PET/MRI in paediatric inflammatory bowel disease – a prospective accuracy study
Abstract
Cross-sectional imaging supplements endoscopy in detecting disease manifestations in inflammatory bowel diseases (IBD). This study aimed to evaluate the accuracy of PET/MRI in a paediatric population suspected of IBD. This prospective study consecutively included patients aged 8–17 years under diagnostic evaluation for IBD. Forty-three patients underwent a PET/MRI scan and subsequent ileocolonoscopy, of whom 26 patients diagnosed with IBD participated in a follow-up scan, hereof 19 with Crohn's disease (CD), five with Ulcerative colitis and two with unclassified IBD. The results of PET alone, MRI alone, and PET/MRI combined were compared to a reference standard of endoscopy and histopathology. Of the 208 intestinal segments analysed, 109 showed inflammation, and 99 had no inflammation. In the per-segment analysis PET had a sensitivity of 0.83 (95% CI 0.73–0.93), specificity of 0.59 (95% CI 0.47–0.71), and area under the receiver operating characteristic curve (AUROC) of 0.73 (95% CI 0.67–0.80). MRI had a sensitivity of 0.52 (95% CI 0.41–0.64), specificity 0.89 (95% CI 0.82–0.96), and AUROC of 0.72 (95% CI 0.66–0.77). PET/MRI had a sensitivity of 0.83 (95% CI 0.74–0.94), specificity of 0.57 (95% CI 0.44–0.69), and AUROC of 0.77 (95% CI 0.71–0.84). At follow-up, PET and MRI scores decreased, and the change in MRI was able to identify patients with a clinical response. The accuracy of the PET/MRI scan in detecting inflammation in the terminal ileum and colon was moderate and not superior to either modality alone. With technological advances and combined reading, PET/MRI may still be valuable in selected cases.
1 INTRODUCTION
Inflammatory bowel diseases (IBDs) are chronic and debilitating disorders that may be particularly devastating in children and include severe intestinal inflammation, extensive intestinal involvement, malabsorption, and growth failure (Dhaliwal et al., 2020; Van Limbergen et al., 2008). Endoscopic procedures are necessary to assess disease severity and extent, but they are invasive, require general anaesthesia or deep sedation in paediatric patients, and carry the risk of complications (Ferreira et al., 2013). Additionally, inflammation may be present in the middle part of the small intestine, which is not evaluated by standard endoscopy (Samuel et al., 2012). Furthermore, endoscopic assessment solely inspects luminal disease activity. Patients with Crohn's disease (CD), ulcerative colitis (UC), or IBD unclassified (IBDU) often undergo repeated endoscopies due to the chronicity and fluctuating behaviour of the disease. Paediatric patients frequently report mental strain associated with the investigative procedures and prefer other procedures to endoscopies (van Wassenaer et al., 2022).
These limitations of the endoscopic procedure call for supplementary investigations. Cross-sectional imaging or capsule endoscopy are recommended to evaluate extra-intestinal manifestations, transmural disease, and small bowel involvement in IBD (Panes et al., 2013), particularly in CD, where up-front biological therapy may be indicated in children at risk of complicated disease (van Rheenen et al., 2020). Magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography combined with CT (PET/CT) are widely available. However, the procedure exposes the patient to higher doses of ionising radiation than CT alone (Jones, 1996; Kastelik-Hryniewiecka et al., 2021). Ionising radiation is a drawback in the paediatric population, as multiple scans may be needed on a life-long basis (Sauer, 2012). The advantages of MRI are the absence of ionising radiation and superior soft-tissue contrast; the disadvantages are a more time-consuming acquisition than PET/CT (Hammer et al., 2013).
The hybrid modality of PET/MRI has been developed in the past decade. Co-registration of the PET scan with MRI sequences reduces inaccurate anatomical localisation of the PET signal but is a challenge in spontaneously moving organs such as the intestine, especially for longer acquisitions. The diagnostic performance of PET/MRI in IBD has been evaluated in adults. Parameters incorporating hybrid-imaging analyses outperform stand-alone parameters in determining disease activity and severity as adding the PET-based metabolic inflammatory volume to the MRI-based disease activity index appears to increase diagnostic performance (Domachevsky et al., 2017). Moreover, PET/MRI may outperform PET/CT in identifying any extra-luminal disease, particularly in patients with CD (Pellino et al., 2016). PET/MRI is generally well tolerated in adults (Li, Langhorst, et al., 2020). To our knowledge, no studies of PET/MRI to evaluate intestinal inflammation in paediatric IBD have been performed.
