The timing of liver resection in patients with colorectal cancer and synchronous liver metastases: a population-based study of current practice and survival
Abstract
Aim
There is uncertainty regarding the optimal sequence of surgery for patients with colorectal cancer (CRC) and synchronous liver metastases. This study was designed to describe temporal trends and inter-hospital variation in surgical strategy, and to compare long-term survival in a propensity score-matched analysis.
Method
The National Bowel Cancer Audit dataset was used to identify patients diagnosed with primary CRC between 1 January 2010 and 31 December 2015 who underwent CRC resection in the English National Health Service. Hospital Episode Statistics data were used to identify those with synchronous liver-limited metastases who underwent liver resection. Survival outcomes of propensity score-matched groups were compared.
Results
Of 1830 patients, 270 (14.8%) underwent a liver-first approach, 259 (14.2%) a simultaneous approach and 1301 (71.1%) a bowel-first approach. The proportion of patients undergoing either a liver-first or simultaneous approach increased over the study period from 26.8% in 2010 to 35.6% in 2015 (P < 0.001). There was wide variation in surgical approach according to hospital trust of diagnosis. There was no evidence of a difference in 4-year survival between the propensity score-matched cohorts according to surgical strategy: bowel first vs simultaneous [hazard ratio (HR) 0.92 (95% CI: 0.80–1.06)] or bowel first vs liver first [HR 0.99 (95% CI: 0.82–1.19)].
Conclusion
There is evidence of wide variation in surgical strategy in dealing with CRC and synchronous liver metastases. In selected patients, the simultaneous and liver-first strategies have comparable long-term survival to the bowel-first approach.
What does this paper add to the literature?
This study has demonstrated that since 2010 there has been a growing popularity in English centres for the liver-first and simultaneous approaches in the management of colorectal cancer patients with synchronous liver-limited metastases. This has resulted in wide variation in surgical approach according to hospital trust of diagnosis.
Background
Colorectal cancer (CRC) is the third most common malignancy in the United Kingdom (UK) 1, and around 20% of patients with colorectal cancer (CRC) have liver metastases at the time of diagnosis 2, 3. The treatment with the best chance of a cure is a complete resection of both the primary and related metastases.
Conventional management of CRC patients with synchronous liver metastases, the ‘bowel-first’ approach, involves resection of the primary CRC followed by liver resection 4. Developments in radiology, surgical techniques and neoadjuvant treatment, coupled with an improved understanding of liver function, have led to alternative surgical sequences 5.
The ‘simultaneous’ approach, resecting the liver metastases and CRC in the same procedure, carries the potential advantage of one anaesthetic and postoperative recovery period, reduced overall hospital stay and possible saving of healthcare resources 6. The ‘liver-first’ approach, where liver metastases are resected usually after a period of downstaging chemotherapy and before resection of the primary CRC, may optimize the chance of a curative resection by achieving earlier control of the liver disease 7.
Patient fitness and the anatomical location and extent of liver metastases and the primary CRC largely govern which strategy to employ. Simultaneous resection is typically performed when the CRC surgery does not involve pelvic dissection and the liver metastases are superficially located and amenable to resection without prolonged portal occlusion 5, 8-10. The liver-first approach is used in patients with rectal cancer that requires neo-adjuvant chemoradiotherapy or with nonobstructive colonic cancer with liver metastases necessitating downstaging 11.
The National Institute for Health and Care Excellence (NICE) recommends that if both the CRC and liver metastases are considered potentially resectable the patient should be referred to a hepatobiliary multidisciplinary team (MDT) 12. The hepatobiliary MDT, in conjunction with the colorectal MDT, will recommend a treatment pathway. Hepatobiliary surgical services in England have been centralized into a hub-and-spoke arrangement 13, and are present on site in 27 of the 145 NHS hospital trusts that manage CRC patients in England 14.
There are no randomized controlled trials comparing the three strategies, and no study has previously described these at a national level. The aim of this study was to describe trends in surgical strategy over time and the factors influencing patient selection. The study also sought to compare long-term survival in patients undergoing either the liver-first or the simultaneous approach compared with the bowel-first strategy in a propensity score-matched analysis.
