Long-term neurodevelopmental outcome of neonates born at term with perinatal haemorrhagic stroke: A population-based study
Abstract
enAim
To assess the long-term neurodevelopmental outcome of neonates born at term diagnosed with perinatal haemorrhagic stroke (PHS) and investigate the associations among brain territorial involvement, clinical risk factors, and neurodevelopmental outcomes.
Method
We conducted a population-based study enrolling 55 neonates born at term with PHS confirmed by magnetic resonance imaging born between 2007 and 2017. Long-term neurodevelopmental outcome was assessed using the Bayley Scales of Infant Development, Second Edition, the Brunet–Lézine test, and the Stanford–Binet Intelligence Scales, Fifth Edition.
Results
Follow-up was available in 50 (91%) of the infants, at a median age of 60 months (interquartile range 35–88). Forty per cent of the infants developed according to population norms, and developmental disabilities were diagnosed less frequently among neonates with frontal lobe PHS. In a multivariable model, parietal lobe PHS increased the risk for cerebral palsy (odds ratio [OR] 6.7; 95% confidence interval [CI] 1.1–41.4) and cognitive impairment (OR: 23.6; 95% CI: 2.9–194.9), while the involvement of the thalamus and/or basal ganglia was associated with epilepsy (OR: 7.0; 95% CI: 1.3–37.7). Seizures on admission were associated with epilepsy (OR: 10.8; 95% CI: 1.8–64.3). Patients with PHS affecting multiple lobes had poor prognosis.
Interpretation
Parietal lobe haemorrhage, the involvement of the thalamus/basal ganglia, PHS affecting multiple lobes, and seizures were independent predictors of chronic neurodevelopmental sequelae, suggesting that the stroke territorial involvement and clinical risk factors influence the outcome of PHS.
Resultado del desarrollo neurológico a largo plazo de los recién nacidos a término con accidente cerebrovascular hemorrágico perinatal: un estudio basado en la población
esObjetivo
Evaluar el resultado del desarrollo neurológico a largo plazo de los recién nacidos a término diagnosticados con accidente cerebrovascular hemorrágico perinatal (PHS) e investigar las asociaciones entre la participación territorial del cerebro, los factores de riesgo clínicos y los resultados del desarrollo neurológico.
Método
Realizamos un estudio poblacional que identificó a 55 recién nacidos a término con PHS confirmado por resonancia magnética nacidos entre 2007 y 2017. El resultado del desarrollo neurológico a largo plazo se evaluó utilizando las Escalas Bayley de Desarrollo Infantil, Segunda Edición, la prueba Brunet-Lézine, y las escalas de inteligencia de Stanford-Binet, quinta edición.
Resultados
El seguimiento estuvo disponible en 50 (91%) de los lactantes, a una mediana de edad de 60 meses (rango intercuartil 35-88). El cuarenta por ciento de los bebés se desarrollaron de acuerdo con las normas de la población, y los trastornos del desarrollo se diagnosticaron con menos frecuencia entre los recién nacidos con PHS del lóbulo frontal. En un modelo multivariable, el PHS del lóbulo parietal aumentó el riesgo de parálisis cerebral (odds ratio [OR] 6,7; intervalo de confianza [IC] del 95 %: 1,1–41,4) y deterioro cognitivo (OR 23,6; IC del 95 %: 2,9–194,9), mientras que la afectación del tálamo y/o los ganglios basales se asoció con epilepsia (OR 7,0; IC 95% 1,3-37,7). Las convulsiones en el comienzo de la presentación clínica se asociaron con epilepsia (OR 10,8; IC 95 % 1,8–64,3). Los pacientes con PHS que afectaba a múltiples lóbulos tenían mal pronóstico.
Interpretación
La hemorragia del lóbulo parietal, la afectación del tálamo/ganglios basales, el PHS que afecta a múltiples lóbulos y las convulsiones fueron predictores independientes de secuelas crónicas del neurodesarrollo, lo que sugiere que la afectación territorial del accidente cerebrovascular y los factores de riesgo clínicos influyen en el resultado del accidente cerebrovascular hemorrágico perinatal.