This prospective study in children and adolescents with suspected IBD aimed to estimate the accuracy of PET/MRI compared to a combined reference standard of endoscopy and histopathology in detecting intestinal inflammation and monitoring treatment response.
2 MATERIALS AND METHODS
2.1 Study design and participants
Patients aged 8–17 years were eligible for inclusion if history, physical examination, blood tests, and faecal samples (including faecal calprotectin) were suggested IBD as the most likely diagnosis. Patients were consecutively included between October 2018 and December 2020 from Hans Christian Andersen Children's Hospital at Odense University Hospital and the Department of Paediatrics at Kolding Hospital. Exclusion criteria were inability to lie still for the PET/MRI scan without sedation, current or prior inflammatory other than IBD or malignant disease, type 1 diabetes, contraindications to MRI, and abdominal surgery during the preceding two months. At visit 1, the clinical work-up, endoscopy with histology, and PET/MRI scan were performed. The treating physician determined the final diagnosis, and patients who did not receive a final diagnosis of IBD were categorised as having Nonspecific symptoms. Patients with IBD were invited for a follow-up scan after 3–6 months (visit 2).
2.2 Clinical work-up
The abbreviated Paediatric CD Activity Index (abbrPCDAI) (Shepanski et al., 2004) and the Pediatric Ulcerative Colitis Activity Index (PUCAI) (Turner et al., 2009) were recorded for each patient at each visit. The abbrPCDAI was used for patients with CD, and the PUCAI was used for patients with UC, IBDU, or Nonspecific symptoms. Levels of faecal calprotectin (FC) and C-reactive protein (CRP) were measured by routine methods at each visit.
To determine the clinical response in the follow-up analysis, we used a cut-off of >5 points for the abbrPCDAI for patients with CD and of >10 points for the PUCAI score for patients with UC or IBDU; or a score of 0 at visit 2. We used a reduction of FC >50% from the value at visit 1 to define biochemical response status.
2.3 PET/MRI scans
PET/MRI scans were performed before endoscopy to avoid imaging findings caused by the endoscopic procedure. The patients were fasted except for plain water 6 h before the examination, and blood glucose levels were measured. A 4 MBq/kg bolus of 2-deoxy-2-[18F]fluoro-d-glucose (2-[18F]FDG) was administered intravenously. After 30 min of rest, the patients were given 500 mL of pineapple juice, which acted as intraluminal contrast (Arthurs et al., 2014), and were asked to drink as much as possible. Patients were scanned in the supine position, starting 80 min after administration of 2-[18F]FDG on a GE Signa 3T PET/MRI scanner (GE Healthcare, Chicago, Illinois, USA). PET imaging used two bed positions with 27% overlap, each with a 5 min acquisition time. Images were reconstructed using a Bayesian Penalised Regularised algorithm in a 256 x 256 matrix with a Dixon-based attenuation correction (MRI sequences listed in Supplementary Table S1).
2.4 PET/MRI image analysis
PET and MRI image analysis was performed on five intestinal segments: terminal ileum, right colon (including cecum and ascending colon), transverse colon, left colon (including descending colon and the sigmoid), and rectum.
2.4.1 PET
Co-registered PET/MRI images were analysed by author R.P. using AW Server software ver. 3.2 (GE Healthcare, Chicago, Illinois, USA) with MRI as anatomical reference.
2-[18F]FDG uptake was visually estimated using a 4-grade scale: 0 = no uptake, 1 = low uptake (barely visible intestinal contour), 2 = moderate uptake (possibly abnormal), and 3 = high uptake (abnormal). The sum of the segmental PET grades represented the global disease burden used in the correlation analysis.
The difference in PET signal from visit 1 to visit 2 was graded for each bowel segment on side-by-side image comparison (decrease means improvement) as −2: significant decrease; −1: mild decrease; 0: no change; 1: mild increase; 2: significant increase. The total change in PET grade (ΔPET) was calculated as the sum of the segmental scores.
2.4.2 MRI
MRI images were evaluated by an experienced radiologist (O. Gr.), who estimated wall thickness and presence of ulceration, mesenteric oedema, comb sign, enlarged regional and central lymph nodes (>10 mm on the short axis), mural contrast enhancement, and restricted diffusion on diffusion-weighted images measured as the minimal apparent diffusion coefficient. We calculated a segmental Pediatric Inflammatory Crohn's Magnetic Resonance Enterography Index (PICMI) score, as described by Focht et al. (Focht et al., 2022), i.e., wall thickness (≥3 mm) × 3 + ulceration (0/1) × 6 + wall-restricted diffusion (0/1) × 9 + mesenteric oedema (0/1) × 6 + comb sign (0/1) × 9. A segmental PICMI score >5 indicated the presence of inflammation and the sum of the segmental scores represented the global MRI score used in the correlation analysis.
