Protocol Biopsies in Delayed Graft Function Kidney Transplants From Brain-Dead Donors
Funding: This study was funded by grants from the Sigrid Jusélius Foundation, Finnish Red Cross Blood Service Research Funds, Finska Läkaresällskapet, Liv och Hälsa Foundation, Finnish Government Funds for Health Research (to I.H. and V.S.), and the Finnish Kidney Foundation (to A.A.).
ABSTRACT
Background
Delayed graft function (DGF) is a significant challenge in deceased donor kidney transplantation, impacting approximately one third of patients and is associated with acute rejection. Post-transplant acute rejection is monitored in kidneys with DGF through sequential protocol biopsies in 7–10-day intervals, according to guidelines based on research done before the current era of immunosuppression treatment. As acute rejection rates have decreased, there is a need to reevaluate the rationale of protocol biopsies.
Methods
We studied the histology of all biopsies taken during the first month post-transplant among recipients of brain-dead donor kidneys with DGF at our institution during 2006–2023. All recipients received corticosteroids as induction. Anti-thymocyte globulin was additionally administered to 3% and 18% receive basiliximab.
Results
A total of 1022 biopsies were studied from 678 recipients. Of the 678 recipients, 178 (26%) had rejection. Most acute rejection episodes occurred 7–10 days post-transplant. In case of no rejection in the first biopsy, rejection was found in 10% of the cases with subsequent biopsies. No risk factors for acute rejection in these later biopsies could be identified in regression analysis. The presence of donor-specific human leukocyte antibodies was associated with higher rates of antibody-mediated rejection during the first post-transplant month (20% vs. 1.3%, p < 0.001).
Conclusions
The first biopsy post-transplant in DGF kidneys has an important role in identifying early acute rejection. Of the patients with no rejection in the first biopsy, only 10% had a rejection in the second biopsy. Cases with borderline findings progressed to rejection in approximately one third of the cases. These data reflect the rate of early TCMR in deceased donor kidney transplants when 79% of patients did not receive an anti-T cell induction antibody.
Abbreviations
-
- ABMR
-
- antibody-mediated rejection
-
- DCD
-
- death by circulatory death
-
- DGF
-
- delayed graft function
-
- DSA
-
- donor-specific antibodies
-
- HLA
-
- human leukocyte antigen
-
- HLA-MM
-
- human leukocyte antigen mismatch
-
- KDIGO
-
- kidney disease improving global outcomes
-
- TCMR
-
- T-cell mediated rejection
1 Introduction
The need for kidney transplantations worldwide continues to grow, and the increasing demand leads to the use of more marginal kidneys from extended criteria donors, including donors after circulatory death (DCD). The use of marginal kidneys increases the risk of delayed graft function (DGF) [1, 2]. The rate of DGF in the literature depends on the definition used, and the reported rates vary between 19% and 55% [3-12], with higher rates being reported in extended criteria and DCD donors. Studying the nature and management of DGF is essential as it is associated with higher risks for graft loss [12-15] and acute rejection [11, 14, 15].
DGF is a complex process, typically thought to originate from ischemia-reperfusion injury, causing acute kidney injury. There are multiple processes associated with the occurrence of ischemic-reperfusion injury: cold and warm ischemia time, activation of the renin-angiotensin-aldosterone system, deficiency of the ACE2 enzyme, and formation of inflammation and fibrosis [16-18]. These cellular processes and consequential injuries are also part of processes resulting in acute rejection.
The occurrence of acute rejection is mostly concentrated to the immediate period following transplantation. Since acute rejection is associated with worse outcomes [19], early recognition is important. Differentiating between other causes of DGF and acute rejection is clinically challenging, and biopsies serve as the diagnostic method. The indication for biopsies in the early post-transplant period is most often DGF, but other findings include loss of graft function, recurrence of primary disease, drug toxicity, or thrombotic microangiopathy [7, 20]. Kidney Disease Improving Global Outcomes (KDIGO) guidelines from 2009 suggest taking protocol biopsies in 7–10-day intervals during DGF since DGF is associated with higher risks of acute rejection [20]. This suggestion is, however, based on low evidence, and is based on studies done before the current era of immunosuppressive treatment [21-23].
