Consequences of impediments to animal movements at different scales: A conceptual framework and review
Abstract
Aim
The persistence of animal populations depends on individuals moving successfully around a landscape, but habitat fragmentation can hinder this by reducing functional connectivity. The proximate cause of population declines in fragmented habitat is dependent on the spatial and temporal scales of movement restrictions.
Location
Global.
Methods
We present a conceptual framework highlighting the relationship between spatial and temporal scales, and three mechanisms through which detrimental impacts can occur when movement is disrupted in fragmented landscapes: limited resource access, restricted demographic exchange and impeded gene flow. We then review the literature to identify the proportion of studies conducted on each mechanism and whether biases existed in how often each was studied among different geographic zones or taxa. A random selection of 250 articles was classified by the mechanism, geographic region and taxon studied in each article.
Results
Our conceptual framework highlighted that each of the three mechanisms tends to be characterized by movement restriction at progressively larger spatial and temporal scales. In our literature review, we found that the overwhelming majority (77%) of articles investigated impeded gene flow, and only 17% and 10% explored restricted demographic exchange and limited resource access, respectively. Work on limited resource access was disproportionately low for particular taxonomic groups, such as reptiles and amphibians.
Main conclusions
Distinguishing which mechanisms are disrupted in a particular system is crucial because addressing each is likely to require a distinct conservation management response. We encourage greater focus on the less-studied mechanisms of restricted demographic exchange and limited resource access.
1 INTRODUCTION
The ability to move around landscapes is critical for the persistence of animal populations (Fahrig & Merriam, 1985; Hanski, 1991). Habitat fragmentation, defined here as a landscape pattern whereby habitat is structurally separated, regardless of the process through which that separateness has come about, reduces structural connectivity within landscapes which can in turn reduce functional connectivity (Brooks, 2003; Pe'er, Henle, Dislich, & Frank, 2011). Structural connectivity reflects the degree to which the physical structure of landscape elements allows individuals to move, while functional connectivity reflects the degree to which movements actually occur based on an interaction between landscape structure and the behavioural and biological characteristics of a species (Betts, Gutzwiller, Smith, Robinson, & Hadley, 2015; Pe'er et al., 2011; Wiens, Schooley, & Weeks, 1997). Landscapes may provide variable degrees of connectivity for species depending on their affinity for habitat edges or their propensity to cross gaps (Pe'er et al., 2011).
Substantial work has been devoted to understanding how habitat fragmentation influences individuals and populations (Fahrig, 2017; Prugh, Hodges, Sinclair, & Brashares, 2008; Tischendorf & Fahrig, 2000). While much of this work has focused on correlations between habitat configuration and the richness, distribution or abundance of organisms (Edge et al., 2017; McGarigal & Cushman, 2002; Prugh et al., 2008), an important subset has focused on the ecological mechanisms through which detrimental impacts can occur in fragmented landscapes (Banks, Piggott, Stow, & Taylor, 2007). It is important to identify which specific mechanisms are impacted and why, so that predictions can be improved (Urban et al., 2016) and targeted interventions can be developed. For instance, impeded gene flow in eastern collared lizards (Crotaphytus collaris collaris) inhabiting isolated rocky outcrops was initially addressed by translocating individuals (Templeton, Robertson, Brisson, & Strasburg, 2001), but the underlying demographic issues were only rectified when fire suppression activities were modified to facilitate dispersal between outcrops (Brisson, Strasburg, & Templeton, 2003; Templeton, Neuwald, Brazeal, & Robertson, 2007).
In this study, we classify the ecological mechanisms affected when habitat fragmentation restricts animal movements. First, we present a conceptual framework outlining the relationship between three core mechanisms that can be impacted by movement disruptions (limited resource access, restricted demographic exchange and impeded gene flow) and scale, both spatial and temporal. Second, we review the literature to explore the relative frequency of studies conducted on each of the three mechanisms, and whether biases exist in the frequency that mechanisms were studied among geographic locations or taxa. Finally, we outline the importance of distinguishing among these three types of mechanisms when identifying negative impacts of habitat fragmentation on movement, so that conservation management can be tailored appropriately.
