Repeated onabotulinum neurotoxin A injections for drooling in children with neurodisability
Abstract
enAim
To evaluate the effect of repeated onabotulinum neurotoxin A injections for the treatment of drooling in children with neurodisabilities.
Method
This was a retrospective cohort study, in which the first, second, and third onabotulinum neurotoxin A injection were compared within children treated between 2000 and 2020. Primary outcomes included drooling quotient, visual analogue scale (VAS), and treatment success defined as ≥50% reduction in drooling quotient and/or VAS 8 weeks after treatment. Each outcome was obtained at baseline and 8 weeks posttreatment.
Results
Seventy-seven children were included (mean age at first injection: 8y 3mo, SD 3y 7mo, range 3–17y; 44 males, 33 females; 51.9% with cerebral palsy, 45.5% wheelchair-bound). The objective (drooling quotient) and subjective (VAS) effect after the second injection was lower compared to the first injection. The third injection showed less objective and significantly less subjective effect compared to the first injection. An overall success rate of 74.0%, 41.6%, and 45.8% were found for the first, second, and third injection respectively.
Interpretation
Although onabotulinum neurotoxin A remained effective throughout the entire treatment course, there is less effect of subsequent onabotulinum neurotoxin A injections compared to the first. Although there might be a loss of effect after repeated injections, there is continued improvement for most children.
What this paper adds
- Repeated injections show a diminished treatment effect after the second injection.
- A continued improvement is seen in most patients.
Inyecciones repetidas de neurotoxina onabotulinum A para el babeo en niños con neurodiscapacidad
esObjetivo
Evaluar el efecto de las inyecciones repetitivas de neurotoxina onabotulinum A, para el tratamiento del babeo en niños con neurodiscapacidades.
Método
Este fue un estudio de cohorte retrospectivo, en el que se compararon la primera, segunda y tercera inyección de toxina onabotulinum A, en niños tratados entre 2000 y 2020. Los resultados primarios incluyeron cociente de babeo, escala analógica visual (EVA) y éxito del tratamiento definido como ≥ Reducción del 50% en el cociente de babeo y / o EVA 8 semanas después del tratamiento. Se obtuvieron los resultados al inicio del estudio y 8 semanas después del tratamiento.
Resultados
Se incluyeron 77 niños (edad media a la primera inyección: 8 años 3 meses, (desviacion estandar 3 años 7 meses), rango 3–17 años; 44 varones, 33 mujeres; 51,9% con parálisis cerebral, 45,5% en silla de ruedas). El efecto objetivo (cociente de babeo) y subjetivo (EAV) después de la segunda inyección fue menor en comparación con la primera. La tercera inyección mostró un efecto menos objetivo y significativamente menos subjetivo en comparación con la primera inyección. Se encontró una tasa de éxito global de 74,0%, 41,6% y 45,8% para la primera, segunda y tercera inyección, respectivamente.
Interpretación
Aunque la toxina onabotulinum A siguió siendo eficaz durante todo el ciclo de tratamiento, las inyecciones posteriores de neurotoxina A de onabotulinum tienen menos efecto en comparación con la primera. Aunque puede haber una pérdida de la magnitud del efecto después de inyecciones.
Injeções repetidas de neurotoxina onabotulínica A para sialorréia em crianças com neurodeficiência
ptObjetivo
Avaliar o efeito de injeções repetidas de neurotoxina onabotulínica A para o tratamento de sialorréia em crianças com neurodeficiência.
Método
Este foi um estudo de coorte retrospectivo, no qual a primeira, segunda e terceira injeções de neurotoxina onabotulínica A foram comparadas em crianças tratadas entre 2000 e 2020. Desfechos primários incluíram o quociente de salivação, escala visual análoga (EVA), e sucesso do tratamento definido como redução ≥50% no quociente de salivação e/ou EVA 8 semanas após o tratamento.
Resultados
Setenta e sete crianças foram incluídas (média de idade na primeira injeção 8a 3m, DP 3a 7m, variação 3–17a; 44 do sexo masculino, 33 do sexo feminino; 51,9% com paralisia cerebral, 45,5% usuários de cadeiras de rodas). O efeito objetivo (quociente de salivação) e subjetivo (EVA) após a segunda injeção foram menores comparados com a primeira injeção. A terceira injeção mostrou menos efeito objetivo e significativamente menos efeito subjetivo comparada com a primeira injeção. Uma taxa geral de sucesso de 74,0%, 41,6%, e 45,8% foi encontrada para a primeira, segunda e terceira injeção, respectivamente.
