Surveillance for dysplasia in patients with ulcerative colitis: Discrepancy between guidelines and practice
Abstract
Background and Aim
The risk of developing colorectal cancer is higher in patients with ulcerative colitis (UC) than in the general population. Guidelines recommend surveillance colonoscopy (SCS) to reduce mortality; however, few studies have assessed physicians’ adherence to guidelines. This study was aimed to clarify the current status of SCS and adherence to guidelines through the characteristics of cancer/dysplasia surveillance for UC patients in Japan.
Methods
A questionnaire was mailed to 541 physicians who attended meetings on inflammatory bowel disease.
Results
The respondents encountered a median of 100 UC cases. Thirty percent of the respondents had never managed a UC patient with cancer. Fifty-one percent of the respondents had never diagnosed colorectal cancer with UC. Forty-seven percent of the respondents considered extensive colitis and left-sided colitis as indications for SCS, and 38% carried out SCS regardless of the disease extent. Sixty-three percent of the respondents started SCS at 7–10 years after UC onset, whereas 20% started SCS at 3 years or less. Fifty-two percent of the respondents obtained targeted biopsies only, and chromoendoscopy was used by 49% of the respondents as a special technique for surveillance. Median number of biopsies at SCS was five per patient; it was three among patients whose biopsy was carried out by physicians who obtained targeted biopsies only and seven among those carried out by physicians who obtained step biopsies and targeted biopsies (P < 0.0001).
Conclusion
A considerable proportion of the respondents did not follow the guidelines when selecting patients for surveillance and carrying out SCS.
Introduction
Patients with ulcerative colitis (UC) have a high risk of developing colorectal cancer (CRC). The risk is thought to increase according to the duration of UC.1 In UC, dysplastic epithelium is considered a marker or precursor of carcinoma.2 Therefore, the dysplasia-carcinoma sequence is believed to be the main pathway of carcinogenesis in UC.3 Clinical guidelines worldwide4-7 recommend surveillance colonoscopy (SCS) to reduce CRC-related death; however, to our knowledge, firm evidence of its efficacy for improving patient survival has not been shown.
Current clinical guidelines on UC recommend annual or biennial SCS in extensive or left-sided colitis cases with a duration of 6–10 years or more.4-7 Furthermore, the guidelines recommend obtaining targeted biopsies using panchromoendoscopy or four random biopsies every 10 cm along the colon.4, 5 However, few reports have been published concerning the proportion of physicians who follow this recommendation.8-12 Therefore, there is a need to evaluate endoscopists’ adherence to the guidelines.
Several questions remain concerning this subject, including what subgroup of patients should be surveyed and what biopsy protocol, random or targeted, is preferred. Although the guidelines suggest how SCS should be carried out, practitioners may not follow them, possibly because of the lack of firm evidence, inconvenience, unawareness, or other reasons.
The present study aimed to clarify adherence to the guidelines through the characteristics of actual cancer/dysplasia surveillance in UC patients by physicians in Japan.
Methods
Questionnaire
The IBD Club Jr. Meeting has been held twice per year for 20 years in Tokyo, Japan and focuses on the clinical management of inflammatory bowel disease (IBD) and related diseases through discussion of IBD case reports and lectures related to IBD. The meeting itself has been supported by Ajinomoto Pharma Co. Ltd, and we shared the attendee list of the meeting. Using this list, a questionnaire was sent by mail to 541 physicians who attended either of the last two IBD Club Jr. Meetings at the time of mailing in 2013. The answers were returned by mail, anonymously. Forty-five questionnaires were returned to us unopened because the recipient's address had been changed. A total of 164 completed questionnaires were returned to us, for a response rate of 33%. The questionnaire was divided into two sections. The first section pertained to ulcerative colitis and surveillance, and the second section addressed the management of low-grade dysplasia, which was omitted from this paper, because of the lack of patients’ permission for publication. There were both multiple-choice questions and open response sections. Numerical data of experience with cases were categorized to five, and other numerical data were analyzed according to the original numbers.