2 CONCEPTUAL FRAMEWORK
2.1 Mechanism and scale are linked
Animal movements can be restricted at a range of spatial and temporal scales. Some movements are undertaken on a daily basis, some are undertaken once in a lifetime and some only occur once in several generations. Movements disrupted by habitat fragmentation on a daily basis are likely to affect a different mechanism than that affected by movement restrictions across multiple generations. For example, unwillingness or inability to routinely cross roads may restrict an animal's home range and influence their daily access to resources (Poessel et al., 2014), but may not impede once-in-a-lifetime dispersal events that facilitate gene flow. Both the temporal and spatial scales at which movement restrictions operate relate to the ecological mechanism affected, and ignoring this relationship could be costly for governments and conservation organizations in terms of conservation outcomes (Scott, Touloumis, Lehsten, Tzanopoulos, & Potts, 2014).
The consequences of small-scale movement restrictions are likely to occur over short time-scales, whereas the consequences of large-scale restrictions may only be evident after many generations. For example, habitat fragmentation can disrupt daily movements when home ranges encompass multiple patches. A gap between parts of a home range could quickly lead to poor condition of individuals if they continually expend extra energy navigating around it (Hinsley, 2000; Smith, Forbes, & Betts, 2013) or death if gap crossings are undertaken in the presence of predators (Eccard, Pusenius, Sundell, Halle, & Ylönen, 2008). Population sizes can respond quickly to changes to the health and survival of individuals (Desy & Batzli, 1989). In contrast, inbreeding depression is likely to be associated with long-term movement impediments, generally requires quite severe levels of habitat isolation and develops over multiple generations (Kenney, Allendorf, McDougal, & Smith, 2014).
2.2 Movement restrictions disrupt three key mechanisms
We consider three mechanisms through which impeded movements can have a direct, detrimental effect on animals: (1) limited resource access, (2) restricted demographic exchange, and (3) impeded gene flow (Figure 1). Habitat fragmentation can spatially segregate resources needed by an individual, reducing their ability to access them. Demographic exchange is restricted when individuals are not able to disperse to new localities or alternative populations to facilitate demographic processes and maintain sustainable demographic parameters within metapopulations or patchily distributed populations. Finally, impeded gene flow occurs when the movement and integration of genetic material from one spatially discrete population to another is restricted.
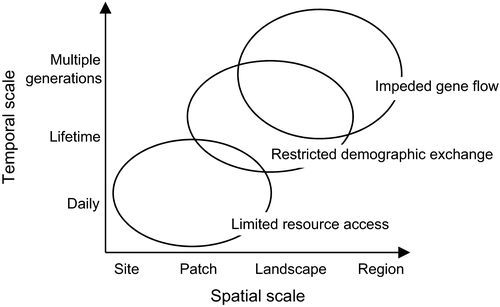
For any given species, each of these mechanisms operates over characteristic spatial and temporal scales (Jeltsch et al., 2013). However, the mechanisms are not mutually exclusive and can overlap in time and space. For example, if dispersal between two patches is prevented, an individual may also have trouble accessing sufficient resources if the occupied patch is depauperate. All three mechanisms do not need to be impeded for a species to go extinct (Harrisson et al., 2013). Individuals may be able to disperse successfully from a source population, but if they continually travel to a sink site harbouring insufficient resources, extinction may result (Dunning, Danielson, & Pulliam, 1992; Pulliam, 1988). While the absolute spatiotemporal scales most relevant to each mechanism will vary among taxa depending on their dispersal abilities, foraging range requirements and generation times, the relative scales applicable to each mechanism remain constant (Jackson & Fahrig, 2012; Thornton & Fletcher, 2014; Wiens, 1989). Below, we consider each mechanism in turn.
2.2.1 Fine-scale mechanism—limited resource access
Individuals must access a variety of resources on a regular basis. We define a resource as any product which improves the growth or fitness of an individual as it becomes more abundant in the environment, and which, when used by an individual, becomes unavailable to others (Tilman, 1982). Food, water and shelter from predators generally need to be sourced on a daily basis, while micronutrients and protected sites for reproduction are needed less frequently. If a single patch cannot adequately meet the needs of an individual, that individual may utilize multiple, fragmented patches (Dunning et al., 1992; Hinsley, 2000). However, accessing spatially separated resources can carry a higher mortality risk, such as through predation or vehicle collisions, than accessing resources within continuous habitat (Cullen et al., 2016; Rodríguez, Andrén, & Jansson, 2001) and incurs increased energetic costs (Hinsley, 2000), especially when individuals deliberately travel longer routes to avoid gaps (Smith et al., 2013).
