Non-interacting impacts of fertilization and habitat area on plant diversity via contrasting assembly mechanisms
Abstract
Aim
The local- and regional-based forms of anthropogenic change reducing grassland diversity are generally identified, but these scale-dependent processes tend to co-occur with unclear interactive effects. Here, we explicitly test how common local and regional perturbations simultaneously affect plant alpha and beta diversity in a multiyear community assembly experiment using fragments of grassland habitat of various sizes. We hypothesized that local disturbances and decreasing patch size would interact, suppressing local diversity while homogenizing composition among patches.
Location
North America.
Methods
We conducted a three-year grassland assembly experiment, factorially manipulating local perturbation (nitrogen addition and mowing) and patch area for 36 patches over 13 ha. We quantified the individual and interactive effects of these local and regional factors on plant alpha and beta diversity within (quadrat scale) and among patches (patch scale). We also used a null model approach to disentangle between stochastic- and niche-based assembly mechanisms.
Results
We detected a gradient of assembly outcomes driven by two non-interacting factors—the effects of N fertilization on alpha (negative) and beta (positive) diversity regardless of spatial scale and the scale-dependant effect of increasing patch size on alpha (positive) and beta (positive) diversity. These effects unfolded over time, with the constraints on richness and composition shifting from dispersal-based during the first sampling year to perturbation-and size-based factors at year two and three. Fertilization effects were driven by a mixture of deterministic (i.e., selection at the species level) and stochastic (i.e., random extinctions) processes resulting in a decline in local richness but an increase in spatial heterogeneity in species composition. Area appeared to influence alpha diversity mainly via stochastic “sampling effect”—larger patches represented a larger sample of the regional pool. Niche-based processes, however, led to convergence in beta diversity among smaller patches driving a positive overall effect of area on beta diversity.
Main conclusion
Our results illustrate how diversity regulation in contemporary grasslands can be simultaneously shaped by local and regional factors acting additively but via contrasting assembly mechanisms that operate at different spatial and temporal scales.
1 INTRODUCTION
Many biological systems are losing diversity in association with human-based environmental change (Hoekstra, Boucher, Ricketts, & Roberts, 2004; Parr, Lehmann, Bond, Hoffmann, & Andersen, 2014; Pe'er et al., 2014). The main drivers are generally well described, often involving the transformation of local processes relating to species interactions within and across trophic levels (Borer et al., 2014; Parker, Burkepile, & Hay, 2006), changes in environmental heterogeneity (Melbourne et al., 2007; Stevens, Dise, Mountford, & Gowing, 2004) and regional processes relating to spatial limitations with habitat loss and patch isolation (Alstad et al., 2016; Holt & Gaines, 1993). While the identity of these drivers can be clear, their mechanistic influences on alpha and beta diversity are less well understood and difficult to test (Catano, Dickson, & Myers, 2017). On modern landscapes, human-induced changes to local and regional processes tend to occur simultaneously with the potential for combined and indirect impacts of perturbations (e.g., dispersal limitation shaping competitive outcomes; Crain, Kroeker, & Halpern, 2008). The end result for diversity can be variable, reducing species richness (alpha), shifting species composition by homogenizing diversity (reduced beta) or increasing species heterogeneity (increased beta) depending on how local and regional changes synergistically combine (Catano et al., 2017; Laurance & Cochrane, 2001).
These issues are especially relevant to plant diversity in grasslands, with both alpha and beta diversity affected by alteration of biogeographic factors relating to habitat loss and isolation interacting with perturbation (Germain et al., 2013; Harvey & MacDougall, 2014). Human-driven effects on local-scale (e.g., plot or site level) processes can involve resource increases and reduced environmental heterogeneity acting to decrease grassland diversity (Harpole et al., 2016; Hautier et al., 2014; Melbourne et al., 2007; Stevens et al., 2004) with the potential for perturbations such as herbivory and mowing to offset competitive exclusion when resources are high (Borer et al., 2014; Collins, Knapp, Briggs, Blair, & Steinauer, 1998; Proulx & Mazumder, 1998). However, regional-scale spatial limitations can alter these local processes, creating potential context-dependent additive, synergistic or cancelling-out effects (Frank, 2005; Hobbs, 2001; Laurance & Cochrane, 2001). For instance, higher colonization rates and higher edge-to-interior ratios associated with larger habitat area could relax the negative effects of fertilization on local plant richness by maintaining local populations of poor competitors in edge areas that would otherwise be excluded because of increased light competition (Borer et al., 2014). Similarly, perturbations may be less likely to cause extinctions in bigger patches with larger populations, via reduced environmental stochasticity (Gilbert & Levine, 2013). Alternatively, if stochastic extinctions dominate, smaller habitats could maintain diversity regionally via higher among-patch turnover in species composition, including the inadvertent protection of subordinate species if dominant competitors have dispersal inefficiencies (MacDougall & Turkington, 2006). In total, these uncertainties suggest a range of possible outcomes for plant diversity in response to interacting anthropogenic change, likely falling along gradients of alpha (local) and beta (compositional turnover) diversity that can be difficult to comparatively test (e.g., does alpha diversity tend to be lower in large fertilized patches versus small unfertilized patches? Do patterns of alpha and beta diversity respond similarly to perturbation interactions?). Understanding the relative impacts of these changes should inform how diversity is regulated in systems subject to co-occurring forms of anthropogenic-based perturbation, a scenario characterizing most grasslands globally (Hoekstra et al., 2004; MacDougall et al., 2014).
