Endoscopic management of colorectal tumors less than 10 mm in size: Current status and future perspectives in Japan from a questionnaire survey
Introduction
Based on the results of the National Polyps Study, Western guidelines recommend endoscopic removal of all detected adenoma for reducing the incidence and mortality of colorectal cancers regardless of lesion size.1 In contrast, Japanese Polyp Guidelines (JPG) state a different opinion compared with Western guidelines2 and do not recommend removing diminutive adenoma, because such a lesion has a very low risk of becoming cancer. In addition, JPG states concerns about complications such as perforation and delayed bleeding related to polypectomy for such lesions.
Until now, endoscopic resection including polypectomy, endoscopic mucosal resection (EMR) and hot biopsy (HB) using an electric current were standard procedures. As an alternative procedure, cold polypectomy without an electric current has recently been introduced to remove small colorectal tumors, because this procedure is considered more effective, safer, and faster.3 However, further research is needed to investigate operator variation in polypectomy outcomes and to establish an evidence base for best practice.
At Endoscopy Forum in Japan (EFJ) 2017, which was held in Otaru, Hokkaido in August 2017, standardization of endoscopic management for colorectal tumors less than 10 mm in diameter was discussed based on a questionnaire survey completed by nine Japanese participants who specialize in gastrointestinal (GI) endoscopy especially in the colorectum. In this editorial, we focus on the current status and future perspectives of endoscopic management for colorectal tumors less than 10 mm in diameter as well as on the report from the lower GI session at EFJ 2017.
Questionnaire Survey on the Current Status of Endoscopic Management of Colorectal Tumors <10 mm in Japan
This questionnaire survey was carried out prior to the meeting. A total of nine Japanese participants responded to the questionnaires as follows.
- Topic 1: Diagnostic strategy for diminutive (<5 mm) and small (<1 cm) polyps
- Q1: What is your indication for image-enhanced endoscopy with magnification for diminutive or small colorectal lesions?
- Q2: What is your indication for chromoendoscopy without magnification for diminutive or small lesions?
- Q3: What is your indication for chromoendoscopy with magnification (pit pattern diagnosis) for diminutive or small lesions?
- Topic 2: Therapeutic strategy for diminutive and small polyps
- Q4: What do you usually use when removing diminutive polyps (<5 mm)?
- Q5: What do you usually use when removing small polyps (6–9 mm)?
The aim of this survey was to clarify the current status of endoscopic management of colorectal tumors less than 10 mm in diameter in Japan.
Topic 1: Diagnostic strategy for diminutive and small polyps
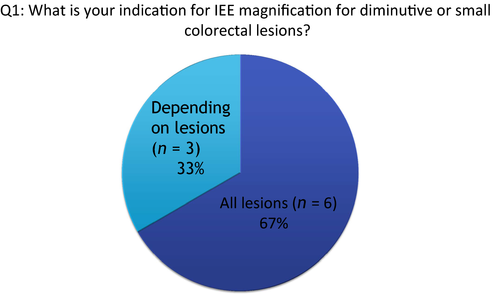
Q1: What is your indication for image-enhanced endoscopy with magnification for diminutive or small colorectal lesions?
Image-enhanced endoscopy (IEE), also known as virtual chromoendoscopy,4 has been developed for detailed diagnosis of GI lesions, as it enhances small vessels and structures on the surface of detected lesions in real-time. Furthermore, use of the magnification function can identify vessel and surface patterns resulting in an improvement of the diagnostic accuracy and confidence levels compared to that of non-magnification endoscopy.
Although IEE technology includes narrow-band imaging (NBI), i-scan, flexible spectral imaging color enhancement (FICE) or blue laser imaging (BLI), all participants had an NBI system and magnification colonoscope at their institutions. Of nine participants, six (64%) responded that ‘all detected lesions’ are candidates for IEE with magnification (Fig. 1). In contrast, three participants responded that they use it ‘depending on lesions’. These participants considered that IEE with magnification is not so essential when diagnosing lesions with typical findings in white light imaging (WLI). For example, sessile or pedunculated type, reddish, lobulation on the lesion surface for adenoma; or sessile type in the sigmoid colon and rectum; pale, mucous on the lesion surface for hyperplastic polyp.
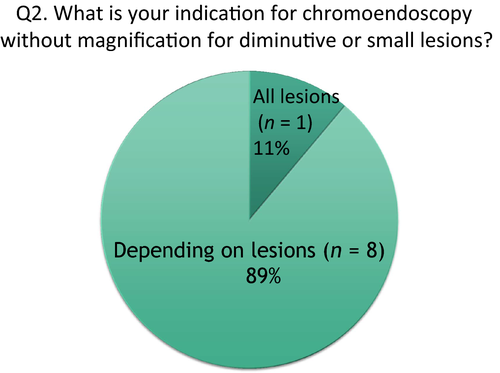
Q2: What is your indication for chromoendoscopy without magnification for diminutive or small lesions?