Food restriction can have an almost immediate effect on the physiological condition of individuals through reduced body condition or increased stress levels (Kitaysky, Piatt, & Wingfield, 2007; MacCracken & Stebbings, 2012). If insufficient food is sourced, reproductive output may be reduced (Hinam & St. Clair, 2008) or the breeding process can be interrupted for the duration of the nutritional restriction (Wingfield et al., 1998).
We consider seasonal migration to be a special case of limited resource access. An entire migratory pathway could be conceptualized as a multipatch “home range” within which the migrants must source their resources. Although their movements are unidirectional and relatively linear, they must be able to access adequate and complementary resources from the different areas of their “home range” in a reasonably energetically efficient way with minimal risky or costly gap crossings. Habitat fragmentation has an impact when barriers are erected along their migratory path or important stopover nodes are lost so they cannot reach their seasonal destination (Simpson et al., 2016; Studds et al., 2017). Thus, long-distance migrants are perhaps less likely to experience impeded gene flow because of their high mobility, and their movement restrictions are most likely to fall within the category of impeded resource access, albeit at a much grander scale than that observed for more-sedentary species.
2.2.2 Intermediate scale mechanism—restricted demographic exchange
Dispersal is a type of movement that tends to occur only once in an individual's lifetime (e.g., dispersal from a natal territory) or regularly but infrequently (e.g., dispersal among breeding sites) (Greenwood & Harvey, 1982). Habitat fragmentation can disrupt these movements (Coulon, Fitzpatrick, Bowman, & Lovette, 2010; Kang, Minor, Woo, Lee, & Park, 2016). Such disruption is likely to be triggered by isolation distances greater than those that restrict efficient daily movements for accessing resources.
While resource access impacts upon individual condition and fitness, dispersal primarily influences population dynamics. When individuals are unable to disperse freely, this can prevent demographic rescue of populations by immigrants (Brown & Kodric-Brown, 1977). Small populations are particularly susceptible to environmental and demographic stochasticity. Even quite low rates of immigration can buffer against these chance events, thereby generating rescue effects (Lecomte, Boudjemadi, Sarrazin, Cally, & Clobert, 2004). Successful dispersal is also necessary to allow unoccupied patches to be recolonized (Holland & Bennett, 2011), reduce kin competition (Hamilton & May, 1977), maintain viable sex ratios (Dale, 2001) and regulate population densities when intraspecific competition is high (Bowler & Benton, 2005). There is a time-lag between restriction of dispersal and observed demographic impacts. This delay is caused by several factors: individuals disperse infrequently, but multiple dispersals are needed before population impacts are noticeable; immigrants may not immediately participate in reproduction; or individuals may be reluctant to emigrate, which may temporarily compensate for the lack of immigration.
2.2.3 Broadscale mechanism—impeded gene flow
Gene flow also requires successful dispersal movements. However, as spatial scale and isolation distance increase, the frequency of dispersal events decreases. While demographic exchange requires relatively regular dispersal events, gene flow can be maintained with very few successful dispersals (Hufbauer et al., 2015; Stacey & Taper, 1992). Effective genetic connectivity among populations may require less than 1% of the population to move within a generation (Drees, Hüfner, Matern, Nève, & Assmann, 2011; Mills & Allendorf, 1996). The introduction of even a small number of genetically distinct individuals can facilitate genetic rescue (Robinson et al., 2017; Vilà et al., 2003). For instance, a highly isolated population of adders (Vipera berus) contained only a few adult males (Madsen, Shine, Olsson, & Wittzell, 1999). Within seven years of temporarily translocating unrelated males into the population, there were eight times more males, recruitment had grown dramatically and genetic variability had increased (Madsen et al., 1999). Severe reductions in habitat connectivity, however, can prevent the arrival of genetically distinct individuals on even rare occasions (Delaney, Riley, & Fisher, 2010; Marsden et al., 2012; Roberts, Angermeier, & Hallerman, 2013).