Method
Study population and data collection
Data from the National Bowel Cancer Audit (NBOCA) 15 for patients diagnosed with primary CRC between 1 January 2010 and 31 December 2015 in English NHS hospital trusts, were linked to the Hospital Episode Statistics (HES) database 2. The NBOCA collects data on all patients with newly diagnosed CRC in England. As the NBOCA involves analysis of data for service evaluation it is exempt from the need for UK National Research Ethics Committee approval.
Patients recorded in the NBOCA dataset with synchronous liver-limited metastases undergoing an elective CRC resection and a liver resection formed the study cohort. Patients were excluded if they underwent emergency CRC surgery or had extra-hepatic disease at diagnosis.
Data regarding hospital trust of diagnosis, surgical urgency, American Society of Anesthesiologists (ASA) grade 3, pathological staging and cancer site were obtained from the NBOCA data. Date of death up to a cut-off date of 17 March 2017 was obtained from linked data from the Office for National Statistics (ONS) 16. Patient socioeconomic status was derived from the Index of Multiple Deprivation (IMD) 17. The IMD ranks 32 482 geographical areas of England according to their level of deprivation measured across seven domains. Patients were grouped into five socioeconomic categories based on quintiles of the national ranking of these areas. The Royal College of Surgeons Charlson Score was used to identify International Classification of Diseases, Version 10 (ICD-10) codes of comorbid conditions in the HES records in the year preceding CRC diagnosis 18.
The site of metastases was identified from HES data according to ICD-10 codes 19. Synchronous metastatic disease was defined by a code for secondary malignant neoplasm of the liver (C787), or elsewhere (C780–C784, C786, C790–C796) recorded 1 year before to 3 months after a diagnosis of CRC. The interval of a year prior to CRC diagnosis was chosen to include patients who are found to have metastases prior to determining the site of the CRC primary. Although NBOCA does not record the site of metastases, data completeness is 93% for M-stage at diagnosis in patients undergoing major resection. As HES data may under-record liver metastases, patients with no HES code for liver metastases who were recorded as having M1 disease at diagnosis in NBOCA data undergoing a liver resection within a year of CRC diagnosis were included.
Procedure information was captured in HES according to the Office of Population Censuses and Surveys classification, fourth revision (OPCS4) 20. All HES records including admissions up to 1 January 2017 were searched for codes indicating a liver resection, portal vein embolization and radiofrequency ablation. Major liver resection was defined as right hemihepatectomy (J021), left hemihepatectomy (J022), extended right hemihepatectomy (J026) or extended left hemihepatectomy (J027). Minor liver resection was defined as resection of a segment of liver (J023), wedge excision of liver (J024), partial excision of liver (J028/9), excision of lesion of liver (J031) and extirpation of lesion of liver (J038/9). Patients were deemed to have a two-stage liver resection when a further liver resection code was recorded within 3 months of the initial liver resection.
Patients were considered to have a simultaneous resection when a liver resection code was recorded on the day of CRC resection, a liver-first approach if a liver resection code was recorded within the year preceding resection of CRC, or a bowel-first approach if a liver resection code was recorded within the year following CRC resection.
Statistical analysis and outcome measures
As this study only includes patients in the staged groups who survive to undergo a second intervention, a traditional survival analysis from date of diagnosis would introduce bias in favour of bowel- and liver-first patients. This is because patients who die after the first procedure would be excluded by definition. Furthermore patients in the staged cohorts have their interventions at different time points following diagnosis. Therefore, a landmark analysis was undertaken to compare survival 21, 22. This method, in which a period of time between a baseline date (date of diagnosis) and study start date (the landmark date) is designated the exposure period and selected a priori, is a standard approach for dealing with these issues. Only deaths after the landmark date were included in the analysis. Patients who died during the exposure window were excluded from analysis, leading to a characteristic plateau in the initial period of the survival curve, as seen in Fig. 3. In this study, individuals who survived for a minimum of 1 year after diagnosis (or, if the second procedure had not been undertaken in the year following diagnosis, more than 90 days from their second procedure) were included in the landmark analysis. Median follow-up from the date of diagnosis was 50 months. Therefore 4-year survival was presented to avoid censoring the majority of patients.