Interpretação
Embora a neurotoxina onabotulínica A tenha permanecido efetiva por todo o curso do tratamento, há menos efeito nas injeções subsequentes de neurotoxina onabotulínica A comparadas com a primeira. Embora possa haver perda de efeito após injeções repetidas, há melhora contínua em um vasto grau de crianças.
What this paper adds
en
- Repeated injections show a diminished treatment effect after the second injection.
- A continued improvement is seen in most patients.
This article's abstract has been translated into Spanish and Portuguese.
Follow the links from the abstract to view the translations.
Abbreviations
-
- Nab
-
- Neutralizing antibodies
-
- VAS
-
- Visual analogue scale
Drooling, defined as the unintentional loss of saliva, is considered atypical when it persists after the age of 4 years.1 Children with cerebral palsy or other neurodisabilities often suffer from drooling.2 Drooling interferes with social interaction, and leads to intellectual underestimation and low self-esteem.3 When treating children with neurodevelopmental disabilities, drooling is easily overlooked. However, patients often consider it as one of their worst affections in relation to social interaction.3
Onabotulinum neurotoxin A (Botox; Allergan, Nieuwegein, the Netherlands) is currently the first-line interventional treatment for drooling because it is effective in the majority of patients, minimally invasive, and there is limited risk for severe adverse events.4 Yet, the effect is temporary, and to maintain effect injections are required at least once per year.4, 5 Moreover, onabotulinum neurotoxin A injections generally take place under general anaesthesia and one injection necessitates multiple hospital visits including an anaesthesiologist visit and to monitor the effect on drooling.6, 7 These drawbacks may lead to discontinuation of treatment. Another possible reason for discontinuation is the diminished or lack of effect after repeated injections.8 Neutralizing antibodies (NAb) or parotid gland compensation may play a role in the decrease of the effect after repeated injections, while alternative literature hypothesize gland atrophy and subsequently a permanent reduction in drooling.9-13
The aim of this study is to evaluate the effect of repeated onabotulinum neurotoxin A injections to reveal the usefulness of repeated injections for the treatment of drooling in neurodisabilities.
Method
A retrospective observational study was conducted in children who were treated with at least two subsequent, equally dosed onabotulinum neurotoxin A injections. The effect of onabotulinum neurotoxin A injections was compared within participants to reduce confounding factors and increase reliability in a heterogeneous population.
Study population
Children aged 4 years or older treated for drooling at the Radboud University Medical Center, Nijmegen, the Netherlands, between 2000 and 2020 were eligible. Children treated for anterior or antero-posterior drooling with at least two onabotulinum neurotoxin A injections with identical dose (25IU/gland) delivered at the same gland(s) were included in this study. The first injection in this study was the first onabotulinum neurotoxin A injection that was administered for the treatment of drooling.
Both injections in the submandibular glands and submandibular and parotid glands (combined injection) were included. In our centre, the parotid glands are not routinely treated because the submandibular glands are thought to be responsible for 70% of the saliva in a resting situation.14 Children were excluded if dosages of the first or second injection were unknown. Assessments of subsequent injections different in onabotulinum neurotoxin A dose or gland localization were excluded, but previous equal injection within the same children were included. Also, children with a progressive condition (i.e. mitochondrial diseases and metabolic diseases) were excluded. The data were saved in an anonymized protected web-based database according to good clinical practice. This study was approved by the medical ethical committee of the Radboud University Nijmegen Medical Centre (CMO: 2020-6145).
Outcome measures
Regular standardized assessments were made by a specialized speech and language therapist and included the severity of drooling using the drooling quotient and a visual analogue scale (VAS) before the injection (baseline) and 8 weeks after the injection. The most recent baseline measurement was used in case of multiple assessments.
Primary outcomes
The drooling quotient and VAS at the 8-week assessment were used as primary outcome variables. Reduction was defined by the baseline minus the outcome at the 8-week assessment. The assessment at 8 weeks was chosen as onabotulinum neurotoxin A injections have shown a maximum effect of clinical reduction in drooling between 2 and 8 weeks.6 A clinically meaningful change (treatment success) was defined as ≥50% reduction in drooling quotient and/or VAS 8 weeks after treatment.4, 5, 15, 16 Treatment success was calculated by either VAS or drooling quotient alone in case of a missing drooling quotient or VAS.
