A practical approach to acute hemiparesis in children
Abstract
Acute hemiparesis in children is a common clinical syndrome presenting to a variety of care settings. The recognition and the differential diagnosis is challenging, particularly in young children. Arterial ischaemic stroke (AIS) is the primary diagnosis to be considered as this requires emergency investigations and management; however, there are several conditions collectively described as ‘stroke mimics’ that need consideration. Accurate diagnosis is essential for appropriate management. Clinical data combined with neuroimaging are important for accurate diagnosis and management. This review and the accompanying illustrative case vignettes suggest a practical approach to differential diagnosis and management of children presenting with acute hemiparesis.
What this paper adds
- Acute hemiparesis in children is a clinical syndrome with diverse causes.
- A systematic approach including clinical data and neuroimaging can help establish accurate diagnosis.
Abbreviations
-
- AIS
-
- Arterial ischaemic stroke
-
- MCA
-
- Middle cerebral artery
Acute hemiparesis is a common clinical syndrome in children that may present to primary, secondary, or tertiary health care professionals. Challenges in clinical practice include difficulty in recognizing acute hemi-syndromes in young children or in children with subtle presentations. For example, whereas older children with vascular stroke syndromes would usually meet the ‘FAST’ (face, arm, speech, and time) criteria used by paramedics to recognize stroke in adults in the community (where patients with face, arm, and speech problems are triaged as likely cases of stroke), younger children may present with a more subtle motor deficits, and diffuse neurological signs such as encephalopathy. Subtle clinical presentations ‘soft signs’ are also a feature of acute vascular events in children with sickle-cell disease, and it is important to consider a central cause in these children rather than attributing limping or writing difficulty to pain. Transient ischaemic attack as a clinical syndrome is not very useful in younger children because of subtle presentations.1
This review considers the clinical approach to a child with lateralized weakness in a practical way, considering a staged approach towards clinical assessment and investigation to reach a diagnosis. It does not consider management of specific individual disorders as this is comprehensively covered elsewhere.
Is it central or peripheral?
This may seem an obvious point, but in our experience insufficient effort is sometimes made to distinguish between central and peripheral deficits, especially in the emergency room. The difficulty of ascertaining the cause of limb weakness in a child with sickle-cell disease, at risk of painful crises or osteomyelitis, has already been discussed. It is important not to be overly swayed by a history of trauma: we have seen several children who sustained falls, presumably as a result of acute weakness, whose orthopaedic or soft-tissue injuries were managed without appreciation of the central neurological signs. Naturally, a competent neurological examination is key here; facial weakness, encephalopathy, or seizures are also all signs that should point the clinician to the central nervous system (CNS). Specific points in neurological examination are helpful indicators of a central lesion. It is important to note that the typical signs of increased tone and exaggerated reflexes on the affected side are late signs in acute hemiparesis. The absence of these signs is not sufficient to exclude a central lesion. In a comatose patient, asymmetry of posture with external rotation of the leg at hip joint may be the only indication of a hemiplegia. Similarly, head version or gaze deviation to one side is a clue to a hemispheric lesion. In older, cooperative children, it may be possible to demonstrate the typical pyramidal distribution of weakness in the limbs, namely predominant weakness of shoulder abduction, elbow extension, and wrist extension in the upper limb, and hip flexion, knee flexion, and ankle dorsiflexion in the lower limb. Careful examination to establish the level of lesion in facial weakness is valuable. Idiopathic lower motoneuron facial palsy (Bell's palsy), particularly if partial and sparing upper face, can be mistaken for an ipsilateral upper motoneuron facial palsy as a component of hemiplegia due to stroke (and vice versa). Facial palsy contralateral to the hemiplegia localizes the lesion to pons and the posterior circulation in vascular events.
The signs in younger children are often more diffuse; however, asymmetry of movements, posture, or tone should be evident on close observation.
Is it a neurosurgical emergency?