Characteristics of the treatment groups were compared using the χ2 test. Due to differences in patient and disease characteristics in patients typically considered eligible for a liver-first or simultaneous approach, the choice of strategy for an individual patient is usually between the bowel-first approach and the liver-first approach, or the bowel-first approach and the simultaneous approach. Therefore two separate long-term survival comparisons were made: bowel first vs liver first and bowel first vs simultaneous. The potential biases to the survival analysis associated with differences in patient characteristics were accounted for by propensity score matching. Propensity score matching can reduce biases associated with multivariable regression modelling because it restricts the comparison to only those patients eligible for either approach 23. Multivariable logistic regression models were used to generate the propensity scores. All variables in Table 1 were candidates for inclusion, with the exception of Charlson score where the individual indicator variable for each comorbidity was entered. One-to-one nearest-neighbour matching without replacement was performed. The one-to-one ratio was chosen to minimize bias in accordance with recommendations 24. Callipers of 0.33 were used (0.2 of the standard deviation of the logit of the propensity score) 25. The distribution of all of the model factors in the bowel-first and the simultaneous group and the bowel-first and the liver-first group was compared. The balance in the covariates across the treatment groups was considered to be achieved if the standardized differences were less than 10% 26.
| Liver first (n = 270) | Simultaneous (n = 259) | Bowel first (n = 1301) | P-value | |
|---|---|---|---|---|
| Gender | ||||
| Male | 173 (64.1) | 141 (54.4) | 814 (62.6) | 0.033 |
| Female | 97 (35.9) | 118 (45.6) | 487 (37.4) | |
| CRC site | ||||
| Right side | 21 (7.8) | 134 (51.7) | 356 (27.4) | < 0.001 |
| Left side | 97 (35.9) | 71 (27.4) | 630 (48.4) | |
| Rectum | 152 (56.3) | 54 (20.9) | 315 (24.2) | |
| IMD quintile | ||||
| 1 (Most deprived) | 27 (10.1) | 45 (17.5) | 153 (11.8) | 0.110 |
| 2 | 53 (19.9) | 51 (19.8) | 233 (18.0) | |
| 3 | 56 (21.0) | 52 (20.2) | 269 (20.8) | |
| 4 | 71 (26.6) | 54 (21.0) | 293 (22.6) | |
| 5 (Least deprived) | 60 (22.5) | 55 (21.4) | 346 (26.7) | |
| Missing | 3 | 0 | 11 | |
| Age (years) | ||||
| < 60 | 122 (45.2) | 73 (28.2) | 397 (30.5) | < 0.001 |
| 60–70 | 88 (32.6) | 81 (31.3) | 472 (36.3) | |
| > 70 | 60 (22.2) | 105 (40.5) | 432 (33.2) | |
| Charlson comorbidity score | ||||
| 0 | 163 (61.1) | 158 (61.7) | 849 (66.6) | 0.097 |
| 1 | 85 (31.8) | 70 (27.3) | 325 (25.5) | |
| ≥ 2 | 19 (7.1) | 28 (10.9) | 100 (7.9) | |
| Missing | 3 | 3 | 27 | |
| Pathological T stage (Primary tumour) | ||||
| T0–2 | 45 (17.3) | 21 (8.2) | 102 (8.0) | < 0.001 |
| T3 | 173 (66.5) | 146 (56.8) | 790 (62.0) | |
| T4 | 42 (16.2) | 90 (35.0) | 382 (30.0) | |
| Missing | 10 | 2 | 27 | |
| Pathological N stage (Primary tumour) | ||||
| N0 | 100 (38.5) | 78 (30.5) | 390 (30.6) | 0.042 |
| N1 | 94 (36.2) | 110 (43.0) | 484 (38.0) | |
| N2 | 66 (25.4) | 68 (26.6) | 401 (31.5) | |
| Missing | 10 | 3 | 26 | |
| ASA gradea | ||||
| 1/2 | 187 (75.1) | 169 (70.4) | 995 (81.0) | < 0.001 |
| 3/4 | 62 (24.9) | 71 (29.6) | 234 (19.0) | |
| Missing | 21 | 19 | 72 | |
| Major liver resection | 127 (47.0) | 40 (15 4) | 535 (41.1) | < 0.001 |
| Combined ablation | 41 (15.2) | 20 (7.7) | 148 (11.4) | 0.026 |
| Two-stage resection | 10 (3.7) | 2 (0.8) | 21 (1.6) | 0.026 |
| Portal vein embolization | 31 (11.5) | 16 (6.2) | 153 (11.8) | 0.030 |
| Hepatobiliary surgery services at site of diagnosis | 72 (26.7) | 138 (53.3) | 269 (20.7) | < 0.001 |
- ASA, American Society of Anesthesiologists; IMD, Index of Multiple Deprivation.