Here, we test the interactions between spatial context and perturbation regimes with a three-year multihectare grassland assembly experiment, with factorial manipulation of local perturbation (nitrogen fertilization and stand removal by mowing) and spatial context relating to patch size and isolation (represented by grassland patches of various sizes randomly distributed over 13 ha). Starting with bare cultivated soil, we quantified the interactive effects of these local and regional perturbations on local species richness (alpha diversity) and pairwise dissimilarity in composition (beta diversity) at both quadrat level (quadrats within patches) and patch level (total patch diversity) in assembling grassland plant communities. We also estimated, using a null model approach, whether beta diversity responses to those perturbations were more likely driven by stochastic drift or niche-based “selection” processes (e.g., drift: larger habitat randomly capturing more species regardless of species identity; selection: nutrients consistently selecting for some species over others; Catano et al., 2017; Chase, Kraft, Smith, Vellend, & Inouye, 2011; Püttker, de Arruda Bueno, Prado, & Pardini, 2015).
Based on the above, we predicted that (1) fertilization will decrease species richness in patches (Collins et al., 1998; Stevens et al., 2004) and lead to higher beta diversity mainly via stochastic extinctions (Chase, 2010; Koerner et al., 2016) and (2) patch size and mowing will increase species richness respectively via stochastic colonization (more colonization on bigger island—e.g., MacArthur & Wilson, 1967) and by offsetting competition for light (Collins et al., 1998), and both will decrease beta diversity by facilitating the recruitment and persistence of a larger sample of the regional pool in each patch. This large-scale experimental design allowed us to untangle the context-dependent drivers (i.e., space and perturbation) of alpha richness and beta diversity, while potentially identifying the mechanisms associated with these outcomes. This includes quantifying treatment effects on mean and variability of ground-level light given the influence of light on diversity in many nitrogen-enriched grasslands (Borer et al., 2014; Harpole et al., 2016; Hautier et al., 2014) and measuring the species-level responses to the treatments that underlie community-level changes to alpha richness and beta diversity. We also explored the temporal trajectory of assembly from one year to the next, given that the relative strength of local and regional influences on community development can vary over time, with random dispersal potentially more influential earlier in the assembly process (Tilman, 2004; Walker & Chapin, 1987).
2 MATERIALS AND METHODS
2.1 Experimental design
Our work commenced in 2012 at the rare charitable research reserve (Cambridge, Ontario, Canada, 43°22′18″N—80°21′44″W, see Figure S1). The experiment was conducted using 36 grassland patches of three different sizes (25,100, 400 m2) that were randomly distributed over 13 ha area (Figure S1). This area had been intensively farmed (cropped) for at least 75 years prior to the start of our work, such that each patch assembled from identical starting conditions of cultivated bare soil. Our design allowed us to account for the effects of both patch size and isolation distance on assembly, with patch location randomly varying in isolation from other patches and from an adjacent 12 ha “mainland” area composed of oldfield plant species (Figure S1, see Harvey & MacDougall, 2014). The average distance among patches (averaged from the pairwise patch distance matrix) was 168 ± 78 m with the closest patches separated by 30 ± 14 m (Figure S1). Plant colonization of the patches was assumed to derive from two sources: colonization by wind dispersal from the oldfield given that all others areas in close proximity were forested or weed-free crop fields (Figure S1) and, to a lesser degree given the legacy of annual herbicide application prior to the start of our experiment, recruitment from seed bank deposited prior to patch establishment. The area between the patches (“matrix”) was continually mowed to a height of 10 cm (~1/month) for the entire experiment to maintain isolation. Before the start of the experiment, we assessed initial within-field variability by measuring soil moisture, N and C. The differences between samples from different locations within the field were relatively small (Table S1).
For three sampling seasons (2012–2014), we imposed one of four perturbation treatments to each patch: addition of nitrogen (10 g N m−2 of urea pellets [46-0-0] once per year in early June), removal of all standing litter at the end of the growing season by mowing to ground level, nitrogen and removal combined, and control. This level of N application is considered to be above estimated current total levels of chronic N deposition globally (Stevens et al., 2015), but in line with deposition rates within some industrialized regions and commonly used in long-term tests of N enrichment (Borer et al., 2014; Bowden, Davidson, Savage, Arabia, & Steudler, 2004; Clark & Tilman, 2008). The late autumn mowing treatment involved mowing to ground level and raking off all aboveground biomass (biomass was senesced by this time) and was implemented to increase bare ground and ground-level light for the following spring. The largest biomass removed was on fertilized plots, given the positive effects of N addition on standing biomass and litter accumulation (see Appendix S2). The treatments were replicated three times per patch size (4 treatments × 3 sizes × 3 replicates = 36 patches).