Studies1-5 in a variety of healthcare settings have shown that there is lack of appreciation of the potentially life-threatening causes of acute weakness in children, with major delays in imaging and diagnosis, even where health care is not limited by resources. The immediate priority in the acute setting is to identify or exclude a neurosurgical emergency: for example acute intracranial haemorrhage, massive arterial ischaemic stroke (AIS), brain tumour, or acute hydrocephalus. Brain computed tomography (CT) will enable these to be identified or excluded. Magnetic resonance imaging (MRI) has better temporal, spatial, and diagnostic resolution but is unlikely to be available as an emergency in most settings. The decision about what imaging modality to select, and with what degree of urgency, is really a matter of clinical judgement. However, in our view any child with encephalopathy or unstable vital signs needs stabilization and brain imaging as a matter of urgency. Inevitably a proportion of children will have both a CT and an MRI; however, unless emergency MRI is easily available, CT will provide sufficient information to manage a sick child safely.
What is the diagnosis?
Acute hemiparesis is the most common presentation of the vascular stroke syndromes, particularly AIS and intraparenchymal haemorrhage. However, around 20% to 30% of children with acute hemiparesis will have a non-vascular diagnosis; collectively these conditions are termed ‘stroke mimics’.6 This is in contrast to adults, where the frequency of stroke mimics is much lower, and it is fairly safe to consider acute hemiparesis as usually having a vascular aetiology. This difference between adults and children is often not readily appreciated by non-paediatricians. Table 1 lists some clinical features that may be helpful in differentiating between vascular stroke syndromes and stroke mimics; however, these are not reliable and imaging is essential to make a clear diagnosis. For any child with hemiplegia, imaging should be performed as soon as possible as this is critical for correct further management.
| Condition | History | Examination | Investigations |
|---|---|---|---|
| Intracranial haemorrhage |
Abrupt onset. Headache, vomiting |
Reduced level of consciousness, rapid evolution of neurological findings. Note potential neurocutaneous signs, e.g. in hereditary haemorrhagic telangiectasia | Imaging may identify aetiology, e.g. arteriovenous malformation |
| Arterial ischaemic stroke | Abrupt or stuttering onset; latter associated with arteriopathy. Preceding chicken pox, febrile illness, cardiac condition, (minor) head injury, neck injury | Consciousness generally preserved unless large middle cerebral artery stroke/brainstem infarct. Note neurocutaneous features, e.g. neurofibromatosis type 1 | Arteriopathy may be evident on imaging |
| Meningitis/meningo-encephalitis | Fever, headache systemic symptoms | Fever, neck stiffness, reduced level of consciousness | Imaging shows multiple areas of involvement, not conforming to vascular territory, variable diffusion-weighted imaging characteristics, cerebrospinalfluid shows pleocytosis, increased protein, organism may be isolated |
| Acute disseminated encephalomyelitis | Fever, headache systemic symptoms; subacute onset | Fever, neck stiffness, reduced level of consciousness, focal neurological deficits | Mainly white matter involvement in a patchy distribution, variable diffusion characteristics, usually not restricted |
| Posterior reversible encephalopathy syndrome | Seizures, drugs (cyclosporine A, tacrolimus, cyclophosphamide) | Reduced level of consciousness, arterial hypertension | Patchy grey and white matter involvement, usually free diffusion |
| Bleeding or oedema associated with CNS tumour | Preceding chronic/subacute history of neurological symptoms followed by acute change | ||
| Metabolic | History of developmental delay, hypotonia, fatigue, encephalopathy, vomiting | Movement disorder, seizures | Symmetric basal ganglia/brainstem involvement, white mater changes, changes not confined to a vascular territory, abnormal biochemistry |
| Reversible cerebral vasoconstriction syndrome | History of thunderclap headache, history of vasoactive drugs | Focal neurological deficit | Vascular imaging shows diffuse segmental narrowing of intracranial arteries |
| Hemiplegic migraine | Headache, history of recurrent episodes with normal imaging, family history of hemiplegic migraine | Hemiparesis generally resolves in 72h (rarely longer) | Normal imaging; genetic testing may be considered |
| Postictala | Preceding focal motor seizure, history of epilepsy | Normal imaging, electroencephalography may be helpful | |
| Non-organica | Fluctuating weakness, disproportionate effect on function | Examination findings not confirming to neuroanatomical localization | Normal investigations |
- a It is important to remember that both postictal Todd's paralysis and non-organic hemiparesis are diagnostic of exclusion, particularly in younger children, and full assessment including investigations is required to exclude other causes. CNS, central nervous system.