The change in MRI score from visit 1 to visit 2 (ΔMRI) was defined as the PICMI score at visit 2 minus the PICMI score at visit 1, i.e., a decrease meant improvement. The minimally necessary change in score was defined as >20 points.
2.4.3 PET/MRI
The segmental PET/MRI grade was determined as PET grade plus zero (+0) if PICMI ≤5 and plus one (+1) if PICMI >5. The sum of the segmental scores was used for the correlation analysis.
The change from visit 1 to visit 2 for PET/MRI (ΔPET/MRI) was determined by categorising and coding the ΔPET as decreased (−1), unchanged (0) or increased (1) while the PICMI score was categorised and coded as >20 points decrease (−1), unchanged, i.e. less than 20 points change (0) or increased, i.e., >20 points increase (1).
Evaluators of the PET/MRI scans were aware of the tentative diagnosis of IBD but blinded to all other data.
2.5 Endoscopic and histopathological evaluation
The patients were on a liquid diet from the day before the endoscopy, and bowel cleansing was achieved with sodium picosulphate (Picoprep®, Ferring Pharmaceuticals, Saint-Prex, Switzerland). Ileocolonoscopy was performed under general anaesthesia by a trained physician. Ileocolic disease activity was assessed with the simplified endoscopic activity score for Crohn's disease (SES-CD) (Daperno et al., 2004) and the ulcerative colitis endoscopic index score (UCEIS)(Travis et al., 2013) respectively. According to clinical guidelines, histopathological evaluation formed part of the routine evaluation performed at the pathology departments in Odense or Vejle (Levine et al., 2014). The assessors were blinded to the PET/MRI scan but not the clinical information.
2.6 The reference standard
A segment was considered inflamed if endoscopy or histopathology indicated inflammation (Supplementary Table S2). Inflammation by endoscopy meant segmental SES-CD score > 0 for patients diagnosed with CD or segmental UCEIS score > 0 for patients with UC, IBDU, or Nonspecific symptoms. Inflammation by histopathology meant the presence of cryptitis, crypt abscesses, or ulceration.
2.7 Statistical analysis
2.7.1 Power considerations
A prerequisite for the statistical comparison was the presence of a reference standard obtained under the same circumstances as the item studied. This limited our options to the colon and distal ileum, where routine endoscopy was performed. To attain precise analysis, we compared well-defined colon and distal ileum segments using a combination of endoscopic and histological studies. Assuming an accuracy for the detection of inflammation by PET/MRI of 95%, 50 patients were sufficient to reject the null hypothesis of an accuracy of 80% with a power of 90% (one-sample Binomial proportion test, type I error: 5% (two-sided)).
2.8 Analysis
Patients with missing data, no PET/MRI or ileocolonoscopy, or other illnesses were excluded from the analysis. Normally distributed data are presented as mean ± standard deviation (SD), and non-normally distributed data as median with interquartile range (IQR). Segmental sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy (together designated diagnostic performance) were derived from 2× 2 tables. Diagnostic performance across segments was derived from linear regression accounting for within-subject clustering. For instance, coding true positives as 1 and false negatives as 0, the intercept describes the expected value of the outcome, and the estimated intercept is simply the mean value of 1's and 0's, i.e., the estimated sensitivity (Vach, 2012). Standard errors were adjusted to allow for within-subject correlation. The receiver operating characteristic curve was analysed segmentally and across all segments, and the area under the curve (AUROC) with a 95% confidence interval (CI) was derived from bootstrapping with 2000 repetitions. Spearman's correlation analysis was used to determine the correlation between scan parameters and FC. Statistical analyses were performed using Stata software ver. 17 (StataCorp LLC, Texas, USA).
2.9 Ethical considerations and trial registration
The parent(s) gave written informed consent. The study was approved by the Regional Committee on Health Research Ethics for Southern Denmark (approval number S-20120161) and registered at the Region of Southern Denmark (record number 18/43605). The study adhered to the tenets of the Declaration of Helsinki. The study was registered with ClinicalTrials. gov (trial identifier NCT03640637) and was reported in accordance with the STARD reporting guidelines (Bossuyt et al., 2015). Study data were collected in a REDCap electronic database (Harris et al., 2009).
2.10 Patient and public involvement statement
Patient representatives evaluated and edited the written patient information.