Small and isolated populations suffer from genetic drift (Holsinger & Weir, 2009), which may lead to either a loss of genetic diversity or an increased frequency of harmful alleles (Amos & Balmford, 2001; Frankham, 2005). Reduced genetic diversity within populations can have deleterious effects such as lower reproductive success, inability to adapt to changing environments and less resistance to disease (Aguilar, Jessup, Estes, & Garza, 2008; O'Brien et al., 1985; Reed & Frankham, 2003). When individuals within a population are highly related, the risk of inbreeding depression increases (Reed & Frankham, 2003). Inbreeding carries negative fitness consequences (Charlesworth & Willis, 2009; Hedrick & Garcia-Dorado, 2016), such as decreased survival, longevity, immune response and hatching rates (Jiménez, Hughes, Alaks, Graham, & Lacy, 1994; Reid, Arcese, & Keller, 2003; Saccheri et al., 1998).
Habitat fragmentation impedes gene flow when movements are prevented for multiple generations (Amos & Balmford, 2001; Storfer et al., 2007). For instance, sealed roads have increasingly segmented the distributions of timber rattlesnakes (Crotalus horridus) over the past seven to eight generations (Clark, Brown, Stechert, & Zamudio, 2010), reducing genetic diversity and increasing population differentiation, with major roads having a greater impact than minor roads (Clark et al., 2010).
3 LITERATURE REVIEW METHODS
Although limited resource access, restricted demographic exchange and impeded gene flow can all lead to local extinctions, the distribution of research effort to understand when and where each occurs is apparently not equal. Conservation solutions are developed based on knowledge of impairments within systems. A bias in knowledge development could potentially lead to important management strategies being overlooked. Biases in research effort already exist among taxa and geographic regions (Griffiths & Dos Santos, 2012). Given that movement strategies are so variable across taxa and regions (Chesser, 1998; Holyoak, Casagrandi, Nathan, Revilla, & Spiegel, 2008), the combination of these biases may actually hinder our ability to generalize findings. We undertook a review of the existing habitat fragmentation literature to determine which of the three mechanisms were most studied, and whether particular mechanisms were more likely to be examined in particular geographic regions or for certain taxa.
3.1 Literature search strategy and inclusion criteria
We searched the ISI Web of Science platform on 13 March 2015 using terms that are associated with movement, resources, demography or gene flow within fragmented landscapes. The search string was designed to deliver a broad and comprehensive list of articles particularly focused on the effects of habitat fragmentation on the mechanisms of interest. Specifically, a search of titles, abstracts and keywords was conducted using the following terms: topic = (“habitat fragmentation” OR “habitat configuration”) AND ((resource* OR food OR forag* OR feed OR “home range”) OR (dispersal OR migration OR immigration OR emigration OR demograph*) OR (“gene flow” OR genetic*) OR (movement*)). The search was limited to articles published from 1994 onwards. The search was then refined to exclude all document types except “article.”
The titles and abstracts of articles were examined to determine whether each warranted inclusion in the literature review. Four criteria were used for inclusion. First, the article must have reported findings from an empirical study in a peer-reviewed journal. Work of a purely theoretical nature, reviews and meta-analyses were excluded; computer simulations were included provided they employed or were tested against an empirical dataset. Second, the focal taxon must have been an animal. Studies on plants were excluded because resource access does not apply to plant movements. Third, the article must have investigated some aspect of habitat fragmentation per se, such as distance to neighbouring patch. Articles that only assessed the effects of habitat extent or patch size were excluded as these are more indicative of habitat loss. We excluded papers that only considered habitat loss because our focus was on structural separateness, not habitat amount. We recognize that measures such as patch isolation may be more appropriately treated as an effect of habitat amount (Fahrig, 2013); however, we have taken a more cautious approach as this suggestion has not yet been widely adopted. Finally, articles were only included if they explored a mechanism linking habitat fragmentation to a consequence for the focal taxon. Specifically, they must have investigated whether limited resource accessibility, restricted demographic exchange or impeded gene flow (as per our definitions above) led to the observed fragmentation effects. Thus, studies that only identified relationships between species abundance or distribution and patch or landscape metrics were excluded.
Due to the large number of results from the initial keyword search (n = 4,017), not all articles were subject to this filtering process. Rather, articles were assessed in a random order until we had a sample of 250 articles that met our inclusion criteria. The proportion of articles that examined each of the three mechanism categories did not vary by more than 1.8% between 200 and 250 articles. Thus, our results form a representative sample from our pool of articles.