An abrupt onset is suggestive of a vascular stroke syndrome; in particular, headache and coma are highly suggestive of intracranial haemorrhage. Arteriopathic AIS may have a stuttering onset.7 Encephalopathy is suggestive of CNS infection or acute disseminated encephalomyelitis. A reduced conscious level is a sinister sign in AIS, indicating significant raised intracranial pressure that can be secondary to ‘malignant’ middle cerebral artery (MCA) territory infarction or associated with brainstem or posterior fossa infarction. Headache can be a feature of AIS (especially with arterial dissection), sinovenous thrombosis, CNS infection, or hemiplegic migraine. It is important to recognize that hemiplegic migraine is a diagnosis of exclusion, especially in the index episode. ‘Thunderclap’ headache is classically a feature of subarachnoid haemorrhage; it is also a feature of reversible cerebral vasoconstriction syndrome, which is associated with stroke (ischaemic and haemorrhagic) in 10% of cases. Reversible cerebral vasoconstriction syndrome is commonly associated with ingestion of vasoactive drugs such as nasal decongestants. Seizures are a common feature of posterior reversible encephalopathy syndrome, also called reversible posterior leukoencephalopathy syndrome, CNS infection, and cerebral sinovenous thrombosis.
Important points in the clinical assessment include cutaneous examination (e.g. for features of neurofibromatosis type 1 or hereditary haemorrhagic telangiectasia), a comprehensive cardiovascular examination, including careful evaluation of peripheral pulses and blood pressure, and systemic features of sepsis, endocarditic, or an underlying genetic or syndromic disorder. A drug history is relevant to many of the differential diagnoses under consideration.
Children presenting with hemiparesis on the background of pre-existing conditions with high risk of AIS, for example sickle-cell disease, congenital heart disease (especially after surgery), or pre-existing arteriopathy, are likely to have AIS but not invariably so. For example, posterior reversible encephalopathy as well as intracranial haemorrhage can be seen in sickle-cell disease. Intracranial haemorrhage needs to be excluded in patients with cardiac disease who are on anticoagulation treatment. Children with moyamoya disease can have prolonged transient ischaemic attack without a completed stroke following triggers such as hyperventilation or dehydration. Hypoglycaemia in children with insulin-dependent diabetes mellitus can manifest with neurological symptoms including hemiplegia. Urgent neuroimaging is critical in these settings to establish the diagnosis and formulate management plans.
It is important to identify venous infarction as a cause of hemiplegia as this will have important management implications. Venous infarction is often haemorrhagic and may be mistaken for primary intraparenchymal haemorrhage. The distribution of injury on imaging may be suggestive. A plain CT scan that is almost universally performed as the first investigation requires careful examination as this will often provide diagnostic information.
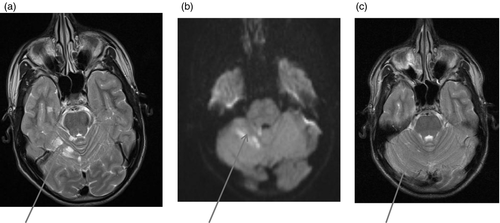
Figures 1-7 provide case vignettes illustrating the range of conditions that may present with hemiplegia.