The optimal timing of early protocol biopsies taken during DGF has not been specified, and the frequency of pathological findings in these early biopsies has not been characterized in detail. Additionally, by considering donor and recipient characteristics, we aim to target sequential biopsies only to risk groups. We study this hypothesis using early indication biopsies taken within 30 days post-transplant of kidneys with DGF at our institution.
2 Methods and Materials
2.1 Study Population and Data Collection
This study was a retrospective observational registry analysis. The study population consists of patients who received a kidney transplant at our institution, Helsinki University Hospital, Finland, from February 9th, 2006 to December 31st, 2023. Helsinki University Hospital is the only transplantation center in Finland. Patients ≤ 16 years, multiorgan transplantations, living donor donation, DCD kidneys (started in 2021 at our institution), and pre-emptive transplantations were not part of the study population. We studied the histology of post-transplant biopsies taken from the kidneys with DGF. Kidneys that did not have biopsies taken (despite DGF) within the first 30 days post-transplant were excluded from the main analyses but used for sensitivity analysis. Inadequate biopsies were excluded. Second or third biopsies included in this study were taken as part of routine monitoring in cases where graft function had not improved significantly after the previous biopsy. Data were collected from electronic medical records and the Finnish Kidney Transplant Registry, a national registry obligated by law to follow-up kidney transplant patients.
All kidney recipients received corticosteroids as induction therapy. In cases with immunologic risk factors, basiliximab or anti-thymocyte globulin was additionally administered. Post-transplant maintenance immunosuppression comprised corticosteroids, an antimetabolite (primarily mycophenolate), and a calcineurin inhibitor (tacrolimus or cyclosporine A). Tacrolimus was chosen in immunologically higher-risk patients (retransplantation, calculated panel reactive antibodies >30%, poor human leukocyte antigen (HLA) match (worse than 2/1 AB/DR mismatch with emphasis on DR-matching) between 2006 and 2019, and in all patients since 2019. T-cell mediated rejections (TCMR) were managed with high-dose intravenous corticosteroids followed by oral corticosteroids. In cases where kidneys with DGF showed signs of borderline rejection, the same treatment protocol was implemented. For cases of antibody-mediated rejection (ABMR), in addition to intravenous corticosteroids, either plasmapheresis or immunoadsorption was performed, followed by rituximab and intravenous immunoglobulin administration.
One Lambda Labscreen mixed and single antigen beads with Luminex were used for pretransplant HLA antibody screening and identification with the use of HLA Fusion software (One Lambda Inc., Canoga Park, CA). Median fluorescence intensity values over 1000 were considered positive.
The Institutional Review Board of the Helsinki University Hospital approved this study (HUS/23/2024). The clinical and research activities being reported follow the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
2.2 Definitions
The definition of DGF used in this study, and in the Finnish Kidney Transplant Registry, is the need for dialysis within 7 days post-transplant.
Calculated panel reactive antibodies were calculated by comparing the known distribution of HLA antigens of Finns to the patient's HLA antibody profile.
2.3 Banff Criteria and Rejection Diagnosis
The histopathological changes used in this study were scored by experienced renal pathologists at our institution at the time of biopsy retrieval based on the Banff scoring system at the time [24]. If needed, lesion scores were used to reclassify the biopsies according to the 2018 reference guide [24]. All biopsies were paraffin-embedded. Along with updates to the Banff reference guides, new and updated lesion scores have some missing data in our study population. The following scores are therefore not included in our study: hyaline arteriolar thickening, arteriolar hyalinosis, inflammation in the area of IFTA, total inflammation, and polyomavirus load. Peritubular capillaritis has missing data because it was systematically scored from 2012 onward at our institution. Sensitivity analysis was performed because of the missing data and the results of these are presented in the Supporting Information.