Desfechos do neurodesenvolvimento em longo prazo de recém-nascidos a termo com acidente vascular cerebral hemorrágico perinatal: um estudo de base populacional
ptObjetivo
Avaliar os desfechos do neurodesenvolvimento em longo prazo de neonatos a termo com diagnóstico de acidente vascular cerebral (AVC) hemorrágico perinatal e investigar as associações entre o envolvimento territorial do cérebro, fatores de risco clínicos e desfechos do neurodesenvolvimento.
Método
Conduzimos um estudo de base populacional envolvendo 55 recém-nascidos a termo com AVC hemorrágico perinatal confirmada por ressonância magnética nascidos entre 2007 e 2017. Os desfechos no neurodesenvolvimento em longo prazo foi avaliado usando as escalas de desenvolvimento infantil de Bayley, segunda edição, o teste de Brunet-Lézine, e as escalas de inteligência Stanford-Binet, quinta edição.
Resultados
O acompanhamento estava disponível em 50 (91%) dos lactentes, com idade mediana de 60 meses (intervalo interquartil 35-88). Quarenta por cento dos bebês desenvolveram-se de acordo com as normas populacionais, e as deficiências de desenvolvimento foram diagnosticadas com menos frequência entre os neonatos com AVC hemorrágico perinatal do lobo frontal. Em um modelo multivariável, o lobo parietal no AVC hemorrágico perinatal aumentou o risco de paralisia cerebral (odds ratio [OR] 6.7; intervalo de confiança de 95% [IC] 1.1-41.4) e prejuízo cognitivo (OR 23.6; IC 95% 2.9-194.9), enquanto o envolvimento do tálamo e / ou gânglios da base foi associado à epilepsia (OR 7.0; IC 95% 1.3–37.7). As convulsões na admissão foram associadas à epilepsia (OR 10.8; IC 95% 1.8–64.3). Pacientes com AVC hemorrágico perinatal afetando múltiplos lobos tiveram prognóstico ruim.
Interpretação
Hemorragia do lobo parietal, o envolvimento do tálamo / gânglios da base, o AVC hemorrágico perinatal afetando lobos múltiplos e convulsões foram preditores independentes de sequelas de neurodesenvolvimento crônicas, sugerindo que o envolvimento territorial do AVC e os fatores de risco clínicos influenciam o desfecho do AVC hemorrágico perinatal.
Abbreviations
-
- CHD
-
- congenital heart disease
-
- PAIS
-
- perinatal arterial ischaemic stroke
-
- PHS
-
- perinatal haemorrhagic stroke
What this paper adds
- Parietal lobe haemorrhagic stroke is associated with an increased risk of cerebral palsy and cognitive impairment.
- The involvement of the thalamus and/or basal ganglia is associated with later epilepsy.
- Seizures on admission among neonates with haemorrhagic stroke are associated with later epilepsy.
- Haemorrhagic strokes involving multiple lobes are associated with poor outcome.
Stroke is 17 times more common in the perinatal period than at any time later in childhood.1 Although the aetiology and presentation of perinatal ischaemic stroke are well studied,2 data on perinatal haemorrhagic stroke (PHS) are limited. PHS is defined as a neonate presenting with neurological symptoms during the first 28 days after delivery with a focal collection of blood within the brain parenchyma.3 It may occur as a primary haemorrhage with or without a possible underlying pathological origin such as a bleeding diathesis or a vascular malformation, or may be secondary haemorrhagic stroke resulting from a transformation of a primary arterial or venous ischaemic injury.4 This definition excludes intracranial haemorrhages in infants born preterm where germinal matrix haemorrhage is common and haemorrhagic stroke occurs beyond the neonatal period.
The estimated incidence of PHS is at least one in 6300 live births.5 PHS is one of the identified causes of cerebral palsy (CP) in childhood, and survivors may also have epilepsy, language disorders, and cognitive and/or behavioural problems often emerging only with maturity.5
A better understanding of the relationship among the risk factors, findings of imaging studies, and long-term neurodevelopmental outcome has been suggested to improve the accuracy and timing of the diagnosis and treatment of PHS.6 Moreover, the pathophysiology of PHS is probably different in newborn infants with different risk factors. Since well-powered, population-based studies investigating the long-term outcomes of this condition are limited, our aim was to investigate the long-term neurodevelopmental outcomes of newborn infants with PHS and to assess the impact of the brain territorial involvement and clinical risk factors on the long-term outcome in this population.
