnosis related to the low incidence of paediatric stroke, high incidence of stroke mimics, low levels of suspicion for AIS among clinicians, the multiplicity of uncommon pathogeneses, low diagnostic utility of non-contrast computed tomography of brain (NCCT), and difficulties procuring rapid magnetic resonance imaging/angiography (MRI/MRA).1 There are no controlled trials of acute therapy for paediatric stroke, but observational studies suggest improved outcome with EVT for AIS due to large vessel occlusion (LVO).2, 3
On the basis of the 2017 Australian Guidelines for the Diagnosis and Management of Acute Childhood Stroke1 and discussions with referring clinicians, we developed an acute stroke pathway, highlighting urgent, local neuroimaging (Figure S1). We aimed to rapidly identify patients who could benefit from EVT and tPA. We surveyed this strategy in a prospective study of children with suspected AIS. Mode of presentation and initial neuroimaging influenced rapidity of diagnosis and delivery of acute therapy in this small cohort. We retrospectively explored these concepts in children with AIS managed at Sydney Children's Hospital (SCH) since 2010.
METHOD
Prospective cohort
We conducted a prospective, observational study of children aged 1 month to 18 years presenting with a first, suspected AIS between February 2018 and April 2020. We included children whether or not the final diagnosis was AIS. Patients with previous AIS, known moyamoya disease, or ‘incidental’ AIS identified unexpectedly by neuroimaging for a different disorder (mostly bacterial meningitis) were excluded.
Contact by clinicians about acute neurological problems prompted urgent face-to-face or telephone consultation by SCH neurologists. Suspicion of AIS by SCH neurologists activated our acute stroke pathway. We encouraged rapid, local neuroimaging to confirm AIS. Our preferred imaging modality was MRI/MRA. When not quickly available, NCCT with computed tomography angiography (CTA) from aortic arch to circle of Willis was expedited. Neuroimaging was electronically available to SCH neurointerventionalists (who also provide 24-hour support to Prince of Wales and Liverpool Hospitals' [adult] stroke services) and SCH neurologists, facilitating communication with local clinicians, diagnoses, and management. Patients with confirmed AIS or uncertain diagnoses were transferred to SCH. Those initially imaged with urgent NCCT/CTA underwent MRI/MRA (using time-of-flight angiography) as clinically indicated. Neuroimaging was reviewed by AKC, blinded to diagnoses, without change in radiological interpretations.
Total AIS cohort
We reviewed inpatient records (International Classification of Diseases codes 163.0–163.9) and neurology outpatient records (using keywords ‘stroke’, ‘infarct’, and ‘infarction’) to identify all children aged 1 month to 18 years with AIS cared for at SCH between 1st January 2010 and 1st February 2021. We selected patients with symptomatic presentations of a first AIS, applying the same inclusion and exclusion criteria as for the prospective cohort. Neuroimaging was reviewed by PIA, unblinded to diagnoses. Studies of concern were reviewed by AKC, blinded to diagnoses.
This total AIS cohort included two subgroups: patients presenting before 2018 and after 2018 (before and after application of the acute stroke pathway). The ‘after 2018’ cohort included patients with AIS in the prospective cohort plus patients with AIS identified after the prospective study concluded.
Data about clinical presentation, imaging, diagnoses, and outcomes were collected. AIS was defined as MRI/MRA with a new ischaemic lesion, or CTA with arterial lesion(s) plus clinical scenario concordant with dysfunction distal to the arterial lesion(s).1 We defined onset of symptoms as the first symptoms attributable to AIS; triage as first report to the emergency department or ward staff for inpatients; suspicion of AIS as the request for first CTA or MRI/MRA; diagnostic imaging as the scan that first confirmed AIS; and time of diagnosis as conclusion of the diagnostic scan. The Pediatric NIH Stroke Scale4 (PedNIHSS) was reported in, or calculated from, documentation at presentation. Outcome was assessed at last neurology review (all >6 months after AIS) using the Modified Rankin Scale,5 since documentation was often insufficient to calculate PedNIHSS.
Patients were divided into two clinical subgroups to explore the effect of mode of presentation upon speed of diagnosis: (1) ‘typical’, those with dominant clinical feature of acute, sustained (at least partial) hemiparesis, with or without dysphasia; and (2) ‘atypical’, those with dominant clinical features other than acute, sustained hemiparesis, for example seizures, ataxia, or transient, often waxing and waning, weakness.