2.4 Statistical Analysis
Categorical variables are described as the number of cases and percentages. Continuous variables are described as median and interquartile range as the data is not normally distributed. To assess statistical significance, the Mann-Whitney U-test was used for differences in the continuous variables, and Pearsons's Chi-squared test was used for categorical data. Fisher's exact test was used in the case of small sample sizes of categorical data. The rates of acute rejection are graphically described using histograms. The evolution of biopsy diagnoses is presented using Sankey diagrams. Potential risk factors for acute rejection in the second biopsy were studied using univariable logistic regression models. Correlations between human leukocyte antigen mismatch (HLA-MM), donor-specific antibodies (DSA), calculated panel reactive antibodies, and the lesion scores glomerulitis, tubulitis, interstitial inflammation, and intimal arteritis, were analyzed using Pearson biserial and Spearman correlation and visualized using boxplots and stacked bar charts.
Statistical analysis was performed using R (R Core Team 2023), RStudio (Posit Team, 2023), as well as the R packages tidyverse, gtsummary, ggsankey, gridExtra, colorspace, patchwork, and writexl.
3 Results
3.1 Characteristics of the Study Population
A total of 3577 kidneys were transplanted during the study period to adult recipients. Early graft function and non-function kidneys were excluded (n = 2556 and n = 84, respectively), as well as cases with missing data on graft function (n = 11). Living donors and DCD donors were not part of the study (n = 5 and n = 25, respectively). Pre-emptive transplantations were excluded from the groups mentioned above. Kidneys that did not have biopsies taken within the first 30 days post-transplant were excluded (n = 209). Inadequate biopsies (<3–5 glomeruli) were also excluded (n = 34). A flow diagram of the study population, along with inclusion and exclusion criteria, is shown in Figure 1. The final study population consisted of 1022 biopsies from the 678 recipients.
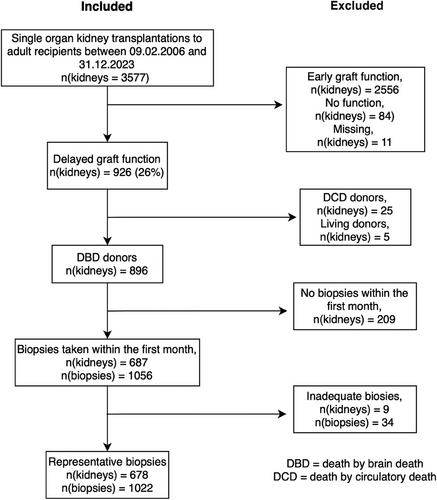
The median recipient age was 56 years and 69% of the recipients were male, while 58% of the donors were male and the median donor age was 61 years. DSAs were present in 11% of the cases while 26% had peak calculated panel reactive antibodies >30%. Acute rejection during the first 30 days was more common among younger recipients, in patients with DSAs, and retransplantations as well as among patients with tacrolimus as their first calcineurin inhibitor (Table 1). One hundred seventy-eight (26%) patients had acute rejection within the first 30 days, of which 3% were ABMR, 1% mixed, 14% TCMR, and 8% borderline (Table 2). In the study cohort, one biopsy had characteristics of coagulative tubular necrosis, and one biopsy showed frank infarction.
| Variable |
Overalla N = 678 |
No ARa N = 500 (74%) |
ARa N = 178 (26%) |
p valueb |
|---|---|---|---|---|
| Recipient age (years) | 56 (48, 65) | 57 (49, 65) | 54 (44, 62) | 0.002* |