METHOD
Population
We conducted a population-based study designed to capture all neonates born at term with perinatal stroke born between 1st January 2007 and 31st December 2017 and cared for in the central Hungarian region, including the capital, Budapest. Findings on neonates with perinatal arterial ischaemic stroke (PAIS) have been published elsewhere.2 In the present study, we describe the findings of neonates diagnosed with PHS.
Magnetic resonance imaging (MRI) scans were performed at a single centre: the MR Research Center of Semmelweis University, Budapest, Hungary. We reviewed the imaging studies of 1400 term and near-term neonates (≥36wks) who had a brain MRI performed before 28 days of age and identified 96 infants with evidence of a focal haemorrhagic event. Patient selection is shown in Figure S1. Inclusion criteria were (a) neonates born at not less than 36 weeks up to 28 days of age and (b) a brain MRI confirming the diagnosis of PHS without any other concurrent abnormality.
Like in previous studies,7 we also excluded neonates with encephalitis, tumour, non-accidental brain injury, and birth asphyxia from the study. We also excluded neonates with exclusively intraventricular, subarachnoidal, subdural, or epidural haemorrhage without intraparenchymal bleeding, as well as neonates with periventricular haemorrhagic leukomalacia. Infants with congenital syndromes with known neurodevelopmental sequelae or other significant brain lesions including neonates with concomitant PAIS or cerebral sinovenous thrombosis were not addressed in this study.
Ethical approval for the study was obtained from the Hungarian Medical Research Council (19934-4/2018/EKU). Informed written parental consent was obtained to recruit patients into the study and perform the neurodevelopmental assessments.
MRI
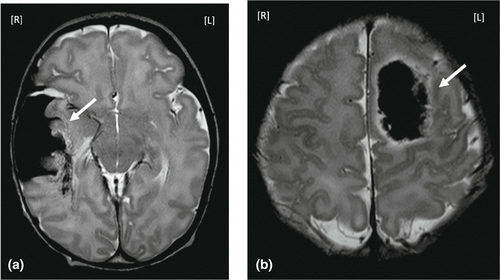
As described previously,2 brain imaging was performed on 3T Philips Achieva and 3T Philips Ingenia MR scanners (Philips Medical Systems) at the MR Research Center of Semmelweis University, Budapest, Hungary. The scanning protocol included diffusion-weighted imaging, conventional T1- and T2-weighted imaging, T2*-weighted imaging, and susceptibility-weighted imaging. In selected cases magnetic resonance angiography and/or magnetic resonance spectroscopy was added, as appropriate. Radiologists trained and experienced in neonatal brain MRI evaluated the MRI. Classification was performed according to the affected brain compartment as described by Govaert et al.7 (Figure 1), except that exclusively extra-axial bleeding without an intraparenchymal haemorrhage was not included in this study. We also noted the involvement of the thalamus and/or basal ganglia since previous reports have described an association between involvement of the thalamus and/or basal ganglia and chronic neurodevelopmental outcomes.
As an additional approach, we measured the size of the PHS by drawing a region of interest covering the area judged to have abnormally high signal intensity on T2*-weighted images using the three-dimensional image analysis package of the MRIcron program.8 Lesion volumes were expressed as total volume (cubic centimetres) and as a percentage of supratentorial brain volume after excluding ventricular volume. The ratio of PHS to the supratentorial brain volume was further subcategorized as small (<5%), moderate (5%–10%), or large (>10%) on the basis of the relative stroke volume.
Clinical data
Clinical characteristics, maternal and neonatal risk factors, and presenting symptoms of PHS were collected from chart reviews. The collected data are described in detail in Appendix S1.9 Bleeding diathesis was investigated in 15 neonates in the present study. Echocardiography was used as indicated to confirm/rule out congenital heart disease (CHD).