Patients were also divided into two radiological subgroups: (1) those with arterial abnormalities identified by CTA or MRA, including LVO, characterized by occlusion of the internal carotid artery, first or second parts of middle cerebral, anterior cerebral or posterior cerebral arteries, vertebral artery, or basilar arteries; arterial dissection; moyamoya; and other arteriopathies (abnormal arterial imaging not classified above); and (2) those without arterial abnormalities on CTA or MRA, which included lenticulostriate vasculopathy6 (stroke after minor head trauma in young children, often with basal ganglia calcification) and other small-vessel strokes.
For patients with AIS due to LVO, we considered EVT within 6 hours of symptom onset, or later with NCCT/CTA features favourable for salvage with reperfusion.7, 8 Treatment with tPA was offered according to Australian Guidelines.1 Inclusion criteria were age older than 2 years; PedNIHSS 4 to 24; and confirmed AIS. Exclusion criteria included multiple risk factors for bleeding; delay of more than 4.5 hours since last known well (patients with ‘wake-up’ stroke were excluded); and AIS due to septic embolism, intracranial arterial dissection, and moyamoya.1 On the basis of the hypothesized pathogenesis and good outcomes among patients with lenticulostriate vasculopathy,6 they were excluded from tPA.
A two-tailed Mann–Whitney U test was applied to compare continuous variables and Fisher's exact test to compare categorical variables between groups.
The SCH Human Research and Ethics Committee approved this study and waived requirement for patient consent (2019/ETH12160 and 2020/ETH01597).
RESULTS
Prospective cohort
Twenty-one consecutive patients were enrolled (Table 1). All were outpatients at onset of symptoms. One had known cardiac disease. Ten presented with acute, sustained hemiparesis (typical presentations) and 11 presented with dominant features other than acute, sustained hemiparesis (atypical presentations), although partial hemiparesis was a component of symptomatology in seven. Eight had AIS. Thirteen had stroke mimics. Nineteen presented to seven hospitals with MRI facilities. None obtained immediate MRI. All had initial NCCT/CTA: 19 in wakefulness, one under general anaesthesia, and one with sedation. Subsequent MRIs were performed in wakefulness in 13 patients and under general anaesthesia in six.
| Age/sex | Diagnosis; typical or atypical presentation; clinical features; timeline and acute management |
|---|---|
| AIS: large vessel occlusion—all had initial NCCT/CTA and subsequent MRI/MRA | |
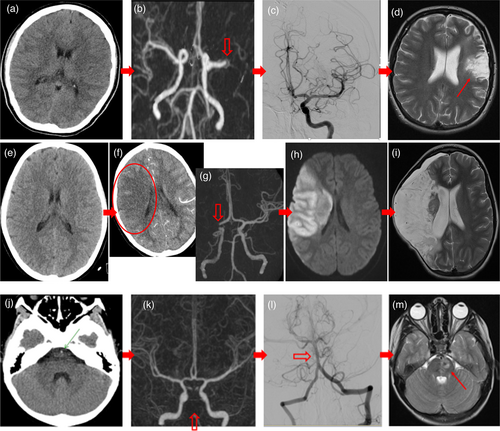
| 17 years/F | Paradoxical embolic left M1 occlusion. Patent foramen ovale. Typ: right hemiparesis and aphasia. Triage at 59 minutes. NCCT/CTA at 95 minutes. tPA started at 111 minutes. Transported to SCH. EVT successfully completed at 251 minutes - TICI 2b. See Figure 1a–d, patient 1. |
| 12 years/M | Cardio-embolic right M1 occlusion. Known cardiomyopathy. Typ: ‘Wake-up’ left hemiparesis. Triage at 87 minutes. NCCT/CTA at 125 minutes revealed loss of grey-white differentiation in right lentiform and caudate nuclei, and occluded right M1. Ineligible for tPA. Transported to SCH. EVT successfully completed at 319 minutes - TICI 2b. Subsequent MRI revealed AIS right lentiform and caudate nuclei. |
| 11 years/F | Idiopathic distal right ICA occlusion. Atyp: long seizure. Triage at 52 minutes. Intubated for resuscitation. Normal initial NCCT (without CTA) at 88 minutes. Transported to SCH intubated and sedated. Left hemiparesis recognized at SCH. NCCT/CTA at 517 minutes (3.37 a.m.), with established large right MCA infarct, confirmed with subsequent MRI/MRA. EVT and tPA not offered. Subsequent hemicraniectomy for malignant cerebral oedema. See Figure 1e–i, patient 2. |
| 8 years/M | Idiopathic basilar artery thrombosis. Atyp: ‘Wake-up’ vomiting and ataxia. Suspected viral illness and discharged. Represented with cranial neuropathies and right weakness. Stroke suspected by telephone consultation at 990 minutes. Local NCCT was electronically reviewed and the dense basilar artery was noted. Transported to SCH for NCCT/CTA at 1258 minutes (0.28 a.m.). Ineligible for tPA. EVT successfully completed at 1331 minutes: TICI 2b. See Figure 1j–m, patient 3. |
| Other AIS—all had initial NCCT/CTA and subsequent MRI/MRA | |