3.2 Data collection and analysis
The 250 articles selected for inclusion (a list of the data sources is found in Appendix S1) were reviewed in full to extract: type of mechanism examined (limited resource access, restricted demographic exchange or impeded gene flow); geographic location of the study (tropics, subtropics as defined by Corlett (2013) and the temperate zone plus higher latitudes); and taxon examined (invertebrate, fish, amphibian, reptile, bird or mammal; see Table S1). Counts across categories may exceed 250 because some articles fell into multiple groupings. A chi-square goodness-of-fit test was used to assess whether research was evenly distributed across the three mechanisms. Fisher's exact tests were used to determine whether the relative proportions of observed mechanisms were different than expected across geographic zones and across taxa. Analyses were conducted using R version 3.2.0 (R Core Development Team, 2015).
4 RESULTS
Our initial keyword search generated 4,017 results, 2,152 of which were passed through our filtering process. From these, 250 articles met our inclusion criteria. Of the filtered articles, 228 (10.6%) were not an empirical study, 632 (29.4%) were not based on animal taxa, 684 (31.8%) did not study the effects of habitat fragmentation and 358 (16.6%) did not investigate the effects of habitat fragmentation on one of the mechanisms of interest.
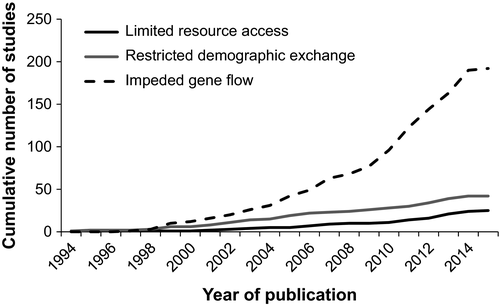
We found significant inequity in research effort across the three mechanisms (χ2 = 195.67, df = 2, p < .001); 192 articles (76.8%) investigated impeded gene flow, 42 articles (16.8%) focused on restricted demographic exchange and 25 articles (10.0%) addressed limited resource access. Nine of these articles studied the effects of habitat fragmentation on multiple mechanisms; eight considered both restricted demographic exchange and impeded gene flow, while one considered restricted demographic exchange and limited resource access. Over the 20-year period, the number of articles investigating impeded gene flow increased from zero per year to 27 per year, while research on limited resource access and restricted demographic exchange increased from zero to five per year (Figure 2).
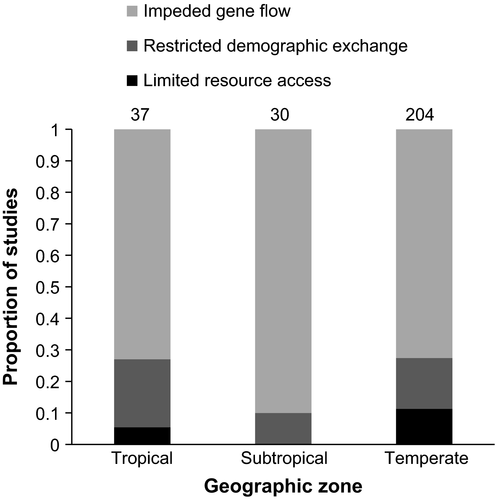
Most work was conducted within the temperate zones or higher latitudes (204 or 75.3%), while 30 (11.1%) articles were from subtropical regions and 37 (13.7%) articles were from the tropics (Figure 3). In all geographic zones, research on impeded gene flow constituted the greatest proportion of articles and the difference in relative frequency of articles on each mechanism was not statistically significant among regions (two-sided Fisher's exact test p = .155; Figure 4).