The drooling quotient is a direct, observational, semi-quantitative method validated to evaluate anterior drooling.17 It is used as measurement for the severity of drooling by observing actual salivation over a 5- or 10-minute period of time during activity or rest. Both are equally validated procedures, but assessment during activity has been shown to distinguish drooling severity better than during rest.17 In this study, the drooling quotient during activity was used. The type of activity varied from playing with blocks and using electronic devices, and was adjusted based on the child’s abilities and interests. The 5- and 10-minute versions were both accepted as they can be used interchangeably.17
Subjective drooling is assessed by a VAS for severity of drooling. Parents or caregivers are asked to indicate drooling severity on a line ranging from 0 (no drooling) to 100 (being the most severe outcome) for the severity of drooling over the past 2 weeks.18
Secondary outcomes
Adverse events documented are described. In addition, the drooling severity and drooling frequency scale are presented in Table S1 (online supporting information).
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics 25 (IBM Corp., Armonk, NY, USA). For each outcome measure (drooling quotient or VAS at the 8-week assessment), a multilevel linear model was used to account for the relation between the number of repeated injections (ordinal variable) within individuals. Sample size estimation was not suitable because of the retrospective nature of the study. This model included fixed effects for baseline (drooling quotient or VAS respectively), age, sex, cerebral palsy, degree of mobility (defined by ambulant or non-ambulant), developmental age (defined as <4y or >4y), and epilepsy and random effects for intercept and participants. Predictors were deemed as fixed as they generally did not change through time. There was no need for imputation of missing data in the outcome variables as multilevel linear models are competent in handling these missing values.19
A paired sample t-test and repeated analysis of variance (ANOVA) was used to analyse the differences in baselines of drooling quotient and VAS and the differences in the 8-week assessments between consecutive injections.
To evaluate potential bias due to a difference in duration between baseline and its respective follow-up assessment (as the 8-wk assessment does not always take place at exactly 8wks after injection), a paired sample t-test was performed. A paired sample t-test was also performed to evaluate differences between injections in the duration between the onabotulinum neurotoxin A injection and the subsequent baseline measurement, because the previous injection may influence the next baseline through ongoing effect.
Results
Of all the children treated with onabotulinum neurotoxin A injections for drooling between 2000 and 2020, 85 were treated with at least two subsequent, identical onabotulinum neurotoxin A injections. One patient turned 4 years old 3 weeks after the first injection and was therefore included for analysis. Nine children were excluded because of a progressive condition or unknown first or second dosage of onabotulinum A injection. In total, 77 children were included in the analysis (mean age 8y 3mo, SD 3y 7mo, range 3–17y; 44 males, 33 females). Twenty-four children (31.2%) had an identical third onabotulinum neurotoxin A injection as well (Table 1). Out of the 356 drooling quotient and VAS measurements, there were 29 (8.1%) missing values for drooling quotient and 29 (8.1%) missing values for VAS. The mean drooling quotient and VAS scores per assessment are shown in Figure 1.
| Patient characteristic | n (%) |
|---|---|
| Age, mean, SD (range), y:mo | 8:3, 3:7 (3–17) |
| Sex | |
| Male | 44 (57.1) |
| Female | 33 (42.9) |
| Main diagnosis | |
| Cerebral palsy | 40 (51.9) |
| Non-cerebral palsya | 37 (48.1) |
| Degree of disabilityb | |
| Ambulant | 42 (54.5) |
| Non-ambulant | 35 (45.5) |
| Developmental age | |
| <4y | 49 (63.6) |
| >4y | 28 (36.4) |
| Epilepsy | |
| Diagnosed with epilepsy | 42 (54.5) |
| No epilepsy | 35 (45.5) |
| Type of injection | |
| Submandibular | 72 (93.5) |
| Submandibular and parotid | 5 (6.5) |
- a Non-cerebral palsy consisted mainly of developmental disorders based on a syndrome, metabolic, or genetic disorder.