Except for one participant, all responded that it ‘depends on lesions’ (Fig. 2). Targeted chromoendoscopy facilitates detection and characterization of particularly flat and depressed colorectal tumor which is associated with an increased risk of cancer. Depressed areas, loss of lobulation or expansion are significant findings for predicting deep submucosal invasive cancer regardless of lesion size. Such findings are enhanced after spraying indigocarmine dye.
As mentioned above, IEE with magnification can predict histology without any dyes. All participants agreed that chromoendoscopy is necessary to diagnose mucosal or submucosal lesions, but it is the next step after IEE with magnification.
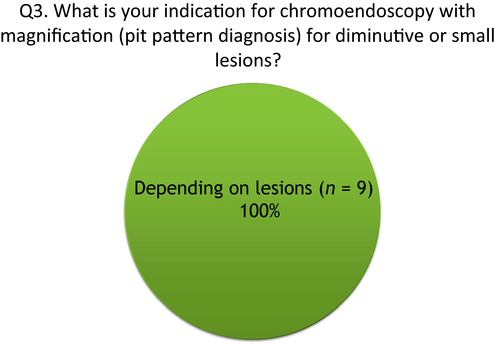
Q3: What is your indication for chromoendoscopy with magnification (pit pattern diagnosis) for diminutive or small lesions?
Indigocarmine dye spraying or crystal violet or methylene blue staining is applied when enhancing superficial patterns. Kudo classification has been established for differentiating types of pit pattern: Type I, II, III, IV and V using a magnification endoscope for predicting lesion histology in the colon.5 Furthermore, Type V pit pattern is subclassified into VI low-grade, VI high-grade and VN pit patterns. For distinction of neoplastic or non-neoplastic lesions, indigocarmine dye spraying is sufficient for most lesions, whereas crystal violet staining is more useful when identifying VI subclassifications and it is required for estimating the depth of cancer invasion.
All participants responded that not all detected lesions are candidates for chromoendoscopy with magnification (Fig. 3). NBI with magnification has a high diagnostic accuracy rate for distinction of neoplastic or non-neoplastic lesions as well as pit pattern.6 For colorectal lesions less than 10 mm, clinical use of NBI with magnification is reasonable compared to that of pit pattern diagnosis. In contrast, diagnostic accuracy of NBI with magnification is not greater than that of pit pattern diagnosis for estimating the depth of cancer invasion. Although the prevalence of submucosal invasive cancers is actually very low (0.05% in diminutive polyps), pit pattern diagnosis should be attempted for making a treatment decision of whether to attempt endoscopic resection or surgery. Therefore, Japanese experts use NBI with magnification followed by pit pattern diagnosis by crystal violet staining and magnification when identifying the degree of dysplasia in adenomas or estimating depth of cancer invasion.
Topic 2: Therapeutic strategy for diminutive and small polyps
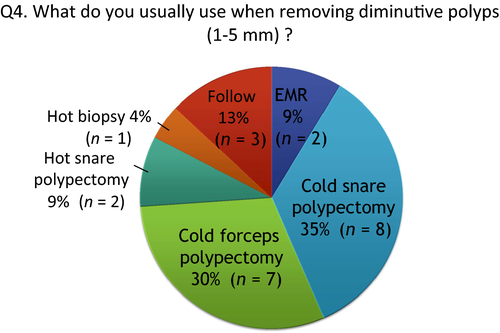
Q4: What do you usually use when removing diminutive polyps (<5 mm)?
Although we can remove diminutive polyps by cold polypectomy, there are two cold polypectomy procedures: cold snare polypectomy (CSP) and cold forceps polypectomy (CFP). Percentage of participants choosing CSP, CFP, hot snare polypectomy (HSP) and hot biopsy (HB) were 35%, 30%, 9% and 4%, respectively, when multiple answers were allowed (Fig. 4). When combining CSP and CFP as cold polypectomy, 65% of participants use cold polypectomy. All the participants who responded that they attempt CFP routinely use jumbo-type forceps which are designed for removing larger tissue samples.3 The frequency of choosing HB was low. According to the report of EFJ in 2013,7 22% of participants responded that they use HB for removing diminutive polyps. Comparing these results, the frequency of choosing HB for removing diminutive polyps seems to be decreasing. In contrast, based on the JPG, 13% chose no resection, even for adenoma.
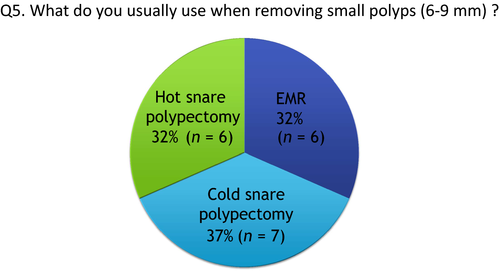
Q5: What do you usually use when removing small polyps (6–9 mm)?