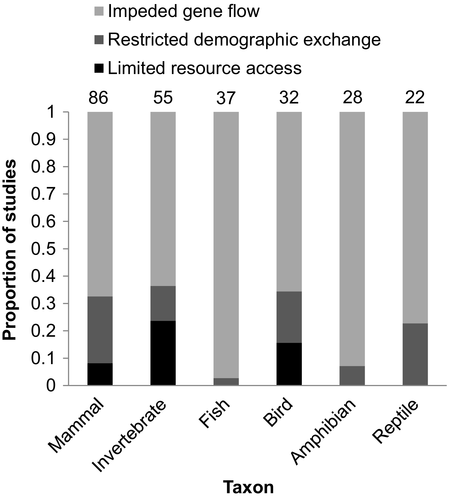
The most frequently represented taxa in our sample were mammals (34.4%) and invertebrates (22.0%) with fish (14.8%), birds (12.8%), amphibians (11.2%) and reptiles (8.8%) receiving less attention (Figure 5). Among invertebrates, Lepidoptera were studied in 14 articles (5.6%). There were significant differences in the frequency with which each mechanism was studied within each taxon (two-sided Fisher's exact test p < .001). Impeded gene flow was the mechanism most studied in all categories. Restricted demographic exchange was most studied in mammals (21% or 24.4% of all mammal articles). Work on invertebrates had the greatest proportion of articles investigating limited resource access (13% or 23.6% of all invertebrate articles), while there was no limited resource access research performed on amphibians, fish or reptiles within our sample.
5 DISCUSSION
Our conceptual framework emphasizes how movements restricted by habitat fragmentation have implications at varying scales and levels of ecological organization. The framework highlights that limited resource access, restricted demographic exchange and impeded gene flow take effect over progressively larger spatial and temporal scales, and the consequences of each become evident after progressively longer time-lags. Although all three mechanisms are likely to be important, we identified through our literature review that impeded gene flow has received the overwhelming majority of research attention.
5.1 Implications and limitations of the conceptual framework
It is crucial to distinguish among the three mechanisms in the conceptual framework when devising solutions that address population declines. In an ideal world, conservation managers would respond to all three mechanisms simultaneously by ensuring that there are sufficient resources within home ranges and individuals can disperse to distant habitat patches. However, there is usually little funding available and few feasible options, so solutions need to be targeted to the specific constraint acting on the population. Failing to consider multiple scales of movement restrictions potentially weakens conservation recommendations because each mechanism may require different conservation responses. Limited resource access could potentially be addressed by improving habitat quality to increase the carrying capacity of the habitat patch (Bonnot, Thompson, Millspaugh, & Jones-Farrand, 2013), but this would not assist if the threat to the local population occupying that patch was primarily caused by a lack of immigrating individuals. For example, habitat restoration in patches or the matrix would do little to restore gene flow between populations of arboreal mammals separated by a highway; a more effective solution may be to install rope bridges that facilitate safe passage (Goldingay, Rohweder, & Taylor, 2013). That solution, however, is of little use if cross-highway movements were adequately frequent to maintain genetic connectivity, but led to high mortality during normal within-home-range movements.
Solutions to different types of movement impediments are not mutually exclusive and could potentially address multiple interrupted mechanisms simultaneously. However, the scale of intervention could become a key determinant of how effectively the solution addresses each of the affected mechanisms (Gunton et al., 2014). For instance, adding corridors (linear strips of habitat) or stepping stones (small patches or isolated trees) to the matrix could help both long-range dispersal and multiple-patch use within a home range (Fischer & Lindenmayer, 2002; Saura, Bodin, & Fortin, 2014). A few, well-placed connections across a broad region may be enough to kick-start demographic or genetic rescue (Miquelle et al., 2015), but a greater number of connections within a more confined area may be needed if resource access is to be improved throughout multiple home ranges (Keeley, Beier, Keeley, & Fagan, 2017; Newmark, Mkongewa, & Sobek, 2010).
Several frameworks have previously been developed for conceptualizing the structural composition of landscapes (Fischer, Lindenmayer, & Fazey, 2004; Forman, 1995; McIntyre & Barrett, 1992; Watson, 2002; Wiens, Stenseth, Van Horne, & Ims, 1993). However, these frameworks were not extended to classify the mechanisms driving isolation effects on animal populations. Jeltsch et al. (2013) explored how different types of movements occurred over characteristic spatiotemporal scales and impacted ecosystems as a whole, such as through interspecific interactions and resource transportation. We take an alternative focus and instead consider how different proximate mechanisms are affected by scale.