- b The degree of disability in children with cerebral palsy was based on the Gross Motor Function Classification System (GMFCS). A GMFCS level of I–III was classified as ambulant and a GMFCS level of IV–V was classified as non-ambulant.
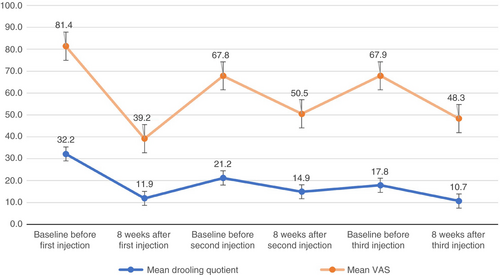
Our analysis showed that the number of injection (injection 1, 2, or 3) was associated with a lower value indicating improvement on the drooling quotient (p=0.016) and VAS (p=0.002) after 8 weeks. This indicates that the subjective (VAS) and objective (drooling quotient) reduction is lower after the second injection than after the first injection (slope [b]=–5.20; 95% confidence interval [CI]=−8.72 to −1.68 and b=−12.74; 95% CI=−19.78 to −5.70 for drooling quotient and VAS respectively). The reduction (∆) in VAS was lower after the third injection compared to the first injection (b=−10.35; 95% CI=−20.62 to –0.87) (Fig. 2). The analysis was corrected for age: 8 years 2 months for (∆) drooling quotient and 8 years for (∆) VAS. The reduction in drooling quotient did not differ significantly between the third and first injection.
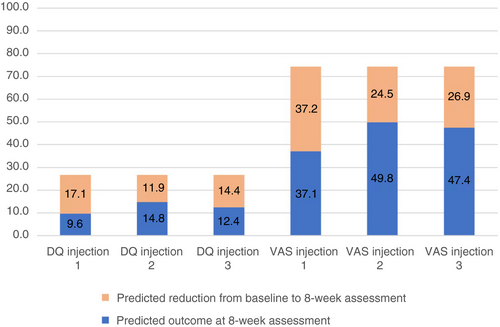
Significant predictors for both drooling quotient and VAS were baseline and age. Namely, a higher baseline drooling quotient or VAS predicted a higher outcome (drooling quotient and VAS) after 8 weeks, as well as a greater reduction in drooling quotient and VAS, and a higher age was associated with less reduction in both drooling quotient and VAS. The degree of motor disability predicted VAS; non-ambulant children (defined by a Gross Motor Function Classification System level of IV or V) have a greater reduction in VAS than ambulant children. No association was found for sex, developmental age, cerebral palsy, and epilepsy.
The 8-week assessment of the drooling quotient and VAS after the second injection and the VAS after the third injection were slightly but significantly higher than after the first injection (Fig. 1). The second and third VAS baselines were significantly lower than the first VAS baseline. In case of drooling quotient, only the second baseline was significantly lower than the first baseline. The third drooling quotient baseline was not significantly different compared to baseline 1.
An overall clinically meaningful change was observed in 74.0%, 41.6%, and 45.8% after the first, second, and third injection respectively.
A paired t-test showed no significant time difference in duration of assessment between the injections. The mean duration between the first injection and the subsequent baseline was 50.3 weeks (SD 40.8). The mean duration between the second injection and third baseline was 61.6 weeks (SD 52.4).
A repeated ANOVA with a Greenhouse–Geisser correction showed no significant difference of the duration between the day of injection to the actually performed 8-week follow-up per each injection (first injection 9.1wks, SD 2.0wks, second injection 9.0wks, SD 1.5wks, third injection 9.5wks, SD 3.8wks).
Potential complications and adverse events
Adverse events after each onabotulinum neurotoxin A injection are reported in Table 2. No serious adverse events were reported. χ2 tests show no significant differences between injections in the overall frequency of adverse events.
| Injection | #1 | #2 | #3 |
|---|---|---|---|
| Adverse events, n (%) | 12 (15.6) | 10 (13.0) | 4 (16.7) |
| Xerostomiaa | 5 | 3 | 1 |
| Diminished feeding or discomfort during feeding | 4 | 1 | 2 |
| Diminished drinking | 0 | 1 | 1 |
| Saliva swallowing problemsb | 6 | 7 | 2 |
| Pneumonia (possibly due to aspiration) | 0 | 1 | 0 |
| Teeth grinding | 0 | 0 | 1 |
| Bruises injection location | 1 | 0 | 0 |
- a Xerostomia included dry mouth and dry lips.