2.2 Plant sampling
Plant sampling in the patches was conducted at peak growing season (late July–early August) from 2012 to 2014. We measured plant cover, plant composition and species richness from various numbers of 4 m2 quadrats randomly located in each patch: two quadrats per 25 m2 patches, three per 100 m2 patches and five per 400 m2 patches, with the differences in sampling intensity by patch size accounted for by rarefaction analyses (described below). The average distance from a quadrat to other quadrats within a patch is 1.3 ± 0.3 m in smallest patches (25 m2), 3.1 ± 0.7 m in intermediate patches (100 m2) and 7.6 ± 1.3 m in largest patches (400 m2).
We sampled aboveground plant biomass in one location in each patch by harvesting a 20 × 100 cm strip clipped to ground level, with clipping occurring in a different corner of each patch in the 3 years of the study where there were no 4 m2 quadrats. Plant biomass was sorted into forbs, grass and litter and dried at 68°C for 48 hr before being weighed.
In 2013, we measured ground-level light at peak biomass with a 1-m-long quantum sensor wand in each 4 m2 quadrat (Apogee instruments, Utah, USA). It is noteworthy that because we measured ground-level light at peak biomass, this measure does not reflect the influence of our mowing treatment on light availability but rather the community-level consequences of our different treatments on light during the growing season.
In this study, we focus on changes in plant richness and composition extracted from the plant cover data, which were sampled in each quadrat for the 3 years period (Figures S2 and S3). Biomass (forb, grass and litter) and light data are used as supplementary information to support our interpretations. All biomass and light analyses are presented in the online supplementary information (Appendix S2, Figures S4 and S5, and Tables S3 and S4).
2.3 Statistical analyses
We tested the interactive effects of our spatial and perturbation treatments on changes to plant diversity, comparing plant species richness (alpha diversity) and pairwise dissimilarity in species composition (beta diversity) at two spatial scales (quadrat level and patch level). Quadrat-level comparisons tested differences in richness and composition of all 4 m2 quadrats as influenced by the fertilization and mowing treatments interacting with patch area. Patch-level comparisons pooled all quadrat data for each patch, testing for differences in numbers of species per patch and differences in composition among patches. We focused our analysis on the last 2 years of sampling (2013 and 2014) because sampling in 2012 occurred before the first mowing event (see Appendix S1 and Table S2 for details on year 1).
Recognizing that the regulation of diversity can be scale-dependant, for each analysis, we looked at quadrat-level and patch-level effects of our treatments. We were especially interested in testing the relative interaction of fertilization and mowing, in the context of habitat area. At quadrat level, we tested the variation in alpha (richness) and beta diversity (among quadrat dissimilarity in plant species composition) among our 120 4 m2 sampling quadrats (see below for blocking details and statistics). At patch level, we tested the variation in alpha and beta diversity among our 36 patches. We extracted patch-level diversity from the averaged plant cover data from each quadrat within a given patch. Again, we used rarefaction to account for differences in sampling effort among patches (see below) to ensure that our patch-level values were comparable.
2.3.1 Alpha diversity
- To compare the accumulation of species across years, we produced species accumulation curves (SAC) for each sampling year and patch size, for quadrat-level vs. patch-level richness accumulation. Comparing SAC among quadrats and patches can provide insights on the main mechanisms driving area-richness patterns such as the potential relative strength of local (biotic interactions and environmental filtering) versus regional processes (Chase & Knight, 2013). For instance, if the effects of patch size on accumulated species richness only appear at patch level and not at the quadrat level, this would suggest that patch size does not influence species richness by increasing plant density (i.e., a quadrat in a large patch does not have higher richness than a quadrat in a small patch) but rather by random sampling effect (i.e., larger patches cover more area and thus contain a larger sample of the regional pool). Here, we used random permutations of the data to determine mean SAC and its standard deviation.
- For richness in both quadrats and patches, we conducted a four-way linear mixed effect model (LME) testing for the interactive effects of patch size, fertilization by nitrogen, mowing and sampling year (2013 vs. 2014). The potential influences of patch isolation were tested by adding distance to the mainland and distance to the nearest patch as covariates. To further test direct effects of the spatial structure of our experimental design (spatial organization of patches), we also added principal coordinate analysis of neighbour matrices (PCNM; Borcard & Legendre, 2002) axes as covariates in the model. PCNM analyses extract spatial contrasts among patches by transforming spatial distances to rectangular data. Those contrasts can provide insights on the spatial dynamics that can otherwise go undetected (e.g., all patches on the edge of the experimental design have higher richness than patches in middle). Prior to being added to the LME, those axes were selected based on a forward selection by permutation procedure (Blanchet et al., 2008). To control for temporal pseudo-replication issues, we added replicates and time as nested random factors: at the quadrat level, our random term was among-year variation conditional on the variation of quadrats nested within patches and at the patch level, our random term was among-years variation conditional on among-patches variation. For each LME model, we used an AIC-based simplification procedure, removing terms sequentially, starting with the highest level of interactions. We first fitted models during model selection using maximum likelihood (“ML”) and the “BFGS” optimization method (Nash, 1990), but the final models were refitted by maximizing the restricted log-likelihood (REML; Pinheiro, Bates, DebRoy, & Sarkar, 2016). Finally, we tested the significance of each term in our LME model using an F-test (Table 1).