Driscoll, Banks, Barton, Lindenmayer, and Smith (2013) outlined how the quality of the matrix in fragmented landscapes influences proximate processes. Specifically, they discussed how features of the matrix influence species’ persistence in the landscape through subsidizing resource availability, altering abiotic microclimates and facilitating movements. For instance, woodland-dependent species may more readily travel between woodland patches interspersed with a pine plantation matrix than one that is more structurally dissimilar to the woodland (Driscoll et al., 2013). Our frame of reference aligns with that of Driscoll et al. (2013); we consider the role of functional connectivity on movement, which is heavily dependent on matrix quality. Thus, both frameworks are complementary for conceptualizing the impacts of habitat fragmentation. In contrast to Driscoll et al. (2013), we explore one of the three core effects of the matrix: how the consequences of movement disruptions affect species. We also elucidate the relationships between the type of mechanism affected by fragmentation and the distance or time-scale over which movements are restricted.
Many mechanisms other than those linked directly to physical isolation are important in fragmented landscapes, such as edge effects (Porensky & Young, 2013; Ries, Fletcher, Battin, & Sisk, 2004; Zurita, Pe'er, Bellocq, & Hansbauer, 2012). Although we do not specifically include these in our framework, they are nonetheless relevant. One component of edge effects is the inability to source sufficient resources, such as sheltered protection from predators (Bennett et al., 2013). Habitat gaps between shelter sites and feeding locations could pose substantial risks. These mechanisms are incorporated into our framework when they are driven by increased isolation, such as when habitat gaps make movement risky. However, these impeded mechanisms could also result from reduced habitat quality, such as when abiotic changes along edge habitat lead to changes in flora (Ries et al., 2004). It is important to distinguish whether habitat fragmentation or related changes such as degradation are impacting species because the conservation solutions differ.
5.2 Distribution of research effort among mechanisms
The most frequently examined of the three mechanisms was impeded gene flow. It is unlikely that the heavy weighting towards genetic articles is an artefact of the keyword choices used for each mechanism because this imbalance was also evident in an informal scan of articles produced by a search of just “habitat fragmentation.” It is unsurprising that the total number of articles increased over time given increasing publication rates and that the number of impeded gene flow articles increased in recent years given the greater availability and affordability of genetic technology (Bolliger, Lander, & Balkenhol, 2014; Storfer, Murphy, Spear, Holderegger, & Waits, 2010). However, given that molecular methods, such as assignment tests and pedigree analyses, can be used to infer dispersal movements (Holderegger & Wagner, 2008; Manel, Gaggiotti, & Waples, 2005), it is surprising that the frequency of articles investigating restricted demographic exchange has not similarly spiked over the past decade. Additionally, it is important to note that an increase in the proportion of publications studying impeded gene flow does not necessarily reflect a corresponding increase in our understanding of the issue.
The key mechanisms we have identified in this study can be considered to form an overlapping, non-nested hierarchy across scales. Each successive mechanism works at progressively larger scales (Wiens, 1989). When habitat is fragmented, it takes time for the evidence of the negative effects to become apparent and the duration of this time-lag is dependent upon where the affected mechanism sits in the hierarchy (Metzger et al., 2009; Wiens, 1989). As the scale of fragmentation increases, impacts on mechanisms may accumulate. Systems that show signs of impeded gene flow may also be subject to impediments to demographic exchange and resource access. Thus, neglecting to take the impacts on these lower-level mechanisms into account could compromise conservation efforts. Additionally, impacts can have synergistic effects on ecological systems (Brown, Harborne, Paris, & Mumby, 2016; Zanette, Smith, van Oort, & Clinchy, 2003). It is currently unknown what interactive effects, if any, operate among the three mechanisms discussed here, but synergisms may partly explain why fragmentation effects are amplified at very low levels of habitat extent (Andrén, 1994; Radford, Bennett, & Cheers, 2005).
5.3 Geographic and taxonomic biases
The overall bias against limited resource access and restricted demographic exchange as a focus of research was disproportionately pronounced in particular taxa, notably reptiles, amphibians and fish. It is difficult to generalize across taxa, but reptiles and amphibians in particular contain a greater proportion of less-vagile species than birds, large mammals and flying insects (Allentoft & O'Brien, 2010; Mac Nally & Brown, 2001; Rivera-Ortíz, Aguilar, Arizmendi, Quesada, & Oyama, 2015). A considerable gap in knowledge about mechanisms operating at small-to-intermediate scales in generally less-mobile taxa could have important implications. For instance, landscape structure across small extents may have a greater impact on sedentary species than on nomadic species (Maron, Goulding, Ellis, & Mohd-Taib, 2012). It is important to gather a body of empirical data across a range of dispersal capabilities, range requirements, generation times and environments (notably aquatic versus terrestrial) both within and between taxa. Without this, it would not be appropriate to generalize findings.