- a Assessed at time of CRC resection.
- Bold indicates statistically significant.
The Kaplan–Meier method was used to compare long-term survival in the matched and prematching cohort. Comparison of survival probabilities in the prematching group was performed with the log rank (Mantel–Cox) test. A Cox regression analysis was performed on the matched cohort using a robust standard error to allow for clustering on the pairs. stata® version 14.1 (StataCorp, College Station, Texas, USA) was used for analyses.
Results
Patient selection and characteristics
Of 1830 patients who underwent both a CRC and liver resection, 270 (14.8%) underwent a liver-first approach, 259 (14.2%) a simultaneous approach and 1301 (71.1%) a bowel-first approach. In 2015, the proportion of patients undergoing either a liver-first or a simultaneous approach increased over the period from 59 out of 249 patients (26.8%) in 2010 to 99 out of 278 patients (35.6%) (P < 0.001; Fig. 1).
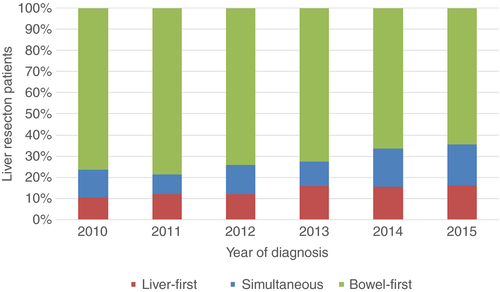
Baseline demographics and clinico-pathological characteristics for patients having both a liver resection and CRC resection according to treatment strategy are outlined in Table 1. Patients in the liver-first group were younger, had a lower T-stage and N-stage and more commonly had a rectal cancer primary than patients in the bowel-first and simultaneous cohorts. In addition, a higher proportion had rectal cancer and underwent a major liver resection. Combined liver ablation, two-stage resection and portal vein embolization were used more frequently in the liver-first cohort.
In comparison, patients in the simultaneous group tended to be older, had a right-sided primary CRC tumour and typically underwent a minor liver resection. These patients were more commonly diagnosed in hospital trusts with on-site hepatobiliary services compared with patients undergoing a bowel-first or liver-first approach.
Variation by hospital trust of diagnosis
There was wide variation in surgical strategy according to the hospital trust of diagnosis (Fig. 2). In 18 out of 132 (13.6%) hospital trusts all patients diagnosed with synchronous liver metastases who underwent a liver resection were treated with the bowel-first approach. There were 19 hospital trusts (14.4%) in which more than 50% of patients underwent either the simultaneous or the liver-first approach.

Survival
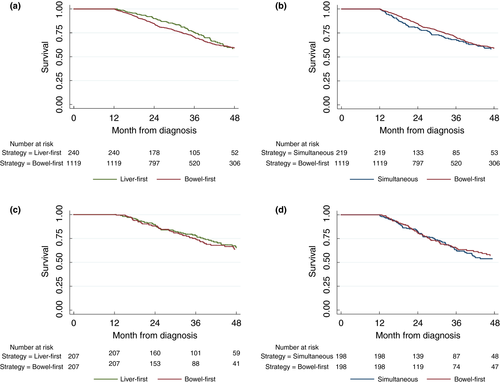
Four-year survival in the landmark analysis for the unmatched cohort was 59% (95% CI: 56–62) in the bowel-first group, 58% (95% CI: 50–66) in the liver-first group and 59% (95% CI: 50–66) in the simultaneous group. Kaplan–Meier survival curves for the unmatched cohorts are shown in Fig. 3a,b.