Statistical analysis and ethics
Numerical data were analyzed using the Mann–Whitney U-test, and categorical data were analyzed using chi-squared tests. A P-value of 0.05 was considered statistically significant. This study was approved by the ethical committee at the Institute of Medical Science, the University of Tokyo.
Results
Respondents
Respondents’ graduation year was equally distributed in general. Among all the respondents, 81% (134/164) and 12% (21/164) were gastroenterologists and gastrointestinal surgeons, respectively; the rest were pediatricians, pediatric surgeons, and a pathologist. Fifty-one percent (83/164) of the respondents worked in non-university hospitals, and 45% (74/164) worked in university hospitals. Thirteen percent (21/161) of the respondents were women. Only 9% (13/152) of the respondents had carried out fewer than 300 colonoscopies; the median number of colonoscopies carried out by the respondents was 3000. In total, the respondents included physicians with a variety of postgraduate periods and most of them had significant experience of colonoscopy.
Experience with UC and UC-associated CRC cases
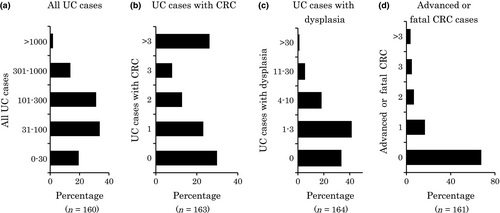
The number of UC cases managed by each respondent ranged from 0 to 1500, and the median was 100. This distribution pattern appeared similar to a normal distribution (Fig. 1a). By contrast, the distribution of UC patients complicated with CRC showed two peaks: 30% (49/164) of the respondents had never managed a UC patient with CRC, whereas 26% (43/164) had managed more than three cases of UC with CRC (Fig. 1b). Thirty-four percent (55/164) of the respondents had never managed a UC case with dysplasia (Fig. 1c). Sixty-eight percent (109/161) had never managed a case of advanced or fatal CRC (Fig. 1d).
Experience in carrying out SCS and diagnosing CRC
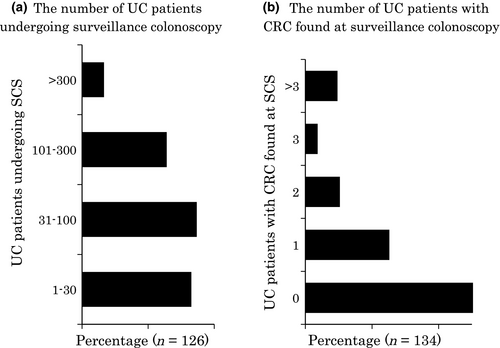
Eighty-nine percent (145/163) of the respondents carried out SCS. Some surgeons and pediatricians commented that they do not carry out colonoscopies themselves. Sixty-eight percent (82/121) of the respondents had carried out SCS on no more than 100 patients (Fig. 2a). Fifty-one percent (69/135) of the respondents had never found CRC, and only 24% (32/135) had encountered two or more UC cases with CRC (Fig. 2b). Thirty-six percent (47/132) of respondents had never found dysplasia (data not shown). Therefore, most physicians had carried out SCS for a relatively small number of UC patients and consequently found few, if any, cases of neoplasia.
Surveillance program
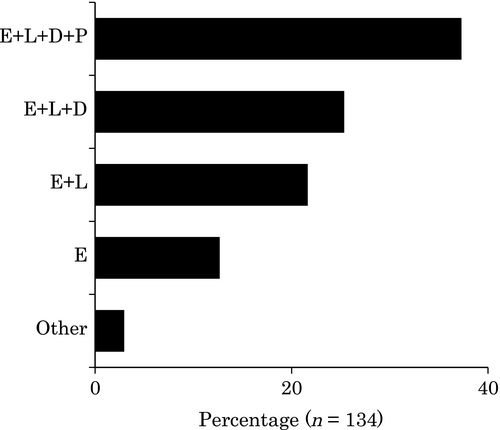
Characteristics of patients included in surveillance programs are shown in Figures 3 and 4. Patients with extensive colitis with or without patients with left-sided colitis, regardless of distal colitis, were selected as candidates for SCS by 59% (80/135) of respondents who carried out SCS, which is consistent with guideline recommendations (Table S1).8-12 However, the most frequent response was that surveillance was done in all patients regardless of disease extent (38%; 51/135). As shown in Table S1, the inclusion of patients with proctitis in the surveillance program is not recommended, and 38% of the respondents did not follow this guideline.