| Recipient sex | 0.001* | |||
| Female | 211 (31%) | 138 (28%) | 73 (41%) | |
| Male | 467 (69%) | 362 (72%) | 105 (59%) | |
| Cold ischemia time (hours) | 20.7 (17.0, 23.8) | 21.1 (17.4, 24.1) | 20.0 (16.0, 23.5) | 0.012* |
| Donor-specific antibodies present | 76 (11%) | 36 (7%) | 40 (23%) | <0.001* |
| Missing | 2 | 2 | 0 | |
| Peak calculated panel reactive antibodies >30 | 175 (26%) | 109 (22%) | 66 (37%) | <0.001* |
| Missing | 3 | 3 | 0 | |
| Recipient kidney disease | 0.909 | |||
| Diabetes | 168 (25%) | 120 (24%) | 48 (27%) | |
| Glomerulonephritis | 174 (26%) | 128 (26%) | 46 (26%) | |
| Hypertension | 32 (5%) | 23 (5%) | 9 (5%) | |
| Polycystic | 126 (18%) | 96 (19%) | 30 (17%) | |
| Other | 178 (26%) | 133 (27%) | 45 (25%) | |
| Donor age (years) | 61 (53, 67) | 62 (54, 67) | 60 (50, 67) | 0.318 |
| Donor sex | 0.363 | |||
| Female | 287 (42%) | 206 (41%) | 81 (46%) | |
| Male | 391 (58%) | 294 (59%) | 97 (55%) | |
| Previous kidney transplant | 123 (18%) | 77 (15%) | 46 (26%) | 0.003* |
| Induction | 0.002* | |||
| Anti-thymocyte globulin | 22 (3%) | 11 (2%) | 11 (6%) | |
| Basiliximab | 120 (18%) | 79 (16%) | 41 (23%) | |
| Corticosteroids | 536 (79%) | 410 (82%) | 126 (71%) | |
| First calcineurin inhibitor | <0.001* | |||
| Cyclosporine A | 325 (53%) | 281 (62%) | 44 (27%) | |
| Tacrolimus | 293 (47%) | 176 (38%) | 117 (73%) | |
| Missing | 60 | 43 | 17 | |
| First antiproliferative | 0.468 | |||
| Azathioprine | 4 (1%) | 2 (0.4%) | 2 (1%) | |
| Mycophenolate | 613 (99%) | 454 (99%) | 159 (99%) | |
| Missing | 61 | 44 | 17 | |
| HLA AB mismatch | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 0.5 |
| HLA DR mismatch | 1 (0, 1) | 1 (0, 1) | 1 (1, 1) | <0.001* |
- Abbreviations: AR, acute rejection; HLA, human leukocyte antigens.
- a n (%); median (25%, 75%).
- b Wilcoxon rank sum test for continuous; Pearson's Chi-squared test for categorical variables, Fisher's exact test.
- * p value statistically significant, <0.05.
| Variable |
Overall N = 678 (100%)1 |
|---|---|
| Biopsy diagnoses | |
| No rejection | 500 (74%) |
| T-cell mediated rejection | 94 (14%) |
| Antibody-mediated rejection | 23 (3%) |
| Borderline changes | 57 (8%) |
| Mixed rejection | 4 (1%) |
| Histological lesion scores | |
| Interstitial inflammation 1;2;3 | 219 (33%); 73 (11%), 36 (5%)2 |
| Tubulitis 1;2;3 | 80 (12%); 24 (4%); 5 (1%)3 |
| Intimal arteritis 1;2;3 | 53 (8%); 30 (5%); 1 (0%)4 |
| Glomerulitis 1;2;3 | 34 (5%); 17 (3%); 14 (2%)5 |
| Peritubular capillaritis 1;2;3 | 34 (8%); 15 (4%); 2 (0%)6 |
| C4d staining 1;2;3 | 32 (5%); 20 (3%); 11 (2%)7 |
- 1n (%)missing n; 218,39,415,520,6269,741.
Two patients had missing data on the presence of DSA at the time of transplantation, and three patients had missing data on peak calculated panel reactive antibodies. Three biopsies had missing information on the number of glomeruli. Missing data on histological lesion scores are found in the footnote of Table 2. Patients with missing data were excluded from relevant analyses.