As has been often identified in literature reviews (Bayard & Elphick, 2010; Lawler et al., 2006; Magle, Hunt, Vernon, & Crooks, 2012), the Southern Hemisphere and the tropical/subtropical regions were underrepresented in the literature we studied. However, the frequency that each mechanism was studied was consistent across geographic locations. The relative importance of different types of habitat fragmentation effects may vary between the northern temperate zones and lower latitudes. Many species undertake long-distance migrations to avoid harsh winters in the northern temperate region, while lower latitudes have a greater proportion of sedentary species (Chesser, 1998; Newton & Dale, 1996a, 1996b). Thus, there are important differences in dispersal ability, life history traits and population demography in species between these regions plus abiotic factors such as climate and productivity which may affect the relative influence of both habitat fragmentation and each of the three mechanisms on species’ responses (Betts et al., 2014; Newbold et al., 2013; Rivera-Ortíz et al., 2015). Therefore, geographic biases in research effort, especially when they occur in conjunction with a bias towards a particular mechanism, have the potential to hamper our ability to draw broad conclusions and develop appropriate conservation strategies accordingly.
6 CONCLUSIONS
Habitat fragmentation poses a significant threat to species, but there is still uncertainty about the mechanisms through which population changes occur (Banks et al., 2007). More research targeting potential proximate causes is needed to clarify our understanding, identify species at risk and assess the benefits to targeted species of proposed conservation solutions. Our framework is designed to stimulate thinking about the mechanisms potentially affected by habitat fragmentation, how the mechanisms relate to the spatial and temporal scales of movement restriction, the hierarchical nature of the mechanisms, and the consequences of having multiple mechanisms impeded simultaneously.
It has long been recognized that taxonomic and geographic biases in research effort have plagued ecology (Clark & May, 2002; Gaston & May, 1992). We found similar biases, such as relatively few studies on invertebrates considering their taxonomic diversity. Here, our focus was not on the proportion of studies conducted in each taxon or geographic zone. Rather, we emphasized the increasing disparity in research effort among mechanisms through which populations are affected (limited resource access, restricted demographic exchange and impeded gene flow) and why this is concerning in the light of the existing taxonomic and geographic biases. Given the global threat of land use change to species, it is paramount that landscape ecologists derive a deeper understanding of the mechanisms through which habitat fragmentation drives ecological change. This could be achieved by finding innovative ways to approach studies of resource access and demography, such as with stable isotope tracers (Robb et al., 2011), drone technology (Whitehead et al., 2014) and radio frequency identification (RFID) systems (Bonter & Bridge, 2011). Focusing research attention on mechanisms, rather than simply patterns of species occurrence and distribution, will garner new insights and progress our understanding of the threats posed by habitat fragmentation.
ACKNOWLEDGEMENTS
We thank David Watson for helpful discussions while conceptualizing the framework. A.J.C was supported by an Australian Postgraduate Award, a BirdLife Australia Stuart Leslie Bird Research Award, a Birds Queensland Research Grant, and student research grants from the Wildlife Preservation Society of Queensland and the Australasian Society for the Study of Animal Behaviour. M.M. was supported by ARC Future Fellowship FT140100516.
REFERENCES
BIOSKETCH
Anita Cosgrove completed her PhD at the University of Queensland. Her research focuses on understanding the impacts of habitat loss and fragmentation on woodland birds, including on their physiology.
Todd McWhorter's research focuses on the emerging area of conservation physiology and medicine. He aims to apply the concepts and methodologies of these fields to pressing problems in conservation of wildlife and biodiversity in general.
Martine Maron's research focuses on the drivers of landscape-level species richness, resource distribution and persistence of bird species in patchy landscapes, and how climate change will influence the persistence of species through its influence on resources.
Author contributions: A.J.C. and M.M. conceived the ideas; A.J.C. collected and analysed the data and led the writing; all authors revised the manuscript.