Percentage of using CSP, endoscopic mucosal resection (EMR) and HSP was 37%, 32% and 32%, respectively (Fig. 5). Multiple bite resection method using CFP is not recommended when removing polyps larger than 5 mm. HSP may be useful for low-grade adenoma because the duration of the procedure is shorter than for EMR. EMR is also carried out for high-grade adenoma, intramucosal cancer or slightly invasive cancers, because this procedure can remove more submucosal tissue for accurate histological evaluation of muscularis mucosa and submucosal layer compared with HSP.
Discussion
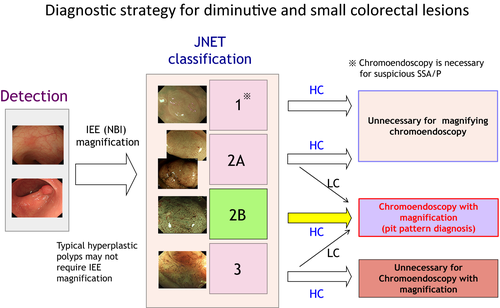
Choice of treatment is determined by endoscopic findings using a step-by-step approach: in the order of WLI, IEE with magnification, chromoendoscopy with non-magnification and then chromoendoscopy with magnification. IEE with magnification, which is equal to that of pit pattern diagnosis for tumor characterization, has advantages of being quicker and of not requiring any dye compared to chromoendoscopy with or without magnification. Therefore, the second step of endoscopic diagnosis is IEE with magnification following lesion detection by WLI. Japanese participants showed a consensus to use Japan NBI Expert Team (JNET) classification8 for making a decision for ideal management and treatment of colorectal tumors. This classification is divided into four types. Type 1, 2A, 2B, and 3 correspond to hyperplastic polyps plus sessile serrated polyps, low-grade intramucosal neoplasia, high-grade intramucosal neoplasia or shallow submucosal invasive cancers, and deep submucosal invasive cancers, respectively. Pit pattern diagnosis is required as the final step for JNET classification Type 2B, or Types 2A and 3 with low confidence lesions which might be intramucosal or invasive cancers. In summary, Figure 6 shows the diagnostic strategy for diminutive and small colorectal lesions.
Recently, the computer-aided diagnosis (CAD) system is gaining attention as an attractive endoscopic diagnostic tool. Some investigators reported that the use of CAD is effective in the management of diminutive and small colorectal polyps.9 However, participants think that some diminutive or small lesions can be diagnosed only by using WLI. Endoscopic criteria or classification using only WLI may be necessary for predicting histology based on location, size, shape or color of lesions.
Based on this questionnaire survey regarding choices of endoscopic resection procedures for diminutive and small tumors, cold polypectomy has become one of the standard polypectomy procedures used by Japanese experts. However, as yet, there are some concerns about risk of local recurrence or poor quality of pathological evaluation in the cold polypectomy procedure (Table 1). In contrast, the frequency of attempting HB is decreasing for removing diminutive polyps. When considering the risk of procedure-related complications, it is difficult to clarify the clinical benefit of HB when cold polypectomy is widely accepted.
| Cold forceps polypectomy Cold snare polypectomy | Hot biopsy EMR Hot snare polypectomy |
|---|---|
Advantages
|
Advantage
|
Disadvantages
|
Disadvantage
|
- EMR, endoscopic mucosal resection.
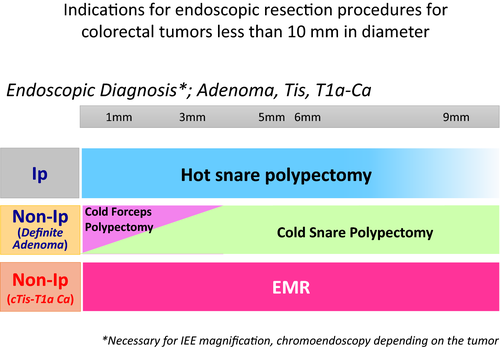
Our consensus on the indications for endoscopic resection for colorectal diminutive or small tumors is shown in Figure 7. Although a diminutive lesion is less likely to be cancer as mentioned by JPG, such a lesion may become cancer in the future as the lesion progresses. Also, there is no surveillance program after a diminutive lesion is left. When the safety and effectiveness of the ‘resect and discard’ strategy10 for reducing the burden on pathology is better clarified and some of the above concerns for cold polypectomy are resolved, the JPG might recommend the eradication of all adenomas to reduce the incidence and mortality of colorectal cancers.
Conflicts of Interest
Authors declare no conflicts of interest for this article.