Neurodevelopmental outcome
In addition to routine follow-up visits between the age of 1 year 6 months to 12 years, we also prospectively performed a systematic neurodevelopmental follow-up assessment of children who were diagnosed with PHS and were available for follow-up in 2018. As described previously,2 a child developing according to population norms was defined as one with symptom-free survival. An infant with chronic neurodevelopmental sequelae was documented if they presented with any one or more of the following outcomes: CP, cognitive impairment, behavioural problems, epilepsy, language disorder, visual field defect, or hearing loss. Developmental tests used during the follow-up visits and the classification of CP and epileptic syndromes10-14 are described in detail in Appendix S2.
Statistical analysis
Descriptive statistics were expressed as frequencies and percentages in the population studied. Mean and standard deviation (SD) or median and interquartile range (IQR) were determined as appropriate. Univariate logistic regression models were fitted to describe relationships between clinical predictors of interest and neurodevelopmental outcome. A multivariable logistic regression model was fitted to ascertain the effect of brain territorial involvement of PHS on the basis of the notion that a patient would probably have a chronic neurodevelopmental outcome while controlling for other clinically relevant factors identified by the univariate analysis, and for emergency Caesarean section on the basis of previous studies.5, 15 Factors found to be clinically relevant by the univariate analysis included CHD and clinical seizure on admission. Models were checked according to the Hosmer and Lemeshow's goodness of fit tests.16 Odds ratios (OR) with 95% confidence intervals (CI) were calculated for each clinical variable. All statistical tests were two-sided, where p-values of <0.05 were considered to indicate statistical significance. Statistical analyses were performed using IBM SPSS Statistics (version 25, IBM Corp.).
RESULTS
Patient characteristics and general findings
A total of 294 000 live births of ≥36 weeks' gestation were registered in the central Hungarian region over the 11-year study period. Acute PHS was diagnosed in 55 neonates, yielding a disease incidence of one per 5300 live births (prevalence 1.87, 95% CI: 1.38–2.36, per 10 000 live births for newborn infants of ≥36 weeks' gestation).
The most common presenting symptom of PHS was seizure activity, occurring in 61% of patients, at a median age of 2 days (IQR: 1–3). The rate of respiratory distress was also high, detected in 39% of the neonates.
Among the 55 infants with PHS, 36 (65%) had a maternal and/or a neonatal risk factor that might have contributed to the development of the PHS. Underlying aetiologies among others included alloimmune thrombocytopenia (n=3), CHD (n=5), and cerebral vascular abnormalities (n=6) including arteriovenous malformation (n=4), cavernoma (n=1), and venous angioma (n=1). In 10 patients (18%) more than one possible primary etiological factor was present, suggesting that the aetiology of PHS might have been multifactorial. Clinical characteristics of the study population are summarized in Table 1.
| Clinical characteristics, risk factors, and presenting symptoms | Patients with PHS, n=55 |
|---|---|
| Gestational age (weeks), mean (SD) | 38.5 (1.6) |
| Birthweight (g), mean (SD) | 3317 (549) |
| Male, n (%) | 34 (62) |
| Apgar score 1min, mean (SD) | 8 (2) |
| Apgar score 5min, mean (SD) | 9 (1) |
| Possible birth trauma, n (%) | 9 (16) |
| Spontanous vaginal birth, n (%) | 38 (69) |
| Elective Caesarean section, n (%) | 11 (20) |
| Emegrency Caesarean section, n (%) | 6 (11) |
| Congenital heart disease, n (%) | 5 (9) |
| Cranial vasculopathy, n (%) | 6 (11) |
| Infection/inflammation, n (%) | 11 (20) |
| CRP in patients with infection/inflammation, median (IQR) | 36 (13.5–85.5) |
| Clinical seizure, n (%) | 34 (62) |
| Postnatal day at first seizure, median (IQR) | 2 (1–3) |
| Hypoglycaemia,a n (%) | 10 (18) |
| Irritability/lethargy, n (%) | 8 (15) |
| Muscle tone abnormalities, n (%) | 8 (15) |
| Respiratory distress, n (%) | 20 (36) |
| Complex resuscitation, n (%) | 4 (7) |
Note
- Frequencies and percentages, mean and standard deviation, or median and interquartile range (IQR) were calculated, as appropriate.