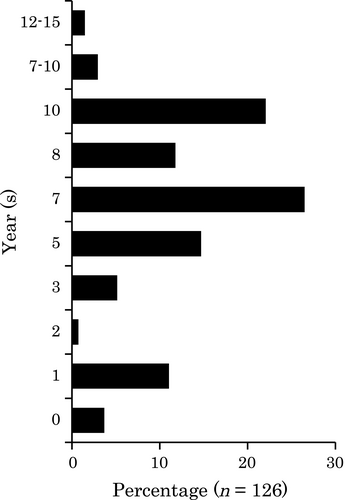
Sixty-three percent (86/137) of the respondents started surveillance at 7–10 years after disease onset. Twenty percent (28/137) of the respondents started surveillance at 3 years or less after UC onset (Fig. 4). Guidelines recommend that SCS should be started between 6 and 10 years from the onset of symptoms (Table S1). Thirty-five percent (48/136) of the respondents started surveillance before 6 years and 1.5% (2/136) more than 10 years after disease onset, timing that did not meet any of the guidelines.
In the current guidelines,4, 5 extent of disease and duration are listed as major risk factors for CRC. However, other factors are needed to improve the efficacy of identifying neoplasia, because many patients require SCS based on these two factors alone. Therefore, the questionnaire asked about such factors. Nine respondents answered with persistence of inflammation. The other answers were family history of CRC in four responses, severe inflammation in three, and primary sclerosing cholangitis in three. Because patients who fulfil the criteria for inclusion in the surveillance program do not always receive SCS, we also asked about the proportion of patients in the surveillance program among the total number of candidates. Thirty-three percent (42/127) of the respondents answered that it was ≥90%, whereas 31% (39/127) answered that it was <70%.
Figure 5 shows the ideal and actual intervals of SCS. Although annual colonoscopy was thought to be ideal by 82% (121/147) of the respondents, only 31% (45/147) of the respondents carried out SCS annually, and the median actual interval was 1.5 years. The reasons why SCS was not carried out as expected are shown in Table 1. The two major reasons were both patient-related: time required to undergo surveillance (e.g. patients would need to take a day off work or school for SCS) and pain. Other reasons were possible symptom exacerbation after surveillance, low frequency of neoplasia discovery, and difficulty in scheduling colonoscopy because of limited capacity. Patient awareness and economic reasons were less frequent, reported by <10% of respondents.
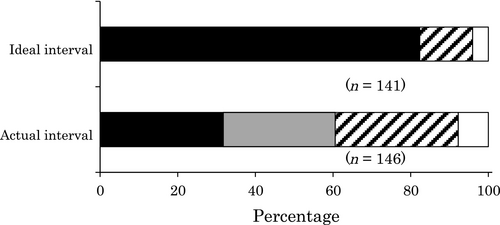
 , 1.5 years;
, 1.5 years;  , 2 years;
, 2 years; , >2 years.
, >2 years.| Cause | Number | |
|---|---|---|
| Patients | Time for surveillance | 99 (70%) |
| Pain caused by surveillance | 90 (64%) | |
| Exacerbation of symptoms caused by surveillance | 49 (35%) | |
| Unawareness of the necessity of surveillance | 13 (9%) | |
| Economical reason | 5 (4%) | |
| Physicians | Low frequency of detecting dysplasia/cancer | 35 (25%) |
| Difficulty in scheduling colonoscopy | 20 (14%) | |
| No reasons | 72 (51%) |
- Multiple answers were allowed (n = 142).
