Endoscopic ultrasound-guided fine-needle biopsy for definitive diagnosis of malignant peritoneal mesothelioma
Abstract
Watch a video of this article
BRIEF EXPLANATION
Malignant peritoneal mesothelioma (MPeM) is a rare disease that is difficult to diagnose pathologically because of nonsensitive cytological evaluation and difficulty in differentiating with other malignant diseases.1 Most MPeM cases are diagnosed using surgically resected specimens, and reports showing the usefulness of endoscopic ultrasound (EUS)-guided tissue acquisition for the diagnosis of MPeM and other peritoneal diseases are limited.2, 3 Herein, we describe the usefulness of EUS-guided fine-needle biopsy (EUS-FNB) for definitive diagnosis of MPeM.
An 82-year-old woman was referred to our hospital for ascites work-up. She had no history of asbestos exposure. She underwent contrast-enhanced computed tomography, which showed no abdominal masses but revealed thickening of the peritoneum with a moderate amount of ascites (Fig. 1A,B); meanwhile, other organs did not manifest abnormality. Furthermore, extremely high levels of hyaluronic acid and cytokeratin fragment 21-1 were detected in ascitic fluid analysis by abdominocentesis; however, ascitic fluid cytology showed no malignancy. For definitive diagnosis, we decided to carry out EUS-FNB. EUS showed hypoechoic thickening in the peritoneum, different from that in the intestinal tract (Fig. 1C). We carried out EUS-FNB of the peritoneum using a 22-gauge Franseen needle (Aquire; Boston Scientific Japan, Tokyo, Japan) without suction during two sessions (Fig. 1D, Video S1). Biopsy showed that atypical cells with acidophilic cytoplasm and anisonucleosis were formed similar to a sheet (Fig. 2A). Immunohistochemically, the cells were diffusely positive for calretinin, vimentin, and mesothelin and negative for carcinoembryonic antigen (Fig. 2B–E). Thus, we established a definitive diagnosis of MPeM. Although we also presented palliative chemotherapy, the patient finally selected best supportive care.

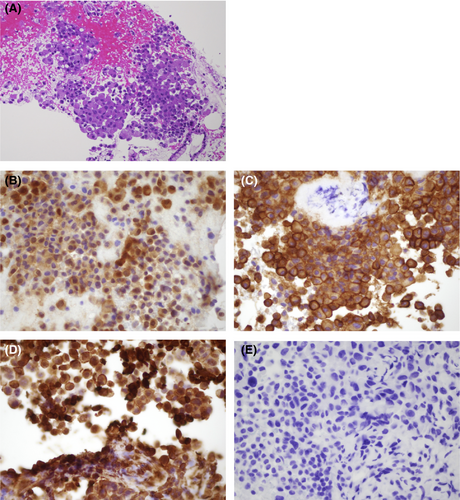
A Franseen needle, which is one of the EUS-FNB needles, is useful in acquiring histological core tissue samples for histological analysis.4, 5 Therefore, EUS-FNB could be an effective and safe procedure to achieve a definitive diagnosis in patients with MPeM.
Authors declare no conflicts of interest for this article.
Acknowledgments
We express our deepest appreciation to Drs Hirohito Naruse, Yoshiya Yamamoto, Kazuteru Hatanaka, Jun Ito, Kenji Kinoshita, Shuichi Miyamoto, Shuhei Hayasaka and Naohisa Tsuchida (Department of Gastroenterology and Hepatology, Hakodate Municipal Hospital) for clinical advice.