- b Problems with saliva swallowing included changes in viscosity, increased choking/gagging, and discomfort during swallowing.29
Discussion
This study evaluated the effect of repeated onabotulinum neurotoxin A injections in children with drooling. Although there was continued improvement after repeated injections in the vast majority of children, this study also revealed a significant (but slight) objective (drooling quotient) and subjective (VAS) decrease in effect when comparing the first injection to the second and third injection. Moreover, treatment success rates seemed to decline between the first and second, but not between the second and third injection. Each injection showed similar adverse events and no serious adverse events, supporting the safe use of onabotulinum neurotoxin A, also after repeated injections.
There are several explanations for the decrease in effect after repeated injections. The second and third baselines for both VAS and drooling quotient were lower than the first baseline. This could be due to an ongoing treatment effect at the baseline measurement of the subsequent injection, gland atrophy, or children who mature and outgrow drooling. However, baselines before each injection have been corrected for in the analysis. Moreover, although one could expect a lower 8-week outcome after the second injection due to: (1) a lower baseline, (2) survivor bias, or (3) presumably gland atrophy, both 8-week drooling quotient and VAS were slightly higher at the second injection compared to the first injection. Therefore, the decrease in effect cannot be neglected.
Another explanation for the decrease in effect could be parotid compensational hypersalivation. One study mentioned dilated ducts and more mucus accumulation in parotid glands after resection of the submandibular glands in rabbits.20 Another study revealed increased parotid salivary flow rate after botulinum neurotoxin A injections in the submandibular glands, which indicated parotid gland compensation.13 Correspondingly, most children in our study received onabotulinum neurotoxin A injections in the submandibular glands only, leaving the parotid glands untreated, which could have led to compensational hypersalivation of the alternative glands. This would, however, have resulted in higher subsequent baseline outcomes which it did not.
Alternatively, NAb against botulinum neurotoxin A have been suggested to play a role in secondary treatment failure, mostly injected in muscles to decrease spasticity.10-12 One case report described secondary non-response of botulinum neurotoxin B injections in drooling after three successful injections. NAb against neurobotulinum toxin B were identified and additional onabotulinum neurotoxin A injection did not show treatment response either.21 Some children included in this study may also have been treated with botulinum neurotoxin A injections in their limbs because of spasticity which may have induced NAb before the first intraglandular onabotulinum neurotoxin A injection. In a recent cross-sectional study, the prevalence of NAb against onabotulinum neurotoxin A in children treated for spasticity was found to be 15.2%. Also, the study found the most influencing factor to be the single dose per session.9 As this study was done retrospectively, data on whether children were treated with botulinum neurotoxin injection for other indications could not be assessed thoroughly.
Although this study certainly revealed a significant reduction in effect between the second and first injection, there was no ongoing decrease in reduction after the second injection while NAb development during onabotulinum neurotoxin A treatment most likely increases with treatment duration.9 Yet, the study was subject to survivor bias, so children with loss of treatment effect after the second injection will presumably not undergo a third injection, indicating that only responders are included for subsequent injections.
In summary, there was presumably an ongoing effect of onabotulinum neurotoxin A on drooling which resulted in a lower second and third baseline outcome. Though there was no ongoing decrease in objective effect after the third injection, the decrease in effect compared to the first injections is not neglectable. Future research should evaluate whether NAb play a role in the decrease in effect after repeated botulinum neurotoxin injections in a prospective setting. Despite the decrease in effect after repeated injections, onabotulinum neurotoxin A still improved drooling in our children during the entire treatment course (Fig. 1).
In case of lack of response after repeated injections, combined injections (submandibular and parotid) could be considered. For children aged older than 10 years or with low expectation to ‘outgrow’ drooling, we consider surgery.
Unlike the objective effect, the subjective effect of the third injection decreased as well. The lack of objective effect could be due to the small sample size, parental expectations, or the burden on carers which might have outweighed the benefit of the procedure. Patients may have forgotten the severity of drooling before treatment, and, as such, have a high VAS score once the drooling has resurfaced again.