3.2 Timeline of Biopsy Diagnosis
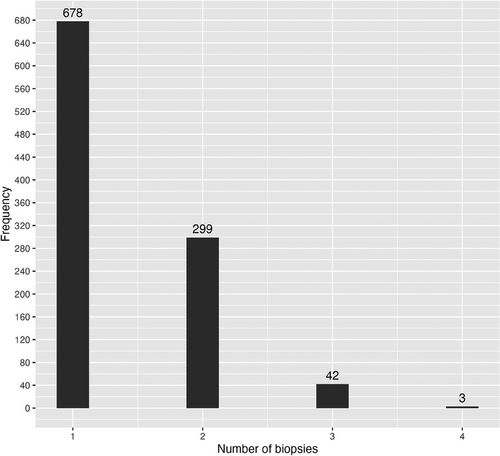
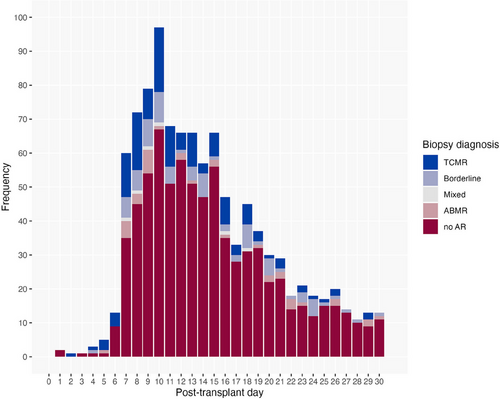
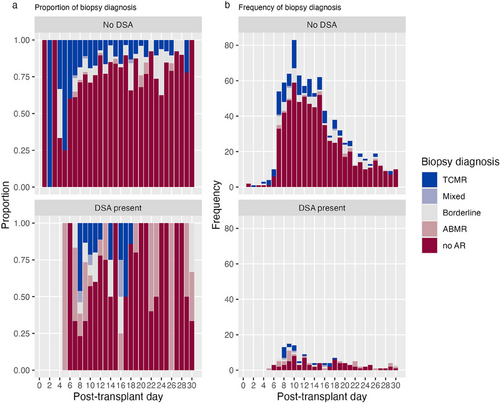
Figure 2 presents the number of sequential biopsies taken. Of the studied patients, 44% had two biopsies taken within the first month. The mean time between biopsies was 10 ± 3.7 days between the first and second biopsy and 9 ± 2.8 days between the second and third. Figure 3 presents the distribution of the biopsy diagnoses based on the post-transplant day. Rejection was most often diagnosed at 7–10 days post-transplant. Figure S1 presents the distribution of all rejection diagnoses based on post-transplant day. Figure 4a,b present the distribution of biopsy and rejection diagnoses based on the presence of DSAs. ABMR was more frequent when DSAs were present (20% vs. 1.3%, p < 0.001).
Sensitivity analysis was performed due to missing data on the lesion score PTC. The incidence of ABMR increased after 2012, when PTC scoring was systematically introduced at our center (Table S1 and Figure S2). Independent of the availability of PTC, rejection was most often diagnosed in early biopsies (Figure S3a,b).
In cases where more than one biopsy was taken post-transplantation, the findings in each sequential biopsy are visualized using a Sankey diagram (Figure 5). The diagram shows that in kidneys with no rejection in the first biopsy, subsequent biopsies revealed rejection in 10% of cases. Among 183 kidneys, the second biopsies found 3 cases of ABMR, 10 of TCMR, 5 borderline, and 165 without acute rejection. Additionally, borderline findings in the initial biopsy progressed to rejection in approximately one-third of the cases, despite treatment with steroids. Of the 42 third biopsies taken, two of the rejections were in kidneys with no previous rejection findings. Table S2 presents characteristics of cases with rejection in the second biopsy. Figure S4a,b presents similar results as Figure 5 regarding the evolution in protocol biopsies, based on the availability of PTC.
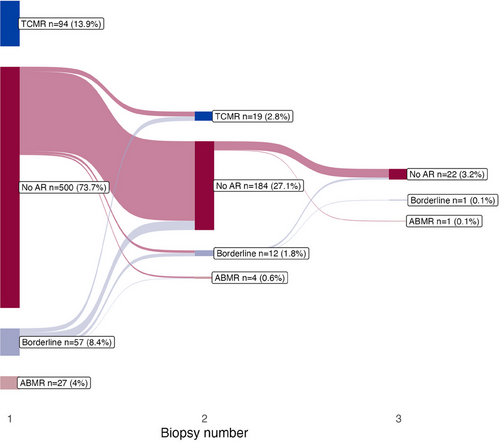
To further investigate the patients who were diagnosed with acute rejection after having no rejection in the first biopsy, we evaluated known risk factors for acute rejection. As there were so few cases of rejection, we studied the risks using univariable logistic regression models. We found no statistically significant risk factors for rejection in these cases (Table S3).