Matched survival analysis
After propensity score-matching was performed across patients included in the landmark analysis, there were 198 matched bowel-first patients in the bowel-first vs simultaneous comparison and 207 matched bowel-first patients in the bowel-first vs liver-first comparison. The patient, tumour and operative characteristics of the bowel-first patients matched to the simultaneous cohort and those of the bowel-first patients matched to the liver-first cohort were quite different. Table 2 shows that the characteristics of the matched bowel-first patients reflect the patients in each of the simultaneous and liver-first cohorts.
| Liver first (n = 207) | Bowel first (n = 207) | Bowel first (n = 198) | Simultaneous (n = 198) | |
|---|---|---|---|---|
| Gender | ||||
| Male | 132 (63.8) | 140 (67.6) | 108 (54.6) | 104 (52.5) |
| Female | 75 (36.2) | 67 (32.4) | 90 (45.5) | 94 (47.5) |
| CRC site | ||||
| Right side | 12 (5.8) | 8 (3.9) | 94 (47.5) | 84 (42.4) |
| Left side | 77 (37.2) | 54 (26.1) | 58 (29.3) | 69 (34.9) |
| Rectum | 118 (57.0) | 145 (70.1) | 46 (23.2) | 45 (22.7) |
| IMD quintile | ||||
| 1 (Most deprived) | 20 (9.7) | 22 (10.6) | 37 (18.7) | 48 (24.2) |
| 2 | 40 (19.3) | 41 (16.8) | 35 (17.7) | 34 (17.2) |
| 3 | 45 (21.7) | 52 (25.1) | 35 (17.7) | 37 (18.7) |
| 4 | 54 (26.1) | 52 (25.1) | 45 (22.7) | 42 (21.2) |
| 5 (Least deprived) | 48 (23.2) | 40 (19.3) | 46 (23.2) | 37 (18.7) |
| Age group (years) | ||||
| 0–64 | 95 (45.9) | 98 (47.3) | 55 (27.8) | 63 (31.8) |
| 65–74 | 66 (31.9) | 65 (31.4) | 65 (32.8) | 57 (28.8) |
| 75–84 | 46 (22.2) | 44 (21.3) | 78 (39.4) | 78 (39.4) |
| Charlson comorbidity score | ||||
| 0 | 131 (63.3) | 126 (60.9) | 121 (61.1) | 128 (64.7) |
| 1 | 61 (29.5) | 65 (31.4) | 56 (28.3) | 52 (26.3) |
| ≥ 2 | 15 (7.3) | 16 (7.7) | 21 (10.6) | 18 (9.1) |
| Pathological T-stage (Primary tumour) | ||||
| T0–2 | 37 (17.9) | 49 (23.7) | 18 (9.1) | 18 (9.1) |
| T3 | 140 (67.6) | 130 (62.8) | 113 (57.1) | 117 (59.1) |
| T4 | 30 (14.5) | 28 (13.5) | 67 (33.8) | 63 (31.8) |
| Pathological N-stage (Primary tumour) | ||||
| N0 | 79 (38.2) | 85 (41.1) | 64 (32.3) | 72 (36.4) |
| N1 | 77 (37.2) | 73 (35.3) | 84 (42.4) | 80 (40.4) |
| N2 | 51 (24.6) | 49 (23.7) | 50 (25.3) | 46 (23.2) |
| ASA gradea | ||||
| 1/2 | 160 (77.3) | 153 (73.9) | 145 (73.2) | 148 (74.8) |
| 3/4 | 47 (22.7) | 54 (26.1) | 53 (26.8) | 50 (25.3) |
| Major liver resection | 98 (47.3) | 106 (51.2) | 27 (13.6) | 26 (13.1) |
- ASA, American Society of Anesthesiologists; CRC, colorectal cancer; IMD, Index of Multiple Deprivation.
- a Assessed at time of CRC resection.
Kaplan–Meier survival curves for the matched cohorts are shown in Fig. 3c,d. Survival analysis on the propensity score-matched groups showed there to be no evidence of a difference in 4-year survival between the bowel-first and simultaneous cohort [hazard ratio (HR) 0.92 (95% CI: 0.80–1.06)], or between the bowel-first and liver-first cohort [HR 0.99 (95% CI: 0.82–1.19)]. Note that in Fig. 3c,d the survival curve of the matched bowel-first patients is different due to the different patient characteristics of the two matched cohorts.