- a Hypoglycaemia was defined as blood glucose level <2.6mmol/L. CRP, C-reactive protein.
Affected brain regions
Diagnostic brain MRI was performed at a median age of 5 (IQR: 3–7) days. PHS was typically unifocal (80%) and unilateral (89%). Stroke occurred in the frontal (24%), temporal (24%), parietal (18%), and occipital lobes (18%), as well as in the basal ganglia and/or thalamus (22%). Eight patients (15%) developed hydrocephalus. The median stroke volume was 10.6cm3 (IQR: 2.2–22.8) with a median stroke percentage of the supratentorial brain volume of 2.7% (IQR: 0.7–6.8). Most of the strokes were subcategorized as small (n=34, 62%), with the minority as moderate (n=15, 27%) or large (n=6%–11%). Detailed frequencies of stroke subtypes are shown in Table 2.
|
PHS types and outcomes Total n=55, n (%) |
Follow-up n=50 | CP n=8, n (%) | Cognitive impairment n=7, n (%) | Behavioural problems n=12, n (%) | Visual/hearing problems n=7, n (%) | Language disorder n=9, n (%) | Epilepsy n=9, n (%) | Ventriculoperitoneal shunt n=9, n (%) | Overall chronic developmental sequelae n=30, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Frontal lobe, n=13 (24) | 12 | 0 (0) | 0 (0) | 2 (15) | 1 (8) | 2 (15) | 0 (0) | 1 (8) | 5 (42) |
| Temporal lobe, n=13 (24) | 12 | 4 (33) | 3 (25) | 4 (33) | 2 (17) | 3 (25) | 4 (33) | 3 (25) | 7 (58) |
| Parietal lobe, n=10 (18) | 9 | 4 (44) | 5 (56) | 3 (33) | 2 (22) | 3 (33) | 2 (22) | 2 (22) | 6 (66) |
| Occipital lobe, n=10 (18) | 9 | 1 (11) | 1 (11) | 3 (33) | 3 (33) | 1 (11) | 2 (22) | 2 (22) | 6 (66) |
| Thalamus ± basal ganglia, n=12 (21) | 12 | 3 (25) | 1 (8) | 3 (25) | 2 (17) | 1 (8) | 6 (50) | 3 (25) | 7 (58) |
| Cerebellum, n=2 (4) | 2 | 0 (0) | 0 (0) | 1 (50) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (50) |
| Multiple territories, n=11 (20) | 10 | 4 (40) | 3 (30) | 4 (40) | 3 (30) | 2 (20) | 6 (60) | 4 (40) | 8 (80) |
| Right stroke, n=28 (51) | 24 | 4 (17) | 5 (21) | 6 (25) | 3 (13) | 5 (21) | 4 (17) | 5 (21) | 14 (58) |
| Left stroke, n=21 (38) | 21 | 3 (14) | 2 (10) | 6 (29) | 2 (10) | 1 (5) | 5 (24) | 4 (19) | 9 (43) |
| Bilateral strokes, n=6 (11) | 5 | 1 (20) | 0 (0) | 0 (0) | 2 (40) | 3 (60) | 2 (40) | 0 (0) | 4 (80) |
| Small strokes, n=34 (62) | 32 | 3 (9) | 3 (9) | 6 (19) | 3 (9) | 5 (16) | 6 (19) | 4 (13) | 16 (50) |
| Moderate strokes, n=15 (27) | 13 | 3 (23) | 2 (15) | 6 (46) | 3 (23) | 2 (15) | 3 (23) | 4 (31) | 8 (62) |
| Large strokes, n=6 (11) | 5 | 2 (40) | 2 (40) | 0 (0) | 1 (20) | 2 (40) | 2 (40) | 1 (20) | 4 (80) |
Note
- Frequencies of specific neurological outcome domains per stroke territory subtypes were calculated for infants with long-term follow-up. Percentages are shown in brackets. The numbers of infants with completed follow-up are shown for at least 18 months per stroke territory. Subtypes are presented in the ‘Follow-up’ column. Small strokes, <5% of supratentorial brain volume; moderate strokes, 5% to 10% of supratentorial brain volume; large strokes, >10% of supratentorial brain volume.