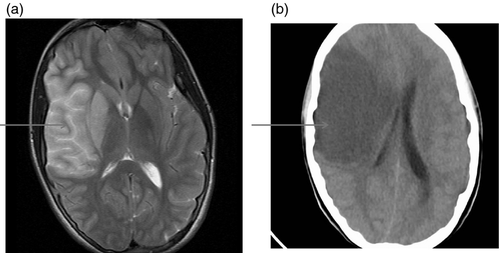
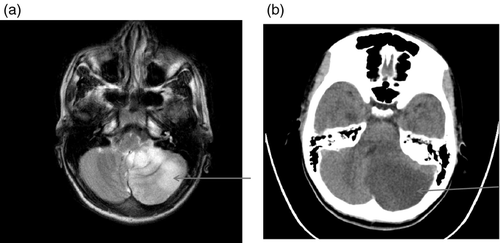
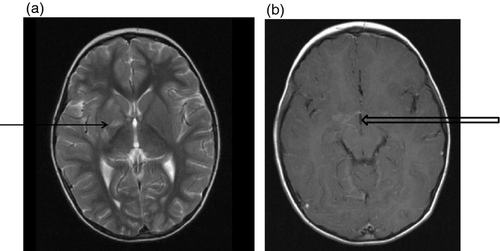
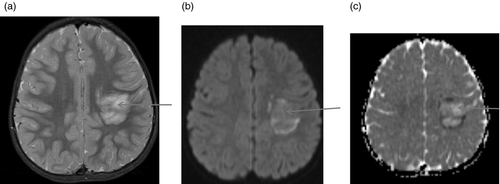
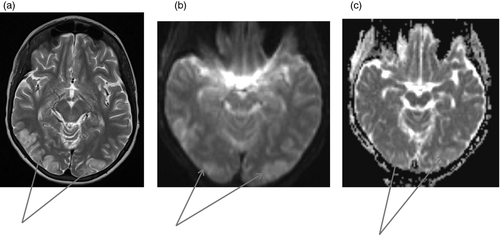
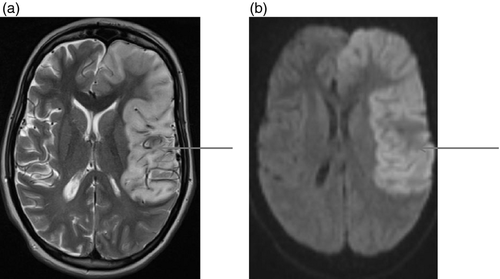
Management
The initial assessment should focus on resuscitation and establishment of homeostasis. Early recognition and management of coma can be lifesaving. It is important to be aware that patients with large MCA territory AIS are at risk of delayed coma in the 24 to 96 hours after onset of the ictus. A deteriorating level of consciousness is a medical emergency, and only close and careful observation will ensure this is recognized promptly. Important practical considerations are whether the child is likely to need transfer to an intensive care unit, as it is clearly optimal to undertake transfer early in a planned and safe way rather than as an emergency. A further practical consideration is whether the child is likely to need urgent neurosurgical intervention (e.g. haematoma evacuation, external ventricular drain, decompressive craniectomy).8 It would be important to consider infection and cover with antimicrobials when there is diagnostic uncertainty. There is no evidence that steroids have any benefit in the management of large MCA strokes, whereas high-dose steroids such as intravenous methylprednisolone clearly benefit in patients with inflammatory demyelination. Anticoagulation with unfractionated or low-molecular-mass heparin needs to be considered early in patients with cerebral sinovenous thrombosis, especially in those with reduced levels of consciousness.
Further investigations
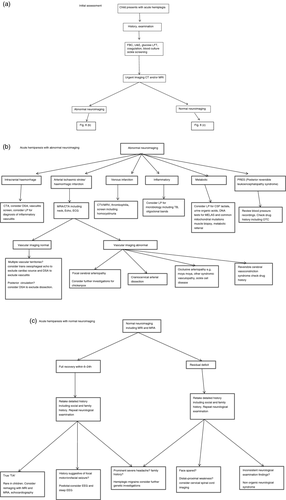
Although the history and examination enables formulation of a differential diagnosis, a definitive diagnosis and the subsequent investigations will be based on imaging findings. It is important to appreciate that it may be difficult to differentiate definitively between conditions such as CNS infection and inflammatory demyelination on imaging, and it is often safest to cover the possibility of infection pending additional results, for example lumbar puncture. Sometimes the final diagnosis only becomes apparent with the evolution of the clinical or imaging picture over time. Figure 8 summarizes further investigations that may be considered according to the likely diagnosis. Testing for prothombotic risk factors is important, particularly where cerebral sinovenous thrombosis is suspected. Current guidelines suggest estimation of protein C, protein S, functional antithrombin, activated protein C resistance, lupus inhibitor, anticardiolipin antibodies, prothombotic mutations (factor V Leiden G1691A, MTHFR C677T, prothrombin G20210A), and measurement of plasma homocysteine.9 Prothombotic risk factors (except homocystinuria) are rarely the primary cause but contribute to the overall risk in the presence of primary risk factors, for example vasculopathy and are more likely to cause venous than arterial thrombosis.
Metabolic stroke is usually the result of metabolic defects that cause energy failure, the most common being respiratory chain disorders. Serum and cerebrospinal fluid lactate is usually but not universally increased. Plasma amino acids and urine organic acids may show suggestive abnormalities such as high alanine, serine, and threonine. Genetic testing for common mitochondrial mutations such as mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) or myoclonic epilepsy with ragged red fibres (MERRF) is generally available; however, muscle biopsy with estimation of respiratory-chain enzyme activities is often required to establish the diagnosis of respiratory chain disorder.
Secondary deterioration
Children presenting with hemiplegia should be observed on a high dependency unit with careful neurological observations. Secondary deterioration, which could be in the form of reduced level of consciousness, new neurological deficit, seizures, or recurrent ‘transient ischaemic attacks’, should prompt urgent clinical and radiological re-evaluation by MRI and MR angiography, or CT and CT angiography if MRI is not available. The aim is to identify development of cerebral oedema and herniation syndromes, extension of infarction, and secondary haemorrhagic transformation urgently. The same questions about neurosurgical intervention and intensive care transfer are applicable and more pressing after secondary deterioration. In the less acute situation of recurrent transient ischaemic attacks that resolve, vascular imaging is critical in identifying progressive vascular disease or recurrent embolic events to guide escalation of treatment, for example immunomodulation.
Hemiplegia with normal imaging
A similar approach starting with history, clinical examination, and relevant investigations is outlined for children presenting with hemiplegia who have normal findings on imaging, including dedicated vascular imaging. Seizures and migraine are the commonest reasons for children to present with a ‘brain attack’.1 Recurrent stereotyped attacks without imaging changes are likely to be epileptic or migrainous postictal Todd's paresis, particularly if the seizure has been unwitnessed; for example, a nocturnal seizure and can pose a diagnostic challenge. Interictal electroencephalography may be helpful but is rarely diagnostic. A period of observation may be required before the diagnosis can be confirmed.
Headache and hemisensory symptoms are characteristic of migraine with aura. Motor weakness is rare but can occur in the context of sporadic or familial hemiplegic migraine. Onset in hemiplegic migraine is slower than an ischaemic event, which is typically hyper-acute rather than developing over minutes. Headache as a presenting symptom is uncommon in AIS except in craniocervical arterial dissection and reversible cerebral vasoconstriction syndrome. Even in these conditions, the headache is a new-onset acute severe headache and may have a distinctive character and location. Mutations (in the genes CACNA1A, ATP1A2, and SCNA1A) have been characterized in patients with familial hemiplegic migraine and can be tested for if compatible family history is obtained.
Alternating hemiplegia of childhood is a disorder presenting with episodic hemiplegia affecting alternate sides of the body and variable dystonia, epilepsy, and developmental delay caused by mutation in ATP1A3. The clinical presentation is characteristic and the diagnosis can be confirmed by mutation analysis.