Table 1. Test of the effect of habitat area, fertilization (F) and mowing (D) on plant species richness (alpha diversity) at both quadrat and patch level. The table shows parameter estimates of the F-tests on two linear mixed effect models following an AIC-based simplification procedure (see Section 2 for details). The initial model at quadrat level tested for the individual and interactive effects of patch area, mowing and fertilization, with distance to mainland, distance to nearest quadrat and continuous time as covariates. The initial model at patch level tested for the individual and interactive effects of patch area, mowing and fertilization, with distance to the mainland, distance to the nearest patch, PCNM axes and continuous time as covariates. To control for temporal pseudo-replication issues, we added replicates and discrete time as nested random factors: at the quadrat level, our random term was among-year variation conditional on the variation of quadrats nested within patches and at the patch level, our random term was among-years variation conditional on among-patches variation Quadrat level Patch level df F-value df F-value Intercept 119 939.28*** 36 1126.74*** Area 27 17.39*** Mowing 27 0.81 Fertilization 34 27.39*** 27 20.77*** Time 119 4.27* PCNM axis 2 27 8.97** PCNM axis 21 2.61 Area×Mowing 27 3.11† - ***p < .001; **p < .01; *p < .05; †p < .1.
2.3.2 Beta diversity
- For quadrat-level and patch-level beta diversity, we performed a four-way permutation analysis of variance (PERMANOVA, 9,999 permutations) testing for the interactive effects of patch size, fertilization, mowing and sampling year (2013–2014) on variation in species composition (Anderson & Walsh, 2013). To control for pseudo-replication issues in the PERMANOVA analyses, permutations were constrained (i.e., stratified) by the appropriate sampling unit: to quadrat nested in patches (quadrat level) or to patches (patch level). PERMANOVA analyses are known to confound effects caused by changes in mean dissimilarity among treatments (centroid, compositional change) versus changes in distance to the centroid (within-treatment dissimilarity, i.e., beta diversity; Anderson & Walsh, 2013). Therefore, for each significant term from the PERMANOVA, we also performed a post hoc one-way PERMDISP analysis (multivariate homogeneity of group dispersions) that tested for differences in within-treatment variation in plant community similarity (Anderson & Walsh, 2013). As an analogue to our use of species richness as a proxy of alpha diversity, observed beta diversity was calculated as the Jaccard dissimilarity computed from the plant composition (presence–absence) matrix (Legendre & Legendre, 2012).
- Beta diversity changes can suggest how perturbations affect community assembly, in deterministic ways based on traits where the same species are consistently selected or stochastically where outcomes on species composition are driven by random sampling effects such that species identity is unpredictable (Chase & Myers, 2011). However, the relative importance of these deterministic versus stochastic mechanisms on beta diversity can be confounded by changes in alpha diversity. For instance, a decline in beta diversity (biotic homogenization) can be caused by a random increase in the number of individuals in the communities (random sampling from the species pool; Olden & Poff, 2003). Significant changes in beta diversity after controlling for such random sampling effects can be interpreted as possible evidence for deterministic, niche-based assembly (Catano et al., 2017; Chase et al., 2011; Püttker et al., 2015). On the contrary, if significant changes in beta diversity are no more detectable after controlling for such random sampling effect, this can be interpreted as possible evidence that the changes were more attributable to stochastic, drift-based assembly (Catano et al., 2017; Chase et al., 2011; Püttker et al., 2015).
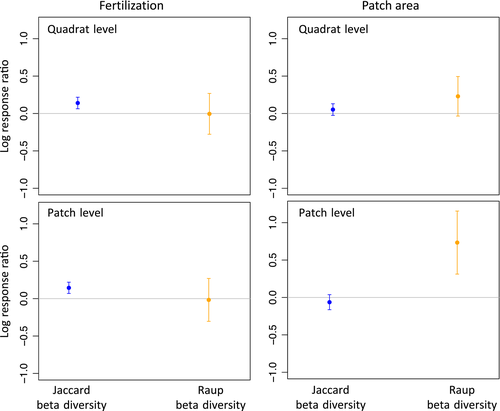
Therefore, as a complementary approach to help disentangle how perturbation influenced beta diversity, we compared our observed changes in beta diversity (hereafter “Jaccard beta diversity”) to changes in beta diversity after controlling for random changes in alpha diversity (Raup-Crick index; Catano et al., 2017; Chase et al., 2011; Püttker et al., 2015). The Raup-Crick index accounts for random sampling by calculating the probability of obtaining the observed or fewer shared species based on random re-sampling from the species pool (Chase et al., 2011). This probability is assessed by comparing the composition dissimilarity in the data against null communities (using 9,999 null community realizations). For each null community, alpha diversity at each site is the same, calculated from observed data, but species composition is randomly sampled from the species pool with the probability of each species being drawn equal to the proportion of sites that they are present (sampling is neutral with respect to species identity; Chase et al., 2011). Indeed, not all changes in alpha diversity are stochastic; however, this null model approach allows us to target and account for these changes in alpha that are purely stochastic with respect to species identity and could confound the influence of deterministic assembly mechanisms on beta diversity. We acknowledge that this incidence-based null model approach does not account for assembly mechanisms that mainly affect changes in relative abundance, which are outside the scope of this study, and that it can be sensitive to very low alpha-diversity level (Chase et al., 2011; Germain et al., 2013; Tucker, Shoemaker, Davies, Nemergut, & Melbourne, 2016). As with the PERMANOVA, for the observed (Jaccard) and expected (Raup-Crick) dissimilarities, we calculated beta diversity (average distance to centroid) using PERMDISP analyses for each treatment (Anderson and Walsh. 2013). We then calculated log response ratios (e.g., ln[βfertilized/βunfertilized] and ln[βlarge.patch/βsmall.patch]) and corresponding 95% confidence intervals.
2.3.3 Species-level change
Changes to alpha or beta diversity are driven by species-specific responses to treatments, yet those underlying effects are rarely tested. We thus conducted an “indicator species analyses” to test whether the interactive effects of our treatments on diversity could be linked to specific species or groups of species. We did this with a multilevel pattern analysis, which chose the combination of treatments with the highest association value (here indicator value) for each species in the community (Cáceres & Legendre, 2009).
2.3.4 Complementary information
Finally, we used plant biomass and ground-level light to support our interpretations on changes in alpha and beta diversity. Because those analyses are performed at patch-level only (and thus the inherent nested structure of our spatial design is irrelevant), we used a simple four-way linear model testing the influences of patch size, fertilization, mowing and sampling year on total plant biomass, relative plant biomass (forb:total biomass ratio) and detritus biomass. As covariates, we also added potential influences on biomass from distance to the mainland, nearest patch and spatial structure of the experimental design (selected PCNM axes, see above). For light availability, we used a three-way linear model testing the influence of patch size, fertilization and mowing. Results for biomass and light availability are presented in Tables S3 and S4 respectively.
All statistical analyses used r 3.1.2 statistical software (R Core Team, 2016). To conduct the LME models, we used the “nlme” package (Pinheiro et al., 2016). SAC, PERMANOVA and PERMDISP analyses were performed using the “vegan” library (Oksanen et al., 2015). The Raup-Crick index was calculated using the “raupcrick” function from the “vegan” library (Chase et al., 2011; Oksanen et al., 2015). The indicator species analyses were conducted with the “indicspecies” package (Cáceres & Legendre, 2009).
3 RESULTS
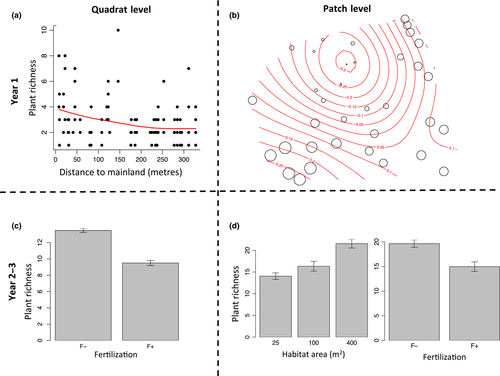
We tested the interaction of local perturbation-based and regional spatial-based factors on the species richness and compositional dissimilarity of assembling plant communities at two different spatial scales (quadrat level vs. patch level). We detected a gradient of assembly outcomes from high to low for both diversity and degree of species dissimilarity, driven by two independent (non-interacting) factors—the effects of N fertilization on alpha (negative, Table 1, Figure 2) and beta (positive, Table 2, Figure 3) regardless of spatial scale and the scale-dependant effect of increasing patch size on alpha (positive, Table 1, Figure 1) and beta (positive, Table 2, Figure 3). These effects unfolded over time, with the constraints on richness and composition shifting from dispersal-based (distance from mainland and proximity to the edge of the experimental design, see Figure 2a,b) during the first sampling year (2012, see Table S2) to perturbation-and size-based factors in 2013–2014 when there were no more detectable effects of distance from the mainland (Figure 2c,d, Table 1). Further, we detected indications of significant influences of deterministic and stochastic processes that both shaped diversity responses to fertilization and patch size, in ways that would have been indistinguishable without the use of multiple complementary analytical approaches (e.g., null model versus species-level indicator analysis—see below).
| Quadrat level | Patch level | |||||
|---|---|---|---|---|---|---|
| df | F | R 2 | df | F | R 2 | |
| Size (S) | 2 | 4.15*** | .03 | 2 | 3.68*** | .07 |
| Fertilization (F) | 1 | 24.33*** | .07 | 1 | 8.49*** | .08 |
| Mowing (M) | 1 | 6.03*** | .02 | 1 | 3.16*** | .03 |
| Time (T) | 1 | 26.27*** | .08 | 1 | 11.58*** | .11 |
| Nearest quadrat | 1 | 1.79* | .01 | |||
| Dist. mainland | 1 | 5.68*** | .01 | 1 | 2.59*** | .02 |
| Nearest patch | 1 | 1.36*** | .01 | |||
| PCNM axis 1 | 1 | 3.44*** | .03 | |||
| PCNM axis 2 | 1 | 3.54*** | .03 | |||
| PCNM axis 21 | 1 | 1.60*** | .01 | |||
| PCNM axis 3 | 1 | 0.95*** | .01 | |||
| S × F | 2 | 3.07*** | .02 | 2 | 1.79*** | .04 |
| S × M | 2 | 3.29*** | .02 | 2 | 1.95*** | .04 |
| F × M | 1 | 2.27** | .01 | 1 | 1.47*** | .01 |
| S × T | 2 | 1.48† | .01 | 2 | 0.85 | .02 |
| F × T | 1 | 5.92*** | .02 | 1 | 2.00* | .02 |
| M × T | 1 | 3.66*** | .01 | 1 | 1.54 | .02 |
| S × F × M | 2 | 3.78*** | .02 | 2 | 1.86*** | .04 |
| S × F × T | 2 | 1.18 | .01 | 2 | 0.82 | .02 |
| S × M × T | 2 | 1.18 | .01 | 2 | 0.81 | .02 |
| F × M × T | 1 | 1.33 | .01 | 1 | 0.55 | .01 |
| S × F × M × T | 2 | 1.23 | .01 | 2 | 0.31 | .01 |
| Residuals | 216 | .65 | 48 | .37 | ||
| Totals | 239 | 1.00 | 71 | 1.00 | ||
- ***p < .001; **p < .01; *p < .05; †p < .1.

Our complementary set of analyses indicate that the effect of N fertilization on alpha and beta diversity was driven by a mixture of stochastic (i.e., random extinctions) and deterministic selection at the species level (for eight of 103 plant species) with the former being dominant and resulting in a decline in local richness and an increase in spatial heterogeneity in species composition. More specifically, nitrogen addition was the strongest driver of plant richness decline (−4 ± 0.8 species at quadrat level and −5 ± 1.2 species at patch level, Figure 2, Table 1), while at the same time leading to higher among-patches composition dissimilarity (beta diversity, F1:70 = 12.92, pPERMDISP < .05 and Table 2). This divergence in community composition among fertilized patches was mainly, but not exclusively, driven by a decrease in richness that was random with respect to species identity (i.e., N addition led to stochastic extinctions, Figure 3). The species-level indicator analysis, however, did suggest some evidence for significant niche-based selection effects of fertilization against only eight of the total 103 species that constituted our regional pool (Medicago lupulina, Taraxacum officinale, Sorghastrum nutans, Desmodium canadense, Schizachyrium scoparium, all pIP < .05), with one completely absent of fertilized quadrats or patches (Trifolium pratense, pIP < .05), while favouring a few (Erysimum cheiranthoides, Sisymbrium altissinum, all pIP < .05).
Although not tested directly, these deterministic selection effects of fertilization were potentially linked to an observed reduction in total mean light availability in fertilized quadrats (Figure S5, Table S4). We also observed higher biomass of annual forb and perennial grass species in fertilized patches compared to the unfertilized ones (Figure S4), but with different species dominating, in terms of plant cover, in different years (Figure S3). In 2012–2013, we mainly observed tall early successional forb species in the larger fertilized patches (e.g., Chenopodium spp., Conyza spp.,), versus 2013–2014 when perennial later-successional grass species became more abundant (Sorghastrum sp., Elymus sp.,).
Patch size was the second main driver of plant richness (+5 ± 2 species in largest relative to smallest patches, Figure 2, Table 1). Our set of complementary analyses suggest that stochastic sampling effects led to higher alpha diversity in larger patches, while niche-based processes were inferred to lead to smallest patches being more similar in composition than larger patches after controlling for stochastic changes in alpha diversity with the null model (Raup-Crick beta diversity—Figure 3). The effects of patch size on diversity were sensitive to spatial scale—that is to say they were only observed for patch level alpha and beta diversity, but not for quadrat level. This means that a 4 m2 sampling quadrat in a larger patch (400 m2) was not on average more species rich than a 4 m2 quadrat in a smaller patch (25 m2) (Figure 1a and Table 1). However, the pooled number of species in a patch was significantly higher in larger (400 m2) than in the smallest patches (25 m2) (Figure 1b and Table 1). This suggests sampling effects on alpha diversity where larger patches simply have more species because they sample more individuals. This supports our finding that beta-diversity patterns with patch size were obscured by random changes in alpha diversity (i.e., no detectable effects on Jaccard beta diversity).
We did not detect any strong interactive effects of our treatments on either plant alpha or beta diversity (see low R2 values in Table 2). However, the species-level analysis indicated that some species of the 103 that composed our regional pool were sensitive to certain treatment combinations, with these results consistent with their life history strategies. All occurrences of Cirsium altissimum (perennial ruderal forb; pIP < .05) were found within large (400 m2) and mowed patches, 70% of Lactuca canadensis (annual–biennal ruderal forb; pIP < .05) were found in quadrats from large (400 m2) and fertilized patches, 71% of Aster ooentangiensis (perennial late-successional forb; pIP < .05) were found in quadrats from intermediate (100 m2) and unfertilized patches, and 91% of Pycnanthemum virginiamum (perennial late-successional forb; pIP < .05) were found in quadrats from unfertilized and unmowed patches. These results indicate that despite overall weak interactive effects of perturbations on the plant community as a whole, multiple-stressor interactions can still affect local occurrences of specific species, as would be expected but rarely tested with analyses of diversity regulation.
4 DISCUSSION
Tests of concurrent influences of spatial and perturbation-based processes on diversity regulation are rare (Catano et al., 2017; Laurance & Cochrane, 2001). Here, we used a unique study design that isolated the scale-dependent individual and combined effects of several key changes typifying anthropogenic landscapes on richness and composition (Chase & Knight, 2013). We found significant additive (non-interactive) effects of fertilization and habitat area on both alpha and beta diversity, but via contrasting assembly mechanisms (niche-based versus stochastic processes) and with the direction and magnitude of these effects varying by scale (quadrat vs. patch). There was evidence that the lack of interaction between fertilization and area derived from the different ways that each affected species occurrences. Fertilization appeared to drive mostly stochastic extinctions thus reducing alpha but increasing beta diversity among patches. Area effects were influenced by a mixture of random sampling effects on alpha diversity and selection effects on beta diversity that led to both higher species richness and increased dissimilarity among larger patches. Dispersal-related predictors (distance to the mainland and from the edge of the experimental site, see Figure 2a,b) had detectable influences on diversity at year one, but this effect was less evident over time as other assembly mechanisms became more dominant. In total, our results illustrate how diversity regulation in contemporary grasslands can be simultaneously shaped by the independent influences of local and regional factors acting additively but via contrasting assembly mechanisms (stochastic- and niche-based processes).
The effect of habitat area on plant richness (alpha diversity) was only detectable when pooling information at the patch level, suggesting that influence of patch size on plant richness was mainly driven by sampling effects: larger patches better sampled the regional pool through dispersal and recruitment from the seedbank. After accounting for these sampling effects on alpha diversity with the Raup-Crick null model, we found that larger patches were more dissimilar in composition than smaller ones presumably through niche-based mechanisms. This result is in contrast with classic expectations of smaller patches experiencing more drift, thus leading to higher dissimilarity (Arroyo-Rodríguez, Pineda, Escobar, & Benítez-Malvido, 2009). On the contrary, in our study, larger patches seem to diverge in composition through stochastic sampling effect on alpha richness and niche-based processes leading to compositional convergence of smaller patches. A previous study on forested fragments showed the same dominance for niche-based processes with habitat loss for small mammals, but no specific process could be identified (Püttker et al., 2015). Although we did not test for specific processes, the selection effect we observed in response to habitat loss can potentially have unfolded via smaller patches being dominated by edge effects and thus being more environmental homogenous and similar in composition compared with larger patches.
On the contrary to patch size, fertilization strongly reduced plant richness (alpha) both at quadrat and patch levels. This is consistent with the expected negative effect of nitrogen on diversity (Stevens et al., 2004, 2015), including reductions of site-level mean light availability in fertilized quadrats and the deterministic selection against fertilized-averse species that was showed by the indicator species analysis (Borer et al., 2014). Deterministic selection by N fertilization should lead to spatial homogenization of the plant community; however, this species-level selection only affected eight species of the 103 present in the regional pool, likely not enough to have a strong imprint at the community level. The null model results rather suggest that fertilized patches were more dissimilar in composition (higher beta diversity) mainly because of stochastic extinctions (see Figure 3). Our results thus help to clarify the often seemingly inconsistent effect of fertilization on compositional turnover (Chase, 2010; Koerner et al., 2016) and suggest that it influences plant diversity via both trait-based selection and stochastic processes, but with the latter being dominant to explain regional beta-diversity changes (Arroyo-Rodríguez et al., 2013; Chase, 2010; Fukami, 2004). Overall, our results suggest that the combined effects of human-derived influences on resources (i.e., eutrophication, late-season mowing affecting light) and habitat loss are additive rather than synergistic or antagonistic, clarifying how the co-occurring influences of habitat loss and eutrophication may combine to affect plant diversity.
The results of our experiment suggest how habitat loss and perturbation on plants may spin-off to affect other processes, especially trophic dynamics given the importance of plant diversity and composition for consumer spatial distribution and diversity regulation in our patches, but elsewhere too (Borer et al., 2014; Haddad, Crutsinger, Gross, Haarstad, & Tilman, 2011; Harvey & MacDougall, 2015a,b). For instance, we know from previous work at this site that fertilization, mowing and habitat size significantly but indirectly affect the assembly of insect food webs through their influences on plant composition and the specialist insects that these plants support (Harvey & MacDougall, 2015b). It is well established that island size can influence trophic dynamics through the loss of major predators (Terborgh et al., 2001)—we observed a similar phenomenon at this site where the diversity of insect predators is compressed on smaller and more isolated patches (Harvey & MacDougall, 2015b). A critical finding of our work is the demonstration that such trophic alterations can be also mediated by indirect bottom-up pathways especially the species composition of plants. We found that fragmentation impacts of predators also arose via regional scale “biogeographic” limitations, with space and isolation shaping the availability of plant resources that in turn determined which herbivore and predator communities are likely to establish (i.e., so-called trophic dependencies where specialist herbivores and their specialist predators do not establish until their food plants do; Harvey & MacDougall, 2014). In short, the deterministic and stochastic factors shaping plant diversity in response to perturbations are likely to also determine effects on the whole food webs.
The temporal and spatial complexity of global environmental change can obscure its causes and consequences for local diversity and function (Flores-Moreno et al., 2016; Simkin et al., 2016). Not only can human-induced perturbations combine additively (Crain et al., 2008), synergistically (Cowles, Wragg, Wright, Powers, & Tilman, 2016) or antagonistically (Jackson, Loewen, Vinebrooke, & Chimimba, 2016), but the direction of the interaction can also change as a function of the local environmental context (Jackson et al., 2016) and the ecosystem function studied (Cowles et al., 2016). This illustrates that predicting diversity changes requires an understanding of the complex interplay between spatial context and disturbance regimes. Although our study was performed at relatively smaller scales than which habitat fragmentation generally occurs, our results illustrate the underlying mechanisms that could otherwise be confounded or hard to detect in nature (Debinski & Holt, 2000). By investigating effects at two contrasting spatial scales (quadrat vs. patch level), we covered two order of magnitude in area (4 m2 to 400 m2) and were able to discern the dominant additive effects of our main diversity drivers: habitat area and fertilization.
The long-term implications of our results suggest that the effects of some perturbations such as habitat loss can accumulate and amplify (Haddad et al., 2015). Our multiyear experiment suggests that the negative effect of fertilization on local richness plateaued after only one year (no change in fertilization effect between 2013 and 2014, no fertilization-by-sampling year term in Table 1), while its positive effect on beta diversity strengthened significantly over all three sampling years (significant fertilization-by-sampling year term in PERMANOVA and PERMDISP, see Table 2). Predicting the effects of multivariate interactions among stressors, over a long period of time, does pose important statistical issues concerning complex interactive terms for which clear mechanistic interpretations can become quickly challenging (Mayfield & Stouffer, 2017). Nonetheless, our study helps illustrate how these effects can unfold across spatial and temporal scales.
ACKNOWLEDGEMENTS
We thank Amber-Lynne Lammers, April Clyburne-Sherin, Brianna Collis, Cara Bulger, Felicia Syer, Jenna Quinn, Jennifer Caws, Katherine McLeod, Kathryn Tisshaw, Peter Kelly and Timothy Skuse for field assistance and logistical supports. Carole Ann Lacroix assisted with plant identification. Jenny McCune provided valuable comments on the initial manuscript. We also thank three anonymous reviewers for comments. We thank two anonymous reviewers for useful comments. This project was financially supported by F.Q.R.N.T., the rare graduate student research fellowship, and the Queen Elizabeth II Graduate Research Fellowship (E.H.); NSERC, CFI, the province of Ontario (Early Researcher Award) and Mountain Equipment Co-op (A.S.M.); and substantial in-kind contributions from the rare scientific research reserve.
DATA ACCESSIBILITY
All r-formatted data and the main r-script to reproduce the results can be downloaded from Github (https://github.com/harveye/ddi_12697).
REFERENCES
BIOSKETCH
Eric Harvey is a postdoctorate fellow at the University of Toronto. His research focuses on the spatial impacts of global changes, such as altered disturbance frequencies, on the structure of food webs and the ecosystem function that they support. He is very interested in bridging ecology and conservation sciences by developing a research programme centred on the empirical testing of ecological theory.
Andrew MacDougall is an associate professor at the University of Guelph. His research focuses on the processes that determine the structure and function of plant communities and how those processes are altered by the covarying impacts of global environmental change.
Author contributions: E.H., A.S.M. designed the research; E.H. conducted the research and processed the data; E.H. analysed the data and wrote the first draft of the manuscript. E.H. and A.S.M. edited the manuscript.