| 14 years/M | Intracranial right ICA dissection. Typ: left hemiparesis and headache. Triage at 146 minutes. CT/CTA at 183 minutes. Ineligible for tPA. Rx aspirin. Transported to SCH. MRI/MRA with small AIS in posterior limb of internal capsule and intracranial ICA dissection. Fluctuating weakness at SCH. Endovascular stent at 48 hours. |
| 10 years/M | Moyamoya. Down syndrome. Atyp: ‘Wake-up’ right arm disuse, initially attributed to pain. Triage at 295 minutes. NCCT/CTA at 606 minutes also revealed new and old infarcts. Ineligible for tPA. MRI/MRA confirmed new left frontal AIS, old ischaemic lesions and moyamoya. |
| 5 years/F | Arteriopathy - narrow proximal right MCA. Subcortical AIS. Typ (but complicated): collapse. Left hemiparesis. Vomiting. Drowsy. Dehydrated. Panhypopitituitarism. Eight weeks after resection craniopharyngioma. Normal MRA before surgery. Triage at 81 minutes. Rehydrated. NCCT/CTA at 130 minutes with new narrowing proximal right MCA without new parenchymal lesion. Relative contraindication to tPA. MRI/MRA at 338 minutes revealed new AIS in right caudate and putamen and confirmed narrow proximal MCA. Normal magnetic resonance venogram. |
| 7 months/F | Cortical small-vessel AIS. Atyp: focal seizure. Mild left hemiparesis (PedNIHSS <4). Six weeks after tuberculosis meningitis. On prednisolone and tuberculosis treatment. Dehydration. Triage at 37 minutes. NCCT/CTA at 434 minutes with previous injury only. Rx hydration. Ineligible for tPA. MRI/MRA at 1387 minutes revealed new small cortical AIS and old injuries. |
| Stroke mimics: presumed first migraine with aura—normal initial NCCT/CTAs and subsequent MRI/MRAs | |
| 15 years/M | Atyp: 5 hours right paraesthesia, confusion, dysarthria and dysphasia. Headache. |
| 15 years/M | Atyp: 30–60 minutes right visual field neglect. Headache. Rx aspirin. |
| 15 years/F | Typ: several hours mild right weakness, photophobia, dizziness, and headache after chiropractic neck manipulation. Rx aspirin. |
| 13 years/M | Typ: 3 hours right weakness, dysarthria, and paraesthesia. Headache. |
| 11 years/M | Typ: 4.5 hours right weakness, dysphasia, paraesthesia after head injury. Headache. Sister with similar history. Benign familial hemiplegic migraine gene testing. |
| 11 years/F | Atyp: 1 hour right paraesthesia, dysarthria and dysphasia. Headache not initially reported. |
| 7 years/F | Typ: 8 hours right hemiplegia and expressive dysphasia. Headache not initially reported. Family history of migraine and moyamoya. Rx aspirin. |
| Other stroke mimics—all had initial NCCT/CTA | |
| 14 years/F | Conversion. Typ: right weakness and paraesthesia. MRI not performed. |
| 8 years/F | First Todd paresis. Atyp: ‘wake-up'a focal seizure followed by 1.5 hours right weakness. EEG suggested childhood epilepsy with centro-temporal spikes. MRI not performed. |
| 8 years/F | SLC2A1 mutation (glucose-1 transporter deficiency). Typ: 3 hours left hemiparesis, dysarthria, and confusion. No headache. NCCT/CTA showed complete right hemisphere oligaemia. Pre-existing cognitive impairment. Normal MRI/MRA the next day. Rx aspirin. |
| 3 years/M | First Todd paresis. Atyp: first focal seizure. 4.5 hours left hemiparesis. Headache. Normal subsequent MRI/MRA. |
| 3 years/M | Acute flaccid paresis due to enterovirus. Atyp: febrile illness with ‘wake-up'a right arm weakness. Developed left arm and diaphragm weakness and headache. Subsequent urgent MRI revealed anterior horn lesions. Rx intravenous immune-globulin. |
| 6 weeks/F | Abusive head trauma. Atyp: long seizure. Left hemiparesis. NCCT/CTA and subsequent MRI showed CNS contusion and subdural blood. Rx anti-convulsants. |
- a ‘Wake-up’, well before bed and symptoms apparent upon waking. Abbreviations: AIS, acute ischaemic stroke; Atyp, atypical presentation; CNS, central nervous system; CTA, computed tomography angiography; EEG, electroencephalography; EVT, endovascular thrombectomy; F, female; ICA, internal carotid artery; M, male; M1, first division middle cerebral artery; MCA, middle cerebral artery; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; NCCT, non-contrast computed tomography; Rx, treatment; SCH, Sydney Children's Hospital; TICI 2b, thrombolysis in cerebral infarction score: complete but slow filling of vascular territory; tPA, tissue plasminogen activator; Typ, typical presentation.