3 RESULTS
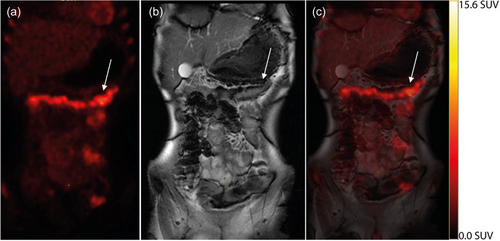
Of the 63 patients screened for participation, 10 were not included. Of these, six declined, two were too ill, one could not lie still, and one encountered administrative problems, leaving 53 eligible patients (Figure 1). After the diagnostic work-up, three patients were diagnosed with ailments other than IBD (chronic granulomatous disease, recurring intestinal abscesses, and lymphoma). One did not complete the scan, and scan data were lost in three patients; thus, scan data were available for 46 patients. Finally, endoscopy was not performed in two patients, and the ileocolonoscopy data was lost in one patient; thus, endoscopy data were available for analysis in 43 patients. In seven of these, the terminal ileum could not be intubated, resulting in (43 × 5) − 7 = 208 intestinal segments available for analysis of diagnostic performance. No clinical interventions were performed in the median of 7 days between the PET/MRI scan and endoscopy. No adverse events occurred in relation to the index or reference tests. Figure 2 depicts illustrative PET, MRI, and PET/MRI images.

3.1 Endoscopic and histopathological findings
Of the 208 intestinal segments, 109 were positive and 99 were negative for inflammation. Segments from the terminal ileum to the rectum were affected (Supplementary Table S3). All disease severity classifications were represented among patients with CD; in contrast no patients in the UC and IBDU groups had severe disease (Table 1).
| Crohn's disease | Ulcerative colitis | IBD undefined | Nonspecific symptoms | Total | |
|---|---|---|---|---|---|
| n (%) | 26 (60.5) | 6 (14.0) | 5 (11.6) | 6 (14.0) | 43 (100.0) |
| Age, median (range) | 14 (8–17) | 16 (13–17) | 14 (9–17) | 12 (9–17) | 14 (8–17) |
| Sex, female n (%) | 10 (38.5) | 1 (16.7) | 0 (0.0) | 3 (50.0) | 14 (32.6) |
| BMI z-score, median (IQR) | −0.94 (−1.57; 0.17) | −0.03 (−0.85; 0.91) | 0.13 (−1.58; 1.49) | −0.24 (−0.78; 1.07)a | −0.36 (−1.3; 0.3)a |
| abbrPCDAI, median (IQR) | 20 (14; 26) | 15 (15; 28) | 10 (10; 25) | 18 (10; 21) | 20 (10; 25) |
| PUCAI, median (IQR) | 18 (10; 25) | 48 (40; 58) | 10 (8; 43) | 15 (9; 16) | 20 (10; 25) |
| Endoscopic disease severity,b n (%) | |||||
| Inactive | 4 (15.4) | 0 | 2 (40) | 5 (83.3) | 11 (25.6) |
| Mild | 4 (15.4) | 3 (50) | 2 (40) | 1 (16.7) | 10 (23.2) |
| Moderate | 9 (34.6) | 3 (50) | 1 (20) | 0 | 13 (30.2) |
| Severe | 9 (34.6) | 0 | 0 | 0 | 9 (20.9) |
| Number of segments | 126 | 28 | 25 | 29 | 208 |
| Calprotectin µg/g, median (IQR) | 1451 (616; 3600)a | 1635 (706; 3206) | 3905 (1254; 5656) | 36 (15; 504) | 1390 (408; 3241)a |
| CRP mg/L, median (IQR) | 22.0 (4.7; 34.5) | 1.3 (0.5; 5.1) | 0.8 (0.5; 9.8) | 2.6 (0.6; 74.3) | 8.2 (1.2; 33.0) |
- Abbreviations: abbrPCDAI, abbreviated Pediatric Crohn's Disease Activity Index; BMI, body mass index; CRP, C-reactive protein; IQR, interquartile range; PUCAI, Pediatric Ulcerative Colitis Activity Index.
- a observation missing
- b Definition of cut-off for the global simple endoscopic score for Crohn's disease (SES-CD) score: inactive: 0–2; mild: 3–6; moderate 7–16; severe: ≥16. Cut-off for ulcerative colitis endoscopic index of severity (UCEIS) score: inactive: 0–1; mild 2–4; moderate 5–6; severe 7–8.
3.2 Accuracy of PET
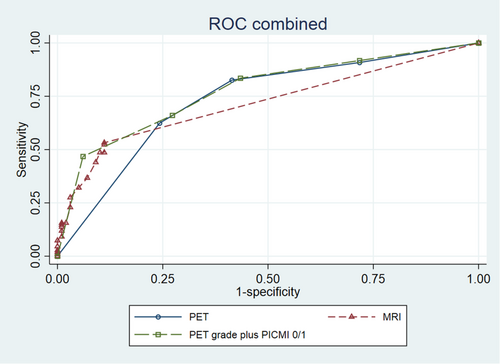
The grade of PET uptake was compared to the reference standard, and the distribution of PET grades is presented in Table 2. Evaluation of PET grade > 1 across segments gave a sensitivity of 0.83 (95% CI 0.73–0.93), specificity of 0.59 (95% CI 0.47–0.71), and AUROC of 0.73 (95% CI 0.67–0.80) (Table 3 and Figure 3). A detailed table of segmental analyses is available in Supplementary Table S4.
| Modality | Value | Reference standard | p-value | |
|---|---|---|---|---|
| Negative (99 segments) | Positive (109 segments) | |||
| PET grade | 0 | 28 (28.3%) | 10 (9.2%) | <0.0001a |
| 1 | 30 (30.3%) | 9 (8.3%) | ||
| 2 | 17 (17.2%) | 22 (20.2%) | ||
| 3 | 24 (24.3%) | 68 (62.4%) | ||
| MRI score, median (10th − 90th percentile) | 0 (0–15) | 6 (0–45) | <0.0001b | |
| PET grade plus MRI 0/1 | 0 | 28 (28.3%) | 9 (8.3%) | <0.0001a |
| 1 | 28 (28.3%) | 9 (8.3%) | ||
| 2 | 16 (16.2%) | 19 (17.4%) | ||
| 3 | 21 (21.2%) | 21 (19.3%) | ||
| 4 | 6 (6.1%) | 51 (46.8%) | ||
- a chi2-test
- b Wilcoxon rank-sum test
| PETa | MRIb | PET/MRIc | PET/MRId | |
|---|---|---|---|---|
| Sensitivity | 0.83 (0.73–0.93) | 0.52 (0.41–0.64) | 0.83 (0.74–0.94) | 0.66 (0.54–0.78) |
| Specificity | 0.59 (0.47–0.71) | 0.89 (0.82–0.96) | 0.57 (0.44–0.69) | 0.73 (0.62–0.83) |
| PPV | 0.69 (0.60–0.79) | 0.84 (0.75–0.92) | 0.68 (0.58–0.78) | 0.73 (0.63– 0.82) |
| NPV | 0.75 (0.61–0.90) | 0.63 (0.50–0.76) | 0.76 (0.61–0.91) | 0.66 (0.52–0.80) |
| Accuracy | 0.71 (0.65– 0.78) | 0.70 (0.62– 0.78) | 0.71 (0.64–0.78) | 0.69 (0.62–0.76) |
| AUROC | 0.73 (0.67– 0.80) | 0.72 (0.66–0.77) | 0.77 (0.71–0.84) | |
- Abbreviation: AUROC, area under the receiver operating characteristic curve.
- a PET grade > 1.
- b PICMI cut-off > 5.
- c PET grade + PICMI 0/1 > 1.
- d PET grade + PICMI 0/1 > 2.
The correlation between global disease burden by PET and faecal calprotectin was not statistically significant in patients with CD (Spearman rho 0.23, p = 0.27) nor in patients with UC/IBDU (Spearman rho 0.49, p = 0.13).
3.3 Accuracy of MRI
Table 2 displays the distribution of MRI scores by the reference standard results and Supplementary Table S5 displays the frequency of the MRI parameters per segment. Across segments, the sensitivity was 0.52 (95% CI 0.41–0.64), specificity was 0.89 (95% CI 0.82–0.96), and AUROC was 0.72 (95% CI 0.66–0.77) (Table 3 and Figure 3). Segmental analyses of accuracy are presented in Supplementary Table S6.
The correlation between global MRI score and FC was moderate in patients with CD (Spearman rho 0.53, p = 0.0072); there was no correlation in patients with UC and IBDU (Spearman rho −0.02, p = 0.96).
There was mural contrast enhancement in 52 (47.7%) of the inflamed segments and eight (8.1%) without inflammation. Mural contrast enhancement was negative in 91 (92%) of the non-inflamed segments and 57 (52%) of the inflamed segments.
Enlarged regional lymph nodes were found in 27 (24.8%) of the 109 inflamed segments and in seven (7.1%) of the 99 segments without inflammation. No enlarged lymph nodes were found in 91 (92%) of the non-inflamed segments, and in 57 (52%) of the inflamed segments.
Central lymph nodes were enlarged in 4 (15.4%) patients with CD, no patients with UC, and 2 (40%) patients with IBDU. A fistula was detected in one patient, and 15 patients had small amounts of free abdominal fluid. Quantitative data on the minimal apparent diffusion coefficient were only available for a few patients and were not analysed further.
3.4 Accuracy of PET/MRI combined
Table 2 presents the distribution of PET/MRI grades across all segments. Segmental parameters are presented in Supplementary Table S7. We evaluated a cut-off >1, which yielded a sensitivity of 0.83 (95% CI 0.74–0.94) and a specificity of 0.57 (95% CI 0.44–0.69). Cut-off >2 resulted in a sensitivity of 0.66 (95% CI 0.54–0.78) and specificity of 0.73 (95% CI 0.62–0.83). The AUROC was 0.77 (95% CI 0.71–0.84) in both cases (Table 3 and Figure 3).
There was no correlation between PET/MRI and FC in CD (Spearman rho 0.39, p = 0.0525) nor in UC and IBDU (Spearman rho 0.42, p = 0.2).
3.5 Imaging of the small bowel
In the jejunum and proximal ileum, the MRI score was elevated in nine (35%) patients with CD and in no patients diagnosed with UC, IBDU, or nonspecific symptoms. PET grade was >1 in 21 (80.8%) with CD, two (33.3%) with UC, one (20%) with IBDU, and 6 (100%) with nonspecific symptoms. Of the seven patients without endoscopy of the terminal ileum, PET grade was >1 in six patients and MRI score was >5 in five.
3.6 Follow-up image analysis
Of the 43 patients included, 26 participated in the follow-up scan: 19 with CD, five with UC and two with IBDU. Concomitant medication is listed in Supplementary Table S8 in clinical responders and clinical non-responders respectively.
ΔPET grade showed a decrease from a median of 10.5 (IQR 7–14) at visit 1 to a median of 8 (IQR 5–11) at visit 2, p = 0.0053. The ΔMRI score decreased from a median of 43.5 (IQR 18–69) to 22.5 (IQR 0–48), p = 0.029 (Supplementary Figure S9).
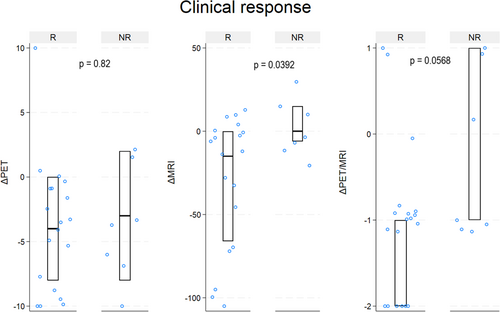
When dividing patients according to the clinical response status, there was a statistically significant difference in ΔMRI score between responders and non-responders (responders median −15 (IQR −66–0) vs. non-responders median 0 (IQR −6–15), p = 0.0392). For ΔPET (responders median –4 (IQR −8–0) vs. non-responders median −3 (IQR −8–2), p = 0.82) and ΔPET/MRI (responders median −1 (IQR −2 to −1) vs. non-responders median −1 (IQR −1–1), p = 0.0568) the difference was not statistically significant. Figure 4 illustrates ΔPET, ΔMRI, and ΔPET/MRI according to clinical response.
There was an overall decrease in FC from visit 1 to visit 2, illustrated in Supplementary Figure S10. When using FC to determine response status, there were no statistically significant differences between the responders and non-responders for ΔPET responders median −6 (IQR −9 to −2 vs. non-responders median −3 (IQR −8–0)), ΔMRI responders median −6 (IQR −69 to −0 vs. non-responders median −6 (IQR −18–3)), or ΔPET/MRI responders median −1 (IQR −2 to −1 vs. non-responders median −1 (IQR −1–0)) (Supplementary Figure S10).
4 DISCUSSION
This study examined the accuracy of PET/MRI in children and adolescents with CD, UC, and IBDU at the time of diagnosis and at follow-up 3–6 months later. Accuracy was estimated based on comparing affected versus non-affected gut segments as determined by endoscopy and histology.
PET grade had the highest sensitivity but low specificity for detecting IBD, while the MRI score had high specificity but low sensitivity. The AUROC did not differ between the two modalities. The combination of PET and MRI did not improve the diagnostic performance. However, it should be kept in mind that in clinical practice, PET and MRI may serve different purposes, e.g., examinations of inflammation versus anatomy, and thus supplement each other.
4.1 Comparison with other studies
To our knowledge, studies with similar aims have only been conducted in adults. An early pilot study in 21 adults with CD compared the diagnostic performance of PET/MRI with that of CRP and FC and found that addition of the PET-based metabolic inflammatory volume to the MRI disease activity index significantly increased the AUROC from 0.632 to 0.917 (Domachevsky et al., 2017). In a study that detected disease activity in the entire GI tract using surgical specimens of the reference standard in 21 adult patients with CD, hybrid PET/MRI we had a sensitivity of 88%, specificity of 93%, and diagnostic accuracy of 91%, which was equal or superior to each modality alone (Catalano et al., 2018). However, in patients with quiescent UC, PET/MRI had a lower diagnostic performance with sensitivity of 75% and specificity of 69% in identifying disease activity (Shih et al., 2018). Still, one study indicated that PET parameters could separate colonic segments with mild disease from segments with moderate-to-severe disease (Tenhami et al., 2021). In extra-luminal disease, PET/MRI outperformed PET/CT in 29 patients with (Pellino et al., 2016).
In our paediatric material, we found a sensitivity comparable to other studies with PET grade but a lower specificity of 0.59 compared to 0.71–0.97 in prior studies (Catalano et al., 2018; Li, Schaarschmidt, et al., 2020; Shih et al., 2018). As we included patients with different diagnoses and nonspecific symptoms, the disease manifestations in our material were likely more heterogeneous than those in other studies investigating patients with known CD or known UC exclusively. In addition, the applied reference standard differed among studies (endoscopy alone, histology alone, or surgery), which may affect PET's negative or positive results.
The specificity of the MRI score was similar to other studies, (Catalano et al., 2018; Li et al., 2019; Li, Schaarschmidt, et al., 2020; Shih et al., 2018) but a sensitivity of 0.52 in our study was significantly lower compared to most (0.75–0.89) (Catalano et al., 2018; Li et al., 2019; Li, Schaarschmidt, et al., 2020) but not all other studies (0.25) (Shih et al., 2018). We observed that the MRI parameters were challenging to evaluate in all bowel segments, possibly due to the relatively long scan duration, increasing the likelihood of patient movements and bowel activity during the scan. For example, we only found ulcerations in one intestinal segment, which was lower than expected. In addition, we used the PICMI score segmentally to evaluate the MRI. The PICMI was designed as a global measure that included intestinal segments from the jejunum to the rectum in children with CD and was not intended for segmental use or children with UC or IBDU. However, we assumed this to be the best-suited paediatric scoring system available. Moreover, the PICMI score included typical signs of UC found on MRI (Maccioni et al., 2005). To enhance acceptance of the scan for these vulnerable paediatric patients, we did not administer an anti-peristaltic agent such as hyoscine N-butylbromide. However, it could reduce bowel movements and thus improve MRI interpretation (Dosdá et al., 2003; Park et al., 2017).
In a meta-analysis including adults and children, PET or PET/CT showed good diagnostic performance with a pooled sensitivity of 0.85 (95% CI 0.81–0.88), a pooled specificity of 0.87 (95% CI 0.84–0.90), and AUROC of 0.93 (Treglia et al., 2013). Our results do not show a superior performance of the combined PET/MRI parameters compared to PET/CT reported in the literature. A PET/CT scan exposes the patient to additional ionising radiation, and the cumulative radiation dose following repeated scans might be significant in children with a chronic condition such as IBD (Sauer et al., 2011). Therefore, PET/MRI may offer an alternative diagnostic modality with less ionising radiation in selected cases, i.e. cases with diagnostic challenges.
4.2 Strengths and limitations of the study
Our study included a sufficient number of patients as delineated in the power analysis, with more than 200 segments evaluated by the index and reference tests, assessed by blinded assessors. We used a combined reference test comprising endoscopic and histopathological signs of inflammation, allowing estimation of both structural and microscopic alterations caused by inflammation. In addition, the patients were included as a consecutive cohort, notably at the time of diagnosis. A subgroup of 26 patients, 19 with CD, five with UC and two with IBDU, underwent a follow-up scan, which showed an overall decline in PET and MRI parameters at follow-up. The MRI score detected patients with clinical improvement, whereas PET and PET/MRI parameters did not.
Our study has several limitations. We used segments from the distal ileum and the colon for statistical analysis. In theory, segments from the small intestine may show another pattern of inflammatory activity, where PET will stand out. We used qualitative visual 0–3 grading of PET uptake rather than a quantitative measure, such as the standardised uptake value (SUV). In doing so, we renounced one of the absolute advantages of PET, namely its inherent ability to quantify molecular disease processes. This approach made it harder to compare with other studies reporting SUVmax or SUVmean values, i.e., tracer uptake in the voxel of the diseased area with the highest uptake or the mean uptake in the entire diseased area. Upon review of the intestinal scans, we concluded that the spontaneous movements of the intestines made it virtually impossible to delineate specific intestinal segments with sufficient precision to allow proper quantification.