3.3 Immunologic Risk Factors and Histologic Lesion Scores
To test the secondary hypothesis that immunologic risk factors show higher frequencies of lesion score values greater than 0, we studied the correlations between these risk factors and the lesion scores. There was a weak positive correlation between peak calculated panel reactive antibodies and glomerulitis (r = 0.115, p < 0.001) and the median peak calculated panel reactive antibodies was higher among glomerulitis score >0. We found no statistically significant correlations between HLA AB-MM and the lesion scores. There was a weak positive correlation between HLA DR-MM and interstitial inflammation (r = 0.197, p < 0.0001), intimal arteritis (r = 0.103, p 0.0001), and tubulitis (r = 0.11, p < 0.0001). A higher percentage of interstitial inflammation scores greater than 0 was observed with increasing HLA DR-MM values, and this trend was also evident for tubulitis and intimal arteritis. There was a weak positive correlation between pre-existing DSAs and glomerulitis (r = 0.141, p < 0.0001), and higher glomerulitis scores could be seen when DSAs were present. These results are presented in Table S4 and Figure S5a-c).
3.4 Patients Without Biopsies
As 209 patients with DGF did not have biopsies taken, we compared the clinical characteristics of the patients who did not have biopsies taken during the first months to those who did (Table S5). The median time from transplantation to the onset of graft function was significantly shorter in the group with no biopsies compared to the patients with biopsies taken (7 and 13 days, respectively). This reflects the practice of taking the first protocol biopsies in case of DGF at 7–10 days post-transplantation, making the biopsy unnecessary when graft function was attained. Furthermore, the use of induction therapy was less frequent in the group without biopsies, and both patients and donors were younger in the group with no biopsies.
3.5 Immunosuppressive Protocols
As acute rejection is heavily influenced by the immunosuppressive therapies used, we compared the rates of rejection and differences in histological scores between patients based on the immunosuppressive protocols. In Table S6 we present the rejection rates and histological lesion scores based on the induction therapy used. The rejection rates were the highest when basiliximab was used, especially the rate of ABMR. As our protocols regarding the use of Tacrolimus changed in 2019, we compared the rejection and histological lesion score rates, as well as the use of other immunosuppressive drugs, based on whether the transplantation was done before or after 2019. Rejection rates were higher when the protocol shifted to the use of mainly Tacrolimus, versus when Tacrolimus was used primarily for patients with immunologic risk factors. The rate for ABMR stayed similar, while TCMR and Borderline rejections became more frequent. The most notable histological difference is the intimal arteritis score, where the rate of higher scores increased. Along with the shift in calcineurin inhibitor use, the use of induction therapies increased, especially basiliximab. The results are presented in more detail in Table S7.
4 Discussion
Our study showcased the practice of taking biopsies of DGF kidneys at 7–10 days post-transplant, which consequently led to the finding of most rejections at this point. This is consistent with previous studies [8, 25]. Through studying sequential biopsies, we also found that in cases where additional biopsies were performed after the first one taken during DGF, rejection seldom occurred in later biopsies within the first month. This suggests that further biopsies may be less important when rejection is absent in the initial biopsy. Moreover, in the majority of cases, no further biopsies were performed after the first one. We could not identify any specific risk factors for acute rejection in later biopsies if the first biopsy did not have rejection. We did, however, find that the pre-existing DSAs are associated with glomerulitis and that ABMR is more prevalent in these kidneys, which is in line with earlier studies [12, 26].
In the present study, most acute rejection episodes occurred in the first biopsy post-transplant. The significance of the first biopsy during DGF has been previously highlighted by a smaller study [8], as well as a recent review [27]. One study, on the other hand, argued that early protocol biopsies do not necessarily offer clinically significant information in low-immunological risk recipients with induction therapy, and suggested postponing the rejection diagnostics until post-operative Day 15 [7]. Another argument for minimizing the number of biopsies taken by protocol during DGF is the complications associated with biopsies. However, the rate of these is considered quite small, and routine biopsies are a safe procedure [28]. A study about the histology of early indication biopsies found that inflammation in the biopsy is associated with alloimmune risk factors but not with nonimmune risk factors. Furthermore, they did not find a correlation between the inflammation in the biopsies and DGF [12]. The identification of risk factors requires further research.
The immunosuppressive regimens in the current era are associated with lower rates of acute rejection [29], and for example, the rates of acute rejection in the United States have decreased steadily during the last decade [3]. Even though ABMR is less common than TCMR in the acute phase, ABMR has been found to be the most common cause of late graft failure [30]. One study explored the histologic differences in DGF kidneys with different induction therapies, and while thymoglobulin was found to be preventative of inflammation compared to basiliximab, no differences in survival were found [31]. Our study observed a higher percentage of acute rejection with both anti-thymocyte globulin and basiliximab compared to corticosteroids alone. It is important to note that in Finland, induction with anti-thymocyte globulin and basiliximab has primarily been used for high-risk patients, which probably explains the association with a higher risk of rejection. Induction practices vary globally and have evolved over time. Previous research has suggested that induction with anti-thymocyte globulin may reduce the risk of acute rejection in the early post-transplant period [7, 32], but is associated with a higher risk of infections [33].
The selection of calcineurin inhibitors during the peri-transplant period requires balancing the risk of graft failure and rejection against the nephrotoxicity associated with these drugs. One article found increased rates of acute rejection with lower calcineurin inhibitor exposure [34]. A meta-analysis evaluating different strategies for calcineurin inhibitor use concluded that reduced exposure was effective in improving renal function without increasing the risk of rejection [35]. The studies referenced by KDIGO in their guidelines on protocol biopsies had variable immunosuppressive protocols. Shapiro et al. studied rejection rates using a protocol consisting of tacrolimus and steroids. Additionally, 64% received mycophenolate as induction. The rate of acute rejection was 46% in the early graft function cohort and 57% in the DGF group [21]. Giral-Classe et al. studied the rate and longevity of DGF using 843 kidney transplants with Cyclosporin A as the calcineurin inhibitor. Mycophenolate was used in less than 10% of cases. The observed rejection rates were 29%–37.4% with higher rates seen with longer DGF duration [22]. Qureshi et al. studied 410 kidney transplantations for the rate of silent acute rejection. Their immunosuppressive protocol changed during the study period, but Cyclosporin A was used in both protocols; 75.6% of the patients received mycophenolate. The observed rejection rate was 50.8% in DGF kidneys [23]. In Finland, tacrolimus has been used in high-risk patients earlier as the first calcineurin inhibitor as opposed to cyclosporin A. Since tacrolimus became the calcineurin of choice for most patients, the observed rejection rates have paradoxically slightly risen in DGF kidneys from brain-dead donors. This trend may be attributed to a decrease in other causes of DGF, such as prolonged CIT, rather than an actual increase in rejection rates. The overall number of DGF cases has decreased over time as CIT has been reduced, leading to a higher relative proportion of rejection-related DGF cases. Over the last decade, our center has seen a dramatic decrease in the incidence of DGF in kidneys from brain-dead donors, partly as a result of successful work to reduce cold ischemia times [36]. New ways to meet the demand for kidney transplants have led to an increase in DCD and extended criteria donations worldwide. DCD is associated with a high incidence of DGF, highlighting the importance of studying DGF, despite the decrease in DGF rates observed with brain-dead donations. In the United States, the rate of DGF is increasing [3], which is probably at least partly related to the increasing rate of DCD and extended criteria donors. We chose not to include DCD donations in our study, since the DCD program started in Finland only a few years ago, and kidneys from brain-dead donors still compose most kidney transplantations at our institution.
Understanding DGF requires knowledge of multiple aspects of this process. A study about the proteomic profile of DGF kidneys in DCD donations found that there is a difference between the proteomic profiles in short versus long DGF duration as well as comparing these two groups to early graft function kidneys [37]. They showed that the molecular and metabolic features make the kidneys less able to cope with the stress reaction caused by the ischemia-reperfusion injury, which prolongs the time for the kidneys to recover post-transplant. Different biomarkers have been studied as predictors for slow graft function and DGF. For example, donor neutrophil gelatinase-associated lipocalin has been studied as a predictor and in some studies shown some predictive power [38, 39]. Because of the complexity, there is no one solution for minimizing the risk of DGF or its associated complications. Even though the rates of acute rejection are currently lower owing to effective regimens of immunosuppression and the availability of early detection of rejection through follow-up laboratory tests and biopsies, we know that DGF is associated with poorer long-term graft outcomes [15, 40] and may also be associated with poorer patient outcomes [14]. Continued research regarding the topic and a striving to understand the mechanisms will therefore aid in the development of clinical guidelines.
There are many strengths in our study; however, there are also limitations to consider. First, the study is retrospective, and thus, the causality of the findings cannot be proved. Our study population is quite large, has an acceptable amount of missing data, and the findings are in line with earlier research. These factors improve the power of our study and strengthen the conclusions. Second, we chose to use the lesion scores from the time of the biopsies and not score them again using the current Banff classification guidelines. This could lead to discrepancies in the results, as the rejection criteria have been updated continuously throughout the study period, especially concerning ABMR [26, 41]. A Spanish study compared the changes in ABMR diagnoses from the Banff 2009 criteria to the Banff 2013 criteria on 68 kidney transplant recipients. They found a change in diagnostic category for 18% of the studied biopsies and concluded that the ABMR diagnosis in the 2013 update reduced the amount of ambiguous ABMR diagnoses [42]. Callemeyn et al. studied the prognostic value of changes to the ABMR criteria in the 2013 and 2017 Banff updates. They found that the 2013 revision improved predictive accuracy, while the 2017 update removed the ABMR diagnosis from kidneys with worse graft survival, that previously were diagnosed as suspicious for ABMR [43]. Furthermore, in the following Banff updates, Notable is, however, that the diagnostic criteria for acute TCMR have not changed significantly. The criteria for borderline findings have been updated multiple times, which has led to heterogeneous descriptions of borderline findings in the literature [44]. In the 2019 updated, the criteria for interstitial inflammation in borderline was raised from i0 to i1 [45]. Based on our data, no conclusions about the optimal cut-offs for borderline changes can be made. The immunosuppression in our cohort is relatively mild, and the findings cannot be directly generalized to other cohorts, in which, for example, lymphocyte-depleting induction is routinely used. In sensitivity analysis regarding the missing data concerning the lesion score PTC, we observed a slight increase in the incidence of ABMR, but not a change in the timeline of rejection diagnosis. Last, different pathologists have analyzed the biopsies over the years, and it is well known that interobserver agreement is far from optimal in kidney transplant pathology [46]. On the other hand, our analyses describe the clinical practice and usefulness of the biopsies at the time of biopsy, not retrospectively.
In conclusion, the first biopsy 7–10 days post-transplant in DGF kidneys is important in identifying early acute rejection. If the first biopsy shows no acute rejection, the risk of finding acute rejection in sequential biopsies is low. These data reflect the rate of early TCMR in deceased donor kidney transplants when 79% of patients did not receive an anti-T cell induction antibody.
Author Contributions
Amanda Ahlmark contributed to the data curation, formal analysis, funding acquisition, investigation, methodology, project administration, visualization, resources, writing -original draft, and writing review & editing. Ville Sallinen contributed to conceptualization, project administration, resources, supervision, validation, and writing – review & editing. Ilkka Helanterä contributed to conceptualization, funding acquisition, methodology, project administration, resources, supervision, validation, and writing – review & editing. Jouni Lauronen contributed to data curation and writing – review & editing. Kaisa Ahopelto contributed to writing – review & editing. Marko Lempinen contributed to conceptualization and writing & editing. Anne Räisänen-Sokolowski contributed to writing – review & editing.
Acknowledgments
Open access publishing facilitated by Helsingin yliopisto, as part of the Wiley - FinELib agreement.
Conflicts of Interest
I.H. holds research grants from Hansa Biopharma and MSD and has received consultancy fees from AstraZeneca, Hansa Biopharma, Takeda, and MSD. J.L. has received consultancy fees from Hansa Biopharma. K.A. has received consulting fees from Hansa Biopharma and Astellas. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Open Research
Data Availability Statement
The individual-level data is not available due to national regulations and laws in Finland regarding the sharing of individual-level patient data. Aggregate-level data can be shared based on a reasonable request.