Patients with typical presentations progressed rapidly through the diagnostic pathway: median delay from triage to AIS diagnosis was 55 minutes, and all with AIS were diagnosed within 4.5 hours of symptom onset (Table 2). Patients with atypical presentations progressed more slowly through the diagnostic pathway: median delay from triage to AIS diagnosis was 219 minutes, and none with AIS were diagnosed within 4.5 hours of symptom onset (Table 2). CTA accurately identified arterial abnormalities concordant with the clinical scenario in seven of the eight patients with AIS. There were no false positives among the stroke mimics, yielding a sensitivity of 87.5% and specificity of 100% in this small cohort. One patient with small-vessel stroke was diagnosed by subsequent MRI. Three of four patients with LVO were successfully treated with EVT without further delay to MRI/MRA (Figure 1). Six patients with AIS had features that precluded tPA, irrespective of delay to diagnosis.1 One patient was treated with tPA.
| Onset to triage (minutes) | Triage to suspicion (minutes) | Suspicion to initial CTA or MRI (minutes) | Triage to AIS diagnosis (minutes) | Onset to AIS diagnosis (minutes) | AIS diagnosis <4.5 hours | |
|---|---|---|---|---|---|---|
| Prospective cohort: typical presentation (AIS n = 4; mimics n = 6) | ||||||
| Median | 70 | 14** | 40 | 55** | 132** | 4/4* |
| Range | 29–146 | 0–120 | 5–81 | 36–152 | 95–183 | 100% |
| Prospective cohort: atypical presentation (AIS n = 4; mimics n = 7) | ||||||
| Median | 57 | 163** | 67 | 219** | 385** | 0/4* |
| Range | 12–610 | 7–780 | 6–268 | 74–1350 | 86–1387 | 0% |
| After 2018 AIS cohort: typical presentation (n = 6) | ||||||
| Median | 111 | 11** | 40 | 44** | 158 | 4/6** |
| Range | 59–146 | 0–150 | 5–283 | 36–934 | 95–1858 | 67% |
| After 2018 AIS cohort: atypical presentation (n = 10) | ||||||
| Median | 67 | 171** | 209 | 1149** | 1294 | 0/10** |
| Range | 33–337 | 25–6346 | 45–5482 | 311–6496 | 417–6615 | 0% |
| Before 2018 AIS cohort: typical presentation (n = 19) | ||||||
| Median | 135 | 189 | 165 | 462** | 573 | 2/19 |
| Range | 18–20252 | 0–1178 | 13–1470 | 30–1782 | 161–21735 | 11% |
| Before 2018 AIS cohort: atypical presentation (n = 16) | ||||||
| Median | 89 | 269 | 214 | 766** | 1180 | 1/16 |
| Range | 0–1290 | 59–3760 | 25–3387 | 202–1800 | 238–7281 | 6% |
| Total AIS cohort: typical presentation (n = 25) | ||||||
| Median | 135 | 130* | 151 | 329** | 495* | 6/25** |
| Range | 0–20.252 | 0–1178 | 5–1410 | 30–1782 | 95–21735 | 24% |
| Total AIS cohort: atypical presentation (n = 26) | ||||||
| Median | 83 | 227* | 214 | 905** | 1264* | 1/26** |
| Range | 0–1290 | 25–6346 | 25–5482 | 202–7147 | 238–7281 | 4% |
| Arterial abnormalities identified by CTA or MRA: typical presentation (n = 15) | ||||||
| Median | 135 | 33** | 84* | 144** | 294* | 6/15** |
| Range | 37–20252 | 0–339 | 5–1289 | 30–1483 | 95–21735 | 40% |
| Arterial abnormalities identified by CTA or MRA: atypical presentation (n = 16) | ||||||
| Median | 101 | 269** | 221* | 620** | 1079* | 0/16** |
| Range | 0–570 | 28–6346 | 25–5482 | 230–1800 | 310–6615 | 0% |
| Large vessel occlusion: typical presentation (n = 5) | ||||||
| Median | 87 | 33** | 35* | 49** | 161* | 3/5 |
| Range | 54–342 | 0–85 | 5–168 | 36–224 | 95–440 | 60% |
| Large vessel occlusion: atypical presentation (n = 6) | ||||||
| Median | 118 | 567** | 224* | 885** | 1264* | 0/6 |
| Range | 52–549 | 149–2785 | 106–485 | 255–3106 | 310–3191 | 0% |
| Arterial abnormalities not identified by CTA or MRA: typical presentation (n = 10) | ||||||
| Median | 134 | 228 | 235 | 1051 | 1118 | 0/10 |
| Range | 0–1410 | 97–1178 | 56–1470 | 234–1782 | 367–3192 | 0% |
| Arterial abnormalities not identified by CTA or MRA: atypical presentation (n = 10) | ||||||
| Median | 65 | 149 | 130 | 1300 | 1398 | 1/10 |
| Range | 24–1290 | 25–3760 | 45–3387 | 202–7147 | 238–7281 | 10% |
- Delays within each component of the pathway for each patient cohort were compared with Mann–Whitney U tests. The last column shows the proportion of patients diagnosed within 4.5 hours of symptom onset, for whom treatment with tissue plasminogen activator could be considered. These proportions were compared for typical and atypical presentations within each cohort using Fisher's exact test. Significant differences are designated by *p < 0.05 and **p < 0.01.