A few patients were too ill to participate in the study, needing prompt endoscopic evaluation without time for a PET/MRI scan before endoscopy. These circumstances excluded the clinically sickest patients from contributing to the study, but we still included nine patients with severe inflammation on endoscopy.
4.3 Meaning of the study: Possible mechanisms and implications
The accuracy of PET/MRI was lower than we expected from our clinical experience. The combination of the PET grade and the MRI score in this study is potentially inferior to the collaboration between nuclear medicine physicians and radiologists in clinical practice. PET/MRI may be thus a valuable imaging tool for diagnostically challenging cases such as colonic CD or IBDU, in patients where a complete endoscopy was not possible or perhaps in episodes of disease flare, where it is essential to distinguish IBD activity from other causes of IBD-like symptoms. In general, children prefer MR enterography or ultrasound to endoscopy. They report increased nervousness, fright, and tiredness in connection with endoscopy compared to MR enterography although overall discomfort from either modality was low (van Wassenaer et al., 2022). Ultrasound examination of the gastrointestinal tract may represent an alternative, noninvasive procedure, with promising results (Hudson et al., 2023; Madsen et al., 2022) and could be examined along the same lines as PET/MR in this study.
4.4 Unanswered questions and future perspectives
PET detected 2-[18F]FDG uptake in the jejunum and proximal ileum in all patients with nonspecific symptoms, i.e. a significantly higher percentage than in patients with IBD. No final diagnosis was given to patients in the nonspecific symptoms category at the time of the study. Still, some may have received a diagnosis later, possibly related to the uptake in the small bowel. The normal physiological 2-[18F]FDG uptake in the small bowel can be affected by muscular activity, lymphoid tissue, diet, and intestinal microbiota, (Kang et al., 2017; Moasses-Ghafari et al., 2021; Yoon et al., 2019). Which we did not investigate in this study. MRI did not detect abnormalities in these patients’ jejunum and proximal ileum.
In the future, standardised quantitative evaluation of the bowel wall with 2-[18F]FDG uptake would increase comparability between studies and allow better early or low-activity disease detection. This requires improved scanner technology with much greater sensitivity, as has recently been achieved with total-body PET scanners. Due to a higher sensitivity than current PET scanners, total body scans lasting 3–5 min with correction for bowel movements and artificial intelligence-based segmentation may provide the needed improvements (Chen et al., 2022; Etchebehere et al., 2022; Sundar et al., 2022).
No uniform standard exists for performing PET, MRI, and PET/MRI scans in paediatric IBD. Institutions differ in scanning protocol, patient preparation and positioning, image acquisition, and interpretation; thus, standardisation is welcomed. Large multicentre studies with standardised parameters and potentially comparing PET/CT, MRI, and PET/MRI head-to-head are necessary to determine the role of PET/MRI in paediatric IBD.
5 CONCLUSION
In our study of patients aged 8-17 years with suspected IBD, the accuracy of PET/MRI to detect intestinal inflammation was not clearly superior to PET and MRI alone. Neither parameter alone nor combined showed the same high accuracy in children and adolescents as reported from studies in adult IBD patients. PET/MRI did not detect clinical response or biochemical response status. New and more advanced techniques may allow for quantification with SUV and facilitate the diagnostic capability of PET/MRI.
AUTHOR CONTRIBUTIONS
Sina Dalby: Data collection and analysis, manuscript preparation. Reza Piri and Ole Graumann: Data analysis and manuscript preparation. Oke Gerke: Study design and supervision, data analysis, manuscript preparation. Thomas Lund Andersen: Study design and supervision, manuscript preparation. Anne-Mette Walsted, Kirsten Risby, Rasmus Gaardskær Nielsen, and Anders Linnemann: Patient recruitment and data collection. Poul Flemming Høilund-Carlsen and Steffen Husby: Study conception, and design and manuscript preparation. All authors revised the manuscript and approved the final version.
ACKNOWLEDGEMENTS
Data management and REDcap access via the Open Patient data Explorative Network, Odense University Hospital, Region of Southern Denmark. Funding: This work was supported by The Region of Southern Denmark, Odense University Hospital PhD fund, The Danish Crohn's and Colitis Organization, Dagmar Marshall's Fund, Takeda Pharma A/S (grant number 2018-102587), Master carpenter Jørgen Holm and wife Elisa F. Hansen's Memorial trust, Aase and Ejnar Danielsen's Fund. The funders did not influence the study.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data underlying this article cannot be shared publicly due to the privacy of individuals participating in the study. The data will be shared on reasonable request to the corresponding author.