Studies which evaluated the effect of repeated botulinum neurotoxin A in children are scarce.22 One prospective study evaluated the effect of repeated botulinum neurotoxin A and B in parotid and submandibular glands for drooling in children with neurodegenerative disorders. Five out of 30 children showed no effect (decrease in Teacher Drooling Scale) on repeated injections, but it was not mentioned at which injection, so we cannot draw definitive conclusions from this article.22 Another study identified a significant decrease in effect after repeated botulinum neurotoxin B injections for the treatment of drooling, but only after the fifth injection. This study, however, only included a small population of 17 children.23 Furthermore, studies on the effect of repeated botulinum neurotoxin A injections for other indications (e.g. spasticity, epiphora, and axillary hyperhidrosis) also showed a consistent effect.24-27 One prospective observational study found a diminished effect after two to three injections in lower extremity spasticity in children with cerebral palsy.28 Nonetheless, botulinum neurotoxin A was given for other indications and this finding is therefore not fully applicable to our aim. Still, it does show the decrease of effect after repeated use of botulinum neurotoxin A in children.
This study also found a treatment success of 74%, defined as ≥50% reduction in drooling quotient and/or VAS at 8 weeks compared to baseline, in this population for the first injection. This is relatively high compared to: (1) treatment success (53–70%) of previous studies at the same centre using similar definitions and (2) treatment success of the second and third injections in the current cohort.4, 5, 29, 30 Two studies, however, evaluated the effect of onabotulinum neurotoxin A in children who received an average of 1.6 to 2.3 previous injections whereas our success rate is calculated solely based on the first injection.4, 5 As our inclusion criteria only allowed children with repeated injections, survivor bias may have caused a higher treatment success rate for the first injection.
In addition, non-ambulant children show better subjective effect compared to ambulant children, but were treated with fewer injections. Reasons for discontinuation of treatment, despite satisfactory response rates, could include the practical burden of repeated hospital visits for wheelchair-bound children.
Age was negatively correlated with treatment effect: older children respond less. This is in contrast to a recent study in which age (more mature) was considered a predictor for successful two-duct ligation treatment of anterior drooling.15 We cannot fully explain this, but one theory is that older children had more exposition to previous onabotulinum neurotoxin A which could have induced NAb. Alternatively, expectations of carers may be higher after repeated injections. However, this may not be the only reason as the objective measure is also negatively correlated with age.
The main strengths of the study were the number of children included, the standardized subjective and objective measurements, and the homogeneity of treatment characteristics. There are, however, also some limitations to the study. First, as this study was done retrospectively, treatment was clinically driven. Children with a good response to onabotulinum neurotoxin A will most likely continue with the same treatment, potentially leading to survivor bias. This may result in an overestimation of the effect of the second and third injection. Second, there was a relatively small number of children included with three injections, which means that the study had relatively little statistical power to address the effect of the third injection. Lastly, our definition of clinical success is defined by at least 50% decrease in the drooling quotient or VAS, which may result in limitations because of its dichotomous nature.30
Conclusion
Onabotulinum neurotoxin A remained effective throughout the entire treatment course and induced lower baseline levels as well. However, this study reveals a reduced subjective and objective effect of subsequent injections compared to the first onabotulinum neurotoxin A injection and, as such, there is possibly a limit to the effect of repeated onabotulinum neurotoxin A for the treatment of drooling in children with neurodisabilities. This reduction may be (partially) explained by NAb or compensational salivation by alternative glands. Importantly, although there might be a loss of effect after repeated injections, there is continued improvement in the vast majority of children. Future prospective research should further evaluate the precise clinical role of NAb and compensational salivation after repeated onabotulinum neurotoxin A injections for the treatment of drooling.
Acknowledgements
Peter Jongerius performed most of the botulinum neurotoxin injections. We want to thank Corrie E Erasmus, Marloes Lagarde, and Sandra de Groot for their contribution in patient care. This study and its analysis were subsidized by Johanna Kinderfonds, Arnhem, the Netherlands, Phelps Stichting voor spastici, Bussum, the Netherlands, and Stichting Rotterdams Kinderrevalidatie Fonds Adriaanstichting, Rotterdam, the Netherlands. All authors report no conflict of interest. None of the authors reported financial disclosures.
Open Research
DATA AVAILABILITY STATEMENT
The protocol, anonymized demographics, and data regarding study outcomes will be shared on request from any qualified investigator.