In Japan, a government-led survey on UC registration has been carried out annually. To fulfil the registration requirements, patients have sometimes been advised to undergo colonoscopy, which is not always aimed to find dysplasia. Seventy-two percent (101/141) of the respondents answered that the survey was helpful in urging patients to undergo SCS.
Biopsy strategy (protocol)
As shown in Figure 6a, 52% (75/143) of the respondents obtained biopsies from visible lesions only (Targeted group), and the rest obtained biopsies from mucosa without recognition of definite neoplasia as well as visible lesions (Step group). In the Step group, only 11% (7/65) of respondents obtained biopsies along the colon every 10 cm, whereas the majority obtained step biopsies according to the anatomical location of the colorectum (e.g. cecum, ascending colon, and transverse colon) (Fig. 6b). Sixty-one percent (37/61) of the respondents in the Step group obtained biopsies from all over the colon, whereas 23% (14/61) obtained rectal biopsies only (Fig. 6c). A median of five biopsies was obtained per patient in all. Median number of biopsies was three and seven in the Targeted group and Step group (Fig. 7), respectively, reflecting a statistically significant difference (P < 0.0001).
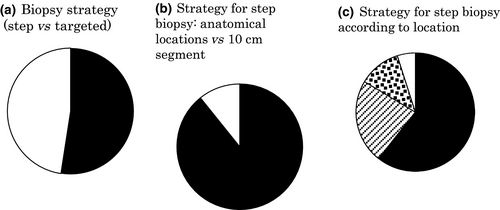
 , 10-cm segment). (c) Strategy for step biopsy according to location (■, whole colorectum;
, 10-cm segment). (c) Strategy for step biopsy according to location (■, whole colorectum;  , rectum only;
, rectum only;  , rectosigmoid only;
, rectosigmoid only;  , others).
, others).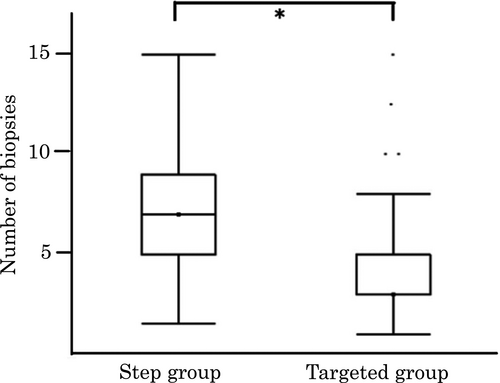
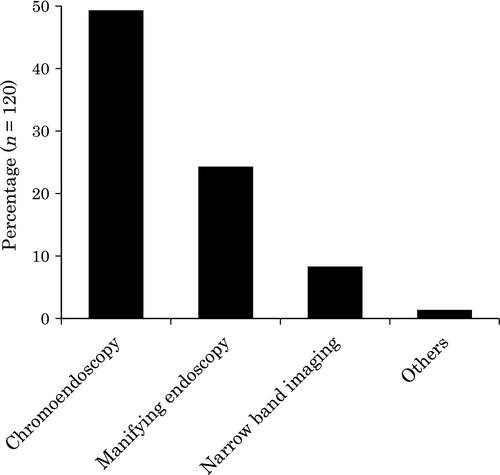
The respondents were asked if they used a special method to identify lesions that were not recognized with normal endoscopic observation. Forty-nine percent (71/145) used chromoendoscopy, and 24% (35/145) used magnifying colonoscopy. In this regard, chromoendoscopy and targeted biopsy were the most common methods used in SCS. Some physicians also used narrow band imaging (8% [12/145]; Fig. 8).
Pathological diagnosis
Thirteen percent (21/157) of the respondents answered that they checked the microscopic specimen themselves. A second opinion regarding pathological interpretation was obtained by 22% (34/157) of the respondents, and another 18% (28/157) did not consult with a pathologist regarding the results. The remaining respondents had not encountered a situation that required consulting a pathologist specialized in dysplasia or cancer in IBD.