Discussion
This population-based cohort study is the first to provide an overview of national practice in the management of patients presenting with CRC and synchronous liver-limited metastases. This study shows a growing popularity of the liver-first and simultaneous approaches, but there is wide inter-hospital variation in patient management. The clinico-pathological characteristics of patients undergoing alternative strategies were quite distinct, confirming that these procedures are generally performed in highly selected patients. There was no evidence of a long-term survival difference of the bowel-first strategy compared with the liver-first or simultaneous strategy.
The findings of this study are strengthened by having a large and recent patient cohort. The NBOCA database has a case ascertainment of 93% 15. Furthermore, since financial reimbursement for NHS hospital trusts is coordinated through the recording of procedures in the HES database, the completeness of liver resection registration in the HES is likely to be high. Previous studies in this area have largely been from single institutions 27 and include patients diagnosed over a long time period 28. Such studies have failed to include, or only have very small numbers in, their liver first or simultaneous groups 9, 28-30, or they group the liver-first and bowel-first patients together as a ‘staged’ cohort 31. The largest previous population-based study of the characteristics and outcomes of patients with synchronous CRC and liver metastases included patients diagnosed over a 20-year period (1982–2011) with only 28 patients in the liver-first group 28.
The present study has some limitations, primarily relating to possible missing data and lack of detail in the HES database. HES may under-record liver metastases, and therefore patients were also included if they were recorded in the NBOCA data as having M1 disease at diagnosis and underwent a liver resection within a year of diagnosis even if they did not have a HES code of liver metastases. In addition, major liver resection is defined as the resection of three or more segments according to the code recorded in the HES database. This has the potential to misclassify patients undergoing nonanatomical resection of multiple single segments as minor liver resection. The HES database does not include information regarding the extent or distribution of liver metastases; this is an important factor in determining management decisions, particularly regarding the use of simultaneous resection. It is, however, unlikely that the burden of liver metastases and other patient and surgical characteristics would vary sufficiently across the country to account for the inter-hospital differences in surgical approach. There are also several important confounders which could not be accounted for in the survival analysis. Reliable data regarding the utilization and patient response to chemoradiotherapy for rectal cancer, and both neoadjuvant and adjuvant chemotherapy, were not available in NBOCA or HES data. In addition, postoperative complications and functional return following the first operation for patients in the staged cohorts were not measured.
Patients were only included in this study if they underwent both CRC resection and liver resection. When comparing both operative mortality and long-term mortality in this cohort, it should be considered that to complete the treatment patients in both the liver-first and bowel-first groups must survive, and recover sufficiently from, the initial operation. There is inevitably patient ‘drop-out’ from such complex and prolonged treatment pathways, which may be significant. Several single-centre cohort studies have reported that between 16% and 35% of liver-first and bowel-first patients fail to proceed to the second operation 27, 29, 32. This may be secondary to either postoperative complications or disease progression. Proponents of a staged approach argue that the assessment of disease progression during the interval between the first resection and the second resection allows those with a poor prognosis to be excluded from surgery 6. A potential disadvantage of the simultaneous approach is that it does not allow for such an assessment period. Studies comparing outcomes between staged and simultaneous cohorts will therefore over-sample patients with favourable tumour biology in the staged population, leading to selection bias in favour of the staged approaches.
We found there to be wide variation in surgical approach according to hospital trust of diagnosis, reflecting no clear consensus as to optimal management. The apparent variation in care seems to align with our previous work demonstrating that patients with simultaneous liver metastases at diagnosis are more likely to receive a liver resection and have a survival advantage compared with patients diagnosed at centres without on-site hepatobiliary services 14. The presence of a hepatobiliary team on site appeared to have an impact upon decision-making, as patients diagnosed at hospital trusts with such services on site were more likely to undergo a simultaneous or liver-first approach. Patients diagnosed at hospital trusts with no hepatobiliary MDT on site may be undergoing CRC resection prior to referral to a hepatobiliary MDT for consideration of liver resection.
The clinico-pathological characteristics of patients undergoing a simultaneous and liver-first strategy were quite distinct, confirming that these are generally performed in highly selected patients. A liver-first approach is commonly used in patients with more extensive hepatic disease 11. This was suggested in the present cohort by the high rate of major liver resection in the liver-first group as well as increased used of techniques to maximize the future liver remnant. Liver-first patients will have been deemed by the colorectal and hepatobiliary MDTs to be fit enough to withstand systemic chemotherapