One-person operated endoscopic submucosal dissection for early esophageal neoplasm using Endosaber
Abstract
Watch a video of this article
Brief Explanation
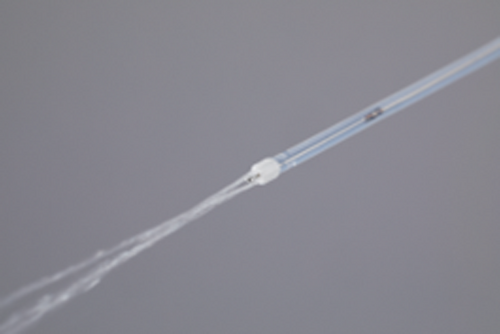
Endoscopic submucosal dissection (ESD) for gastrointestinal tumors is a widely accepted treatment.1 New endo-knives have been invented to improve the ESD procedure.2, 3 We invented a one-person operated ESD (OPO-ESD) using a novel endo-knife, Endosaber (Sumitomo Bakelite, Tokyo, Japan; Fig. 1) for a mock esophageal lesion in an ex vivo porcine model; this required no other devices or assistance.4, 5 Here, we present a case of successful OPO-ESD for early esophageal neoplasm (Video S1).
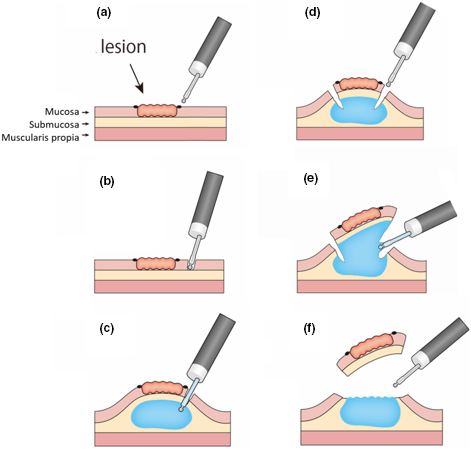
The patient was a 70-year-old man with an esophageal lesion in the posterior wall of the middle thoracic esophagus. A biopsy revealed intraepithelial neoplasia. Therefore, ESD was indicated for this lesion. Marking dots were made around the lesion (Fig. 2a). The first pre-cut was then made near the oral marking of the lesion (Fig. 2b), after which concentrated glycerin and fructose were injected from the pre-cut hole through the Endosaber using a water-jet system (Fig. 2c). Circumferential mucosal incision and submucosal dissection, which are the same as those in the conventional ESD procedure, were made (Fig. 2d). Injection could be administered whenever needed (Fig. 2e). Hemostasis for bleeding or prophylactic coagulation for visible vessels were made with the tip of the Endosaber. En-bloc and complete resection were achieved without complication (Fig. 2f). The total procedure time was 20 min. Histopathological examination showed superficial squamous cell carcinoma with curative resection.
The OPO-ESD was feasible for the treatment of early esophageal neoplasm. All procedures were completed by one operator without any assistance. Only one Endosaber was required. Since no other devices or assistance is needed, the total cost of the endoscopic procedure may be reduced. Further, the total procedure time may be shortened by the lack of need to change devices.
There is still little evidence on the efficacy and safety of OPO-ESD; further studies are required to evaluate these aspects.
Authors declare no conflicts of interest for this article.
Acknowledgement
We thank Akiko Fujisawa for cooperating with illustration.
Funding Information
None.