Discussion
Several published studies on this topic have been carried out in other countries. We did a literature search on PubMed using three keywords (ulcerative colitis, surveillance, and questionnaire) and identified five papers, two from the USA and one each from the UK, Netherlands, and New Zealand.8-12 The results of those studies and the present study are summarized in Table S2. The present study is the first of its kind in Asia, and the participants included not only gastroenterologists but also surgeons and pediatricians. Furthermore, the participants in the present study had a general interest in IBD, as they were attendees at an IBD-related meeting. The gastroenterologists in previous studies may have included hepatologists and gastrologists. As described in the Results, the median number of colonoscopies carried out by the respondents was 3000, and most of the respondents could be considered experienced in carrying out colonoscopy. Therefore, our results may be interpreted as reflective of the current status and concepts of SCS carried out by physicians with a special interest in IBD in Japan, and they may not reflect the current status of cutting-edge researchers.
In the present study, a notable proportion of the respondents had not managed many cases of UC-associated CRC. Thirty percent of the respondents had never managed CRC cases, and one-third had never managed UC cases with dysplasia. Only one-third of respondents had managed an advanced, metastatic, or fatal case. Therefore, a significant proportion of the respondents may carry out SCS according to guidelines, references, or expert opinions without encountering a relevant case in their own practice. To our knowledge, no similar findings have been documented elsewhere. Inexperienced physicians could unknowingly treat UC patients with dysplasia or CRC and not provide appropriate medical treatment. To overcome this situation, we advise that patients with dysplasia or cancer should be treated by experienced physicians, and that a platform should be created whereby inexperienced physicians can easily consult with experienced physicians. Another possible solution is that inexperienced physicians be educated, through reference books or meetings, in the management of these patients.
Subjects of the SCS program
Current guidelines worldwide have a gap among them regarding indicated patients for SCS. In European and British guidelines,5, 6 distal colitis (involving the rectum and sigmoid colon only) is included in the surveillance program, whereas it is excluded in the American College of Gastroenterology (ACG) guidelines.4 In the Japanese guidelines, only extensive colitis is indicated for SCS.7 According to our results, 25% of respondents followed European guidelines, 21% followed American guidelines, and 13% followed Japanese guidelines.
In three previous papers investigating similar questions,9, 10, 12 the proportion of respondents who included cases of extensive colitis and left-sided colitis in the surveillance program ranged from 24% to 68%, and our results are consistent with these reports. However, the proportion of physicians who examined all UC patients in the surveillance program ranged from 2% to 10% in previous studies and was 37% in our results. This finding indicates that SCS is carried out more extensively by physicians in Japan than in Western countries.
When to start surveillance
There is also a gap in the guidelines worldwide concerning the starting period of SCS. According to the ACG and Japanese guidelines, SCS should begin between 8 and 10 years4, 7 after UC onset, whereas the European guidelines recommended starting at 6–8 years.5, 6 Most of the respondents (68%) in the present study responded that the surveillance should start 7–10 years after the onset of UC, and the most frequent answer was 7–8 years (41%).
Because this question was not multiple-choice, this result was similar to that of previous studies.
Surveillance interval
In the ACG and Japanese guidelines, annual or biannual SCS is recommended, whereas the European Crohn's and Colitis Organisation guidelines suggest carrying out surveillance at 1, 3, or 5-year intervals according to the individual patient's risk. In our results, approximately 80% of the respondents answered that the ideal interval of surveillance was 1 year, whereas that proportion ranged from 22% to 56% in previous studies. This difference may be a result of Japanese physicians’ tendency for more aggressive SCS. We included two intervals of SCS in our questionnaire: optimal and actual. Previous studies do not appear to have assessed the actual interval, but they did not describe this point of their methodology clearly. Our results show that the optimal interval is not always equal to the actual interval. Therefore, the actual interval should be assessed to evaluate real adherence to guidelines. In the present study, the physicians attributed this gap largely to patients’ complaints regarding time and pain. However, the same question should be asked of patients to determine whether these reasons are actually the cause for their behavior.