- Abbreviations: AIS, acute ischaemic stroke; CTA, computed tomography angiography; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging.
Total AIS cohort
Fifty-one patients with a first, symptomatic AIS were identified: eight in the prospective cohort; 16 in the ‘after 2018’ cohort (including the eight from the prospective cohort); and 35 in the ‘before 2018’ cohort. Nineteen (37%) were female. Age at presentation ranged from 6 months to 16 years (median 10 years, mean 8 years 6 monts, SD 5 years 4 months), including nine (18%) younger than 2 years. Only four (8%) had a previous history of cardiac disease, in keeping with reduced cardiac surgery at SCH from 2011. Sixteen (31%) presented to SCH and 35 (69%) were transported from 18 other hospitals (median 59 km, range 8–921 km).
Among these 51 patients, 31 (61%) had arterial pathologies identified by CTA or MRA (Table S1): 11 LVOs, six arterial dissections, five with moyamoya (bilateral cerebral arteriopathy of childhood with collaterals9), and nine other arteriopathies (all unilateral focal–cerebral arteriopathies without collaterals9). Twenty (39%) had AIS without arterial abnormalities on CTA or MRA: eight with lenticulostriate vasculopathy and 12 with other small-vessel strokes.
‘Typical’ versus ‘atypical’ presentations
Two broad patterns of clinical presentation and progress through the diagnostic pathway were observed. Approximately half the patients had typical presentations (acute, sustained hemiparesis) and approximately half had atypical presentations (Tables 1, 2, and S1). Among the total AIS cohort, atypical presentations included nine with posterior circulation features, for example ataxia or vomiting; seven with transient, often waxing and waning, hemiparesis; five with seizures; three with headache or pain; one with confusion; and one collapsed.
Patients with typical presentations progressed more rapidly through the diagnostic pathway than with atypical presentations. As detailed in Table 2, delays from triage to suspicion of AIS, triage to AIS diagnosis, and onset to AIS diagnosis were less among patients with typical presentations than atypical presentations in the prospective, ‘after 2018’, ‘before 2018’, total AIS, arterial abnormalities identified by CTA or MRA, and LVO cohorts. Greater proportions of patients were also diagnosed within 4.5 hours of symptom onset among patients with typical presentations than atypical presentations in the same cohorts. In contrast, progress through the diagnostic pathway was similar with typical and atypical presentations among patients without arterial abnormalities identified by CTA or MRA.
Among patients with typical presentations, progress through the diagnostic pathway markedly improved and a significantly greater proportion were diagnosed within 4.5 hours of symptom onset after activation of the acute stroke pathway (4 out of 6 after 2018, 2 out of 19 before 2018; p = 0.015 by Fisher's exact test). In contrast, progress through the diagnostic pathway was similar for patients with atypical presentations before and after 2018, with long delays to suspect AIS.