- Abbreviation: CP, cerebral palsy.
Neurodevelopmental outcome
Long-term neurodevelopmental outcome data were available in 50 (91%) of the 55 children with the last follow-up visit occurring at a median age of 60 months (IQR: 35–88). Fifteen children were assessed by the Brunet–Lézine test (n=13) or the Bayley Scales of Infant Development, Second Edition (n=2) at a median age of 24 months (IQR: 19–34), while 30 children were evaluated by the Stanford–Binet Intelligence Scales, Fifth Edition at a median age of 74 months (IQR: 60–108). Finally, five children were followed by a paediatric neurologist without using a formal neurodevelopmental assessment owing to their severe neurodevelopmental delay. Of the five infants lost to follow-up, one died from complications of stroke and CHD in the neonatal period, two children with neurodevelopmental findings meeting population norms between 12 months and 18 months of age were lost to further follow-up thereafter, and two patients were lost to follow-up after the neonatal period. All infants lost to follow-up had unifocal, unilateral territorial involvement on MRI not involving the basal ganglia and/or thalami.
Twenty of the 50 infants (40%) with longitudinal follow-up had been developing according to the population norms. In individuals with chronic neurodevelopmental sequelae, the most common outcomes were behavioural problems (24%), epilepsy (22%), and language disorders (18%). As out of the 11 patients (22%) initially diagnosed with epilepsy two subsequently remained seizure free for more than a year without antiepileptic treatment, the rate of active epilepsy was 18% among the individuals in the long-term. Unexpectedly, CP was not as frequent a finding as in neonates with PAIS2 since it was only documented in eight patients (16%); yet in those, CP was more severe as 75% of the patients (six out of eight) presented with tetraparesis. Children developed visual field defect (8%) and hearing loss (6%) infrequently. Thirteen individuals (26%) were recorded with more than one type of developmental sequelae.
Risk factors associated with adverse neurodevelopmental outcome
None of the neonates with frontal lobe PHS developed later CP, cognitive impairment, or epilepsy during the follow-up period, and the overall rate of impaired neurodevelopmental outcome was also less (38% vs 50–66% in other lobes).
Univariate analysis of stroke territory subtypes and their relation to neurodevelopmental outcome domains revealed several associations between brain territorial involvement and neurodevelopmental outcome domains (Table S1). Among the clinical risk factors, CHD conferred significantly higher odds for cognitive impairment and clinical seizure on admission was associated with developing epilepsy beyond the neonatal period (Table S1).
Multivariable logistic regression analysis revealed that parietal lobe PHS is a significant independent predictor of CP (OR 6.7; 95% CI 1.1–41.4) and cognitive deficit (OR 23.6; 95% CI 2.9–194.9) while infants with basal ganglia and/or thalamus involvement had seven times higher odds (95% CI 1.3–37.7) for epilepsy. Strokes involving multiple lobes were significant independent predictors of impairment in several neurodevelopmental domains including CP (OR 6.4; 95% CI 1.0–40.5), epilepsy (OR 10.8; 95% CI 1.8–64.3), and the need for ventriculoperitoneal shunt placement (OR 5.7; 95% CI 1.0–30.7; Tables 3–5).
| Cerebral palsy | Cognitive impairment | |
|---|---|---|
| Parietal PHS, aOR (95% CI) | 6.7 (1.1–41.4) | 23.6 (2.9–194.9) |
| Emergency Caesarean section, aOR (95% CI) | 0.5 (0.03–9.8) | 0.3 (0.009–9.0) |
| CHD, aOR (95% CI) | 1.5 (0.09–25.8) | 6.8 (0.4–110.6) |
| Seizure, aOR (95% CI) | 5.2 (0.5–54.5) | 1.5 (0.1–15.6) |
Note
- Statistically significant associations are shown in bold type.