Biopsy protocol
As shown in Table S1, chromoendoscopy with random biopsies (four-quadrant biopsies every 10 cm) and targeted biopsies of any visible lesion are recommended in European guidelines, and four-quadrant biopsy specimens obtained from approximately every 10 cm of colon are recommended in American guidelines. In our study, 34 of 75 respondents who biopsied targeted lesions alone used a dye-spraying technique routinely, and no respondents collected four-quadrant biopsy specimens. Therefore, only 24% (34/143) of respondents followed either of the guidelines.
The number of biopsies obtained by the respondents in the present study was smaller than that reported in other studies (more than 10). The reason for this difference is not clear; however, 70% of the respondents used chromoendoscopy and 35% used magnifying colonoscopy. Therefore, we speculate that endoscopists may be more confident in finding neoplasia with those novel techniques. Comparison of SCS efficacy between Western countries and Japan in future studies would be of value.
Recently, an outstanding randomized control study on SCS was published.13 Watanabe et al. demonstrated that the targeted biopsy strategy was not inferior to the random biopsy strategy in the detection rate of neoplasias. This study was investigated before its publication, and greater numbers of physicians will carry out SCS with targeted biopsies alone in the near future.
Limitations
The present study has some limitations, and the findings may be biased. A questionnaire does not always reflect real practice, and the response rate was low, at 33%. Future studies with more participants may be needed.
Conclusions
Although guidelines suggest that patients with extensive colitis and left-sided colitis are candidates for SCS, only half of the respondents followed this recommendation. A third of respondents started SCS <7 years or >10 years after UC onset, which did not accord with the guidelines. Approximately half of the respondents obtained biopsies of targeted lesions only. Median number of biopsies obtained per patient was five, and the number was significantly larger in patients treated by physicians who carried out stepwise biopsies. Given the lack of a high level of evidence in the guidelines, further studies are needed to establish optimal surveillance methods for dysplasia.
Acknowledgments
The authors thank the respondents for contributing their time to answering our questionnaire.
Conflicts of Interest
Author M.S. was supported by grants from Mitsubishi Tanabe Pharma Co., Ltd. Author T.H received a research grant from Mitsubishi Tanabe Pharma Corporation, Ajinomoto Pharmaceuticals Co. Ltd, Eisai Co. Ltd, AbbVie GK, JIMRO Co. Ltd, Asahi Kasei Kuraray Medical Co, Ltd, Zeria Pharmaceutical Co. Ltd, Daiichi-Sankyo, Kyorin Pharmaceutical Co. Ltd, Nippon Kayaku Co. Ltd Astellas Pharma Inc., consulting fee from EA pharma Co. Ltd, AbbVie GK, and lecture fee from Mitsubishi Tanabe Pharma Corporation, AbbVie GK, EA Pharma Co. Ltd. Author T.S. received lecture fee from Mitsubishi Tanabe Pharma Corporation, AbbVie GK, Zeria Pharmaceutical Co. Ltd, and Eisai Co. Ltd. Author M.W. received a research grant from Asahi Kasei Kuraray Medical Co., Ltd, Ajinomoto Pharma Co., Ltd, AbbVie GK, Eisai Co., Ltd, Kyorin Pharmaceutical Co., Ltd, Mitsubishi Tanabe Pharma Co., Ltd, Otsuka Pharma Co., Ltd, Kyowa Hakko Kirin Co., Ltd, Zeria Pharmaceutical Co., Ltd, UCB Japan Co., Ltd, JIMRO Co., Ltd, Takeda Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd, Ono Pharmaceutical Co., Ltd, Gene Care Research Institute Co., Ltd, Astellas Pharma Inc., MSD K.K., and lecture fees from Mitsubishi Tanabe Pharma Co., Ltd, Eisai Co., Ltd, Ajinomoto Pharma Co., Ltd, Takeda Pharmaceutical Co., Ltd. All the other authors declare no conflicts of interest for this article. The IBD Club Jr. Meeting was financially supported by Ajinomoto Pharma Co. Ltd.