Acute neuroimaging: NCCT/CTA versus MRI/MRA
Seventeen patients with AIS were initially imaged with CTA and all had subsequent MRI/MRA. Thirty-four patients with AIS were initially imaged with MRI/MRA and 17 had subsequent CTA. CTA and MRA findings were concordant in 29 out of 30 patients, eight of whom also had concordant digital subtraction angiography (DSA). Initial CTA revealed LVO in seven patients. Four proceeded to urgent EVT, with concordant DSA during the procedure. Subsequent MRI/MRA revealed resolution of the occlusions and residual ischaemic lesions. Three with delayed diagnoses and large, established infarcts (early computed tomography scores of 1–2 on the Alberta Stroke Program10) were not offered EVT. Subsequent MRI/MRAs confirmed the LVO and the established strokes. MRA and DSA were concordant in seven patients. One infant had a normal MRA, but CTA and DSA revealed moyamoya-type changes. Ten had MRI/MRA alone.
Delay from suspicion of AIS to obtaining initial neuroimaging with CTA was significantly less than with MRI/MRA in all cohorts (Table 3). In addition, delays from triage to diagnosis and onset to diagnosis were reduced among patients with initial CTA compared with those with initial MRI/MRA in all cohorts, except patients without identified arterial abnormalities.
| Onset to triage (minutes) | Triage to suspicion (minutes) | Suspicion to initial CTA or MRI (minutes) | Triage to AIS diagnosis (minutes) | Onset to AIS diagnosis (minutes) | AIS diagnosis <4.5 hours | |
|---|---|---|---|---|---|---|
| After 2018 cohort: initial CTA (n = 11) | ||||||
| Median | 81 | 54 | 56** | 367 | 517 | 4/11 |
| Range | 37–295 | 0–750 | 5–310 | 36–1354 | 95–1408 | 36% |
| After 2018 cohort: initial MRI/MRA (n = 5) | ||||||
| Median | 119 | 28 | 480** | 1249 | 1858 | 0/5 |
| Range | 33–1560 | 15–6346 | 150–5482 | 298–6496 | 845–6615 | 0% |
| Before 2018 AIS cohort: initial CTA (n = 6) | ||||||
| Median | 124 | 212 | 57* | 280 | 507 | 2/6 |
| Range | 85–431 | 0–2785 | 13–321 | 30–3106 | 161–3191 | 33% |
| Before 2018 AIS cohort: initial MRI/MRA (n = 29) | ||||||
| Median | 133 | 205 | 217* | 721 | 1069 | 1/29 |
| Range | 0–20252 | 7–3760 | 25–3387 | 144–7147 | 238–21735 | 3% |
| Total AIS cohort: initial CTA (n = 17) | ||||||
| Median | 111 | 85 | 53** | 367 | 517* | 6/17** |
| Range | 37–431 | 0–2785 | 5–321 | 30–3106 | 95–3191 | 39% |
| Total AIS cohort: initial MRI (n = 34) | ||||||
| Median | 129 | 192 | 224** | 747 | 1128* | 1/34** |
| Range | 0–20252 | 7–6346 | 25–5482 | 144–7147 | 238–21735 | 3% |
| Arterial abnormalities identified by CTA or MRA: initial CTA (n = 12) | ||||||
| Median | 112 | 85 | 46** | 49* | 312* | 6/12** |
| Range | 52–342 | 0–2785 | 5–321 | 30–3106 | 95–3191 | 50% |
| Arterial abnormalities identified by CTA or MRA: initial MRI (n = 19) | ||||||
| Median | 135 | 186 | 217** | 508* | 1069* | 0/19** |
| Range | 0–20252 | 7–6346 | 25–5482 | 144–6496 | 278–21735 | 0% |
| Large vessel occlusion: initial CTA (n = 7) | ||||||
| Median | 87 | 85 | 49 | 74 | 440 | 3/7 |
| Range | 52–342 | 0–2785 | 5–321 | 36–3106 | 95–3191 | 43% |
| Large vessel occlusion: initial MRI (n = 4) | ||||||
| Median | 103 | 186 | 174 | 508 | 790 | 0/4 |
| Range | 54–549 | 56–1310 | 106–485 | 224–1490 | 278–1640 | |
| Arterial abnormalities not identified by CTA or MRA: initial CTA (n = 5) | ||||||
| Median | 54 | 150 | 56* | 1350 | 1387 | 0/5 |
| Range | 37–431 | 34–689 | 45–310 | 367–1400 | 417–1831 | 0% |
| Arterial abnormalities not identified by CTA or MRA: initial MRI (n = 15) | ||||||
| Median | 125 | 226 | 247* | 1167 | 1329 | 1/15 |
| Range | 0–1410 | 25–3760 | 78–3387 | 202–7147 | 238–7281 | 7% |
- Delays within each component of the pathway for each patient cohort were compared with Mann–Whitney U tests. Small sample size precluded comparisons for patients with LVO. Small numbers precluded comparisons in the LVO cohort. The last column shows the proportion of patients diagnosed within 4.5 hours of symptom onset, for whom treatment with tPA could be considered. These proportions were compared for initial imaging with CTA versus MRI within each cohort using Fisher's exact test. Significant differences are designated by *p < 0.05 and **p < 0.01.
- Abbreviations: AIS, acute ischaemic stroke; CTA, computed tomography angiography; LVO, large vessel occlusion; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; NCCT, non-contrast computed tomography of brain.
Patients without arterial abnormalities identified by CTA or MRA
Twenty patients had AIS without arterial abnormalities identified by CTA or MRA (or DSA in three). Diagnoses relied upon parenchymal ischaemia on MRI. Eight had lenticulostriate vasculopathy and 12 had other small-vessel strokes. These patients were diagnosed more slowly than those with arterial abnormalities, irrespective of mode of presentation or initial imaging modality (Tables 2 and 3).
Recognizing urgent NCCT/CTA may not identify small-vessel strokes, and that MRI is required to confirm diagnoses, we explored therapeutic options and outcomes in these patients. All were ineligible for EVT. Four were eligible and 16 were ineligible for tPA, irrespective of delay to diagnosis (age <2 years [n = 6]; lenticulostriate vasculopathy [n = 8]; PedNIHSS <4 [n = 5]; wake-up stroke [n = 2]; recent surgery [n = 1]; and AIS due to tumour embolism [n = 1]). Six presented to the emergency department more than 4.5 hours after symptom onset, and 4.5 hours had elapsed by the time stroke was suspected and the first NCCT/CTA or MRI/MRA was requested in 12. Only one was diagnosed within 4.5 hours of symptom onset. None were treated with tPA. Even so, outcomes were relatively good, with Modified Rankin Scale scores of 0 for 12 patients and 1 for seven patients. One infant had pre-existing injury from tuberculous meningitis and consequences of the stroke were not assessable.
Five were initially imaged with NCCT/CTA. Four had atypical presentations, three were excluded from tPA irrespective of delay to diagnosis (including two with lenticulostriate vasculopathy for whom the clinical scenario and punctate basal ganglia calcification on NCCT allowed presumptive AIS diagnoses), and 4.5 hours had elapsed by the time stroke was suspected and NCCT/CTA was requested in two and performed in four. Since treatment with tPA was precluded, urgency to obtain subsequent MRI/MRAs was reduced (Table 3).
DISCUSSION
Our acute stroke pathway aimed to rapidly identify patients with LVO likely to benefit from EVT, and to increase the proportion of patients diagnosed within 4.5 hours of symptom onset, so tPA treatment might be considered. This approach was a subtle departure from the previous focus upon comprehensive, but often delayed, diagnosis. Although urgent MRI/MRA was our preferred imaging modality, this was commonly unavailable, so many patients were imaged with NCCT/CTA. We undertook the prospective study and review of our patients with AIS to evaluate this approach.
Mode of presentation markedly influenced the rapidity with which AIS was suspected and diagnosed. AIS was quickly suspected in patients with typical presentations (acute, sustained hemiparesis) among the prospective cohort (both strokes and mimics); the ‘after 2018’ cohort (after activation of the acute stroke pathway); those with arterial abnormalities detected by CTA or MRA; and those with LVO. This expedited progress through the diagnostic pathway and facilitated time-limited interventions. Similar, less-marked differences were also apparent among patients presenting before 2018, when tPA was not offered and rapidity of diagnosis was less emphasized.
Approximately half the patients in the prospective and total AIS cohorts had atypical presentations. We noted slower suspicion for AIS and slower neuroimaging in these patients, which delayed diagnoses and limited therapeutic options. For example, among the 10 with atypical presentations after 2018, two were initially discharged from the emergency department with presumed non-neurological diagnoses (‘gastroenteritis’ with basilar artery thrombosis, and ‘anxiety’ with transient weakness and middle cerebral arteriopathy), and stroke was unsuspected until revealed by MRI in one patient presenting with seizures. These observations suggest atypical presentations are common with paediatric AIS. This concept provides a target for ongoing education to improve our diagnostic acumen.
A crucial component of our acute stroke pathway is urgent, local neuroimaging. We anticipated difficulties obtaining urgent MRI/MRA, so we were anxious to explore the utility of acute NCCT/CTA. NCCT/CTA was quickly available in most hospitals, including after hours, and images were readily accessible electronically by SCH clinicians, facilitating liaison with treating clinicians, diagnoses, and management. CTA was at least as accurate as MRA in identifying arterial abnormalities underlying AIS, and we found no false positives among patients with stroke mimics in the small prospective study.