- Abbreviations: CHD, congenital heart disease; PHS, perinatal haemorrhagic stroke.
| Epilepsy | |
|---|---|
| Thalamus ± basal ganglia, aOR (95% CI) | 7.0 (1.3–37.7) |
| Emergency Caesarean section, aOR (95% CI) | 1.3 (0.09–18.7) |
| CHD, aOR (95% CI) | 2.9 (0.2–49.1) |
| Seizure, aOR (95% CI) | 8.8 (1.0–81.7) |
Note
- Statistically significant associations are shown in bold type.
- Abbreviation: CHD, congenital heart disease.
| Cerebral palsy | Epilepsy | Ventriculoperitoneal shunt placement | |
|---|---|---|---|
| Multiple strokes, aOR (95% CI) | 6.7 (1.0–40.5) | 10.8 (1.8–64.3) | 5.7 (1.0–30.7) |
| Emergency Caesarean section, aOR (95% CI) | 1.8 (0.1–26.7) | 1.3 (0.09–19.7) | –a |
| CHD, aOR (95% CI) | 3.8 (0.2–61.5) | 3.1 (0.2–50.9) | –a |
| Seizure, aOR (95% CI) | 4.7 (0.5–45.2) | 8.3 (0.9–79.8) | 0.5 (0.1–2.7) |
Note
- Statistically significant associations are shown in bold type.
- Abbreviation: CHD, congenital heart disease.
- a On the basis of the findings of the univariate analysis, these variables were not entered into the multivariable analysis.
When a logistic regression model was fitted, there was no relationship between infarct size category and neurodevelopmental outcomes. This finding suggests that the location of the stroke is more important in predicting the neurodevelopmental outcome than the volume of the lesion itself.
Finally, among the clinical risk factors, clinical seizure on admission was associated with the risk of later developing epilepsy (OR 8.8; 95% CI 1.0–81.7). Indeed, neonates with clinical seizure on admission developed epilepsy more frequently than those without clinical seizure on admission (30% vs 5%, p=0.03).
DISCUSSION
We describe the findings of a population-based study of neonates with PHS born at term over an 11-year period in central Hungary. Similarly to that reported in the literature,5, 17 the incidence of PHS was 1 per 5300 live births in our patient population as well.
Few studies have evaluated the association between risk factors and long-term neurodevelopmental outcomes in neonates born at term with PHS.4, 5 However, data on the associations among brain territorial involvement, risk factors, and long-term outcome are lacking. In our study, 40% of the children were developing according to the population norms similarly to the rate of typical development in children with PAIS.18 This finding might be explained by the potential of the developing brain for reorganization. Nevertheless, to the best of our knowledge this is the first study demonstrating that developmental outcomes in neonates born at term with PHS also depends on stroke territory. Indeed, parietal lobe PHS, PHS involving the basal ganglia/thalamus, and multiple lobe PHS were independent predictors of chronic developmental sequelae.
Recent advances in neuroimaging modalities allow the prompt diagnosis of PHS and findings on the brain MRI may also predict neurodevelopmental outcomes. This is of clinical relevance as early prognostication and parental counselling are of great importance in optimizing supportive care and rehabilitation strategies. In addition, providing cautious reassurance to parents when there is a high likelihood of a good outcome might help in coping with the unfolding events. As a novel finding, none of the patients with frontal lobe PHS developed CP, epilepsy, or cognitive impairment during the study period. This finding must be interpreted with caution though, as the status of higher cognitive functions such as emotions, problem solving, impulse control, and social interactions regulated by the frontal lobe mostly become evident during school age or beyond. However, in our study, two-thirds of the children with frontal lobe haemorrhage were followed up until school age. Thus, if the findings on the lack of CP and basic cognitive impairment in children with frontal lobe PHS are confirmed in the future, parents could be reassured about these aspects of neurodevelopmental functions. Of note, two patients with long-term follow-up were lost before the 24-month visit and, therefore, they might have missed the diagnosis of CP.
In over half of the patients (60%), neurodevelopmental outcome was significantly affected though. It is important to note that, while long-term neurodevelopmental outcomes were comparable to those of neonates with PAIS,19 the rate of CP was lower (16% compared with 25%–50% in PAIS20). However, in patients with PHS that developed CP, the condition was more severe as 75% of the infants with CP had tetraparesis. The reason for this finding is unclear. A possible explanation might be that, while a large proportion of patients with PAIS had main branch middle cerebral artery stroke concomitantly involving anatomical structures such as the basal ganglia, the posterior limb of the internal capsule, the thalamus, and the cerebral cortex, in PHS such extended involvement was less frequent. Nevertheless, we have found that parietal lobe PHS and PHS affecting multiple lobes were independent predictors for CP. The increased risk for CP in infants with parietal lobe PHS might stem from the fact that nerve fibres in the corticospinal tract partly originate from and/or cross over the parietal lobe. In patients with multiple lobe PHS, the extent of the damage to the central nervous system probably explains their increased risk for developing CP.
Parietal lobe PHS is not only associated with an increased risk for CP, children with it also had a higher likelihood for cognitive impairment. Again, this finding is similar to those found in PAIS, where the posterior branch middle cerebral artery stroke and posterior cerebral artery stroke increased the risk for cognitive impairment.18 The contribution of the parietal lobe to the recollection aspects of episodic memory21 and language delay20 may relate to this phenomenon, yet exact causative mechanisms need further studies.
Finally, as seen in cases of neonatal central nervous system injury of different aetiology such as hypoxic-ischaemic encephalopathy or PAIS,22 PHS involving the thalamus and/or basal ganglia and multiple lobe PHS increased the risk of epilepsy later. This is in line with previous findings on patients with ischaemic stroke where the risk of developing epilepsy is higher when more substantial damage to the brain occurs.22 Additionally, we also noted that clinical seizures on admission were associated with epilepsy. In these patients, continuous electroencephalogram monitoring and initiation of appropriate early interventions are warranted, especially because epilepsy might negatively affect the plasticity of the developing brain.23
Our study also had limitations. First, the analysis was in part retrospective and performed on data obtained over several years. This carries obvious inherent disadvantages. Second, owing to the uncommon diagnosis of PHS, we collected data from all the neonatal intensive care units in central Hungary with probably somewhat different clinical approaches to patient management. Nevertheless, the harmonization by the universal health care system in Hungary might have attenuated some of the consequences of the multicentre nature of the data collection. Finally, only a smaller number of neonates had bleeding diathesis evaluation. However, testing for the presence of bleeding diathesis in patients with PHS is not routinely suggested in the literature.
Strengths of the study include the large number of neonates enrolled and the confirmation of the diagnosis by MRI in all patients. Second, a large proportion of patients (91%) had detailed neurodevelopmental follow-up for at least 18 months and up to early school age. This is particularly important in studies on perinatal brain injury because speech and other higher cognitive functions can only be appropriately assessed later in childhood.24
CONCLUSIONS
In summary, we found that most of the children affected by PHS exhibit long-term neurodevelopmental sequelae. Our findings also revealed that developmental disabilities were diagnosed less frequently among neonates with frontal lobe PHS. On the other hand, parietal lobe PHS increased the risk of CP and cognitive deficit, and the involvement of the thalamus and/or basal ganglia was also associated with epilepsy. Finally, patients with strokes involving multiple lobes had poor outcome, and seizures on admission were associated with the diagnosis of epilepsy beyond the neonatal period. As PHS is a rare condition, rigorously controlled data collection by national or regional registries using an adequate oversight structure remain the main avenues for hypothesis generation and perhaps even testing for treatment and outcome of neonates with this condition.
ACKNOWLEDGEMENTS
We thank the health professionals in the neonatal intensive care units of the First Department of Pediatrics and of the Department of Obstetrics and Gynecology, Semmelweis University, as well as the medical practitioners and nurses in the neonatal intensive care unit of Szent János Hospital and North Buda United Hospitals for treating neonates with the diagnosis of perinatal stroke and for providing data for this study. Financial support for this work was provided by the Semmelweis University grant (EFOP-3.6.3-VEKOP-16-2017-00009) and by the New National Excellence Program of the Ministry for Innovation and Technology from the Source of the National Research, Development and Innovation Fund (ÚNKP-21-3-II-SE-5).
CONFLICT OF INTEREST
The authors have stated that they had no interests that might be perceived as posing a conflict or bias.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