In keeping with adult AIS data,7, 8, 10, 11 NCCT/CTA identified patients with LVO and favourable patterns for salvage, facilitating timely treatment with EVT. This is potentially important, because LVO commonly underlies the most devastating strokes, and EVT is the most beneficial acute therapy for adult stroke AIS due to LVO; for these patients, each minute saved from onset to EVT grants an average of 4.2 days of extra healthy life (more among young adults).12 CTA also facilitated rapid, accurate diagnoses of arterial disorders for which we consider tPA, for example extracranial dissection or arteriopathy, and for which we currently avoid tPA, for example intracranial dissection or moyamoya. These observations suggest NCCT/CTA is a useful, readily available, screening test for paediatric AIS, and can direct urgent EVT and thrombolysis in those with identifiable arterial abnormalities (61% of AIS in this small cohort and probably more in centres with cardiac surgical services).
We were concerned that patients with AIS without arterial abnormalities detectable by CTA, who are dependent upon MRI for diagnosis, might be disadvantaged by initial imaging with NCCT/CTA. Although their progress through the diagnostic pathway was slower than those with arterial abnormalities, it was very similar with initial NCCT/CTA or initial MRI/MRA. These patients were not candidates for EVT. Most (80%) were also excluded from tPA,1 unrelated to delay from symptom onset. Additionally, their diagnoses were often delayed beyond 4.5 hours, further precluding treatment with tPA. Even so, it is conceivable that a minority of patients with AIS without identified arterial abnormalities could benefit from tPA, and initial NCCT/CTA could delay MRI-dependent diagnosis and treatment. Reassuringly, this cohort showed relatively good long-term outcomes (median Modified Rankin Scale 0, range 0–1), suggesting minimal deleterious consequences from initial imaging with NCCT/CTA.
Several cautions need be applied to these observations. The small number of patients and partly retrospective data acquisition restrict extrapolation to the wider paediatric population. That observations were comparable in separate patient cohorts, one of which was prospectively studied, may partly mitigate this concern. We used surrogate markers for progress through the diagnostic pathway, which may not accurately represent clinical complexities for individual patients. For example, request for the first CTA or MRI served as a surrogate marker for clinical suspicion of AIS. Although accurate for patients initially imaged with CTA, this potentially underestimated delay to suspicion of stroke in some with initial MRI, among whom MRI was sometimes requested without documentation of suspected stroke or unexpectedly revealed AIS. Also, among patients with initial, benign NCCT/CTA who were ineligible for tPA, subsequent MRI/MRA was sometimes not expedited, because it was unlikely to alter management. This potentially delayed some diagnoses without implying less effective delivery of treatment. These methodological shortcomings, however, potentially support our conclusions.
Another concern relates to radiation exposure. The radiation dose for our helical NCCT/CTA (0.5 mm slices and overlapping, multiplanar reconstruction with 3 mm slices) in the average 10-year-old child is approximately 2.6 mSv, and 1.8 mSv for NCCT alone. The average natural yearly radiation exposure for Australians is 1.5 mSv.13 We felt this radiation dose was justified when it facilitated rapid diagnosis of AIS and enabled urgent treatment. Patients with stroke mimics, however, were exposed to radiation with little benefit. Partly modifying this concern, we note that urgent CTAs were largely performed without general anaesthesia or sedation, with less anaesthetic risk and resource utilization than with less-available, often-delayed MRI/MRA.
In conclusion, this study highlights the influence of clinical presentation upon rapidity of diagnosis of paediatric AIS. Patients presenting predominantly with acute, sustained hemiparesis were identified quickly, which facilitated time-limited therapies. In contrast, patients presenting with dominant symptoms other than hemiparesis were diagnosed more slowly, reducing treatment options. This subgroup provides a target for improved diagnostic strategies. These observations also suggest readily available NCCT/CTA accurately identifies patients with AIS due to LVO and facilitates timely EVT. CTA also identifies patients with arterial abnormalities underlying most, but not all, paediatric AIS, facilitating thrombolysis in most, but not all, eligible patients. These roles for NCCT/CTA are particularly relevant for the many health services with limited access to urgent MRI, but with potential to offer acute intervention. We hope improved clinical acumen, effective use of acute stroke protocols, lower radiation computed tomography perfusion techniques, and upgraded MRI resources will enhance our future management of childhood AIS.
ACKNOWLEDGMENTS
The authors have stated that they had no interests that might be perceived as posing a conflict or bias. The authors are grateful to the many families and clinicians involved in the care of these patients. Open access publishing facilitated by University of New South Wales, as part of the Wiley - University of New South Wales agreement via the Council of Australian University Librarians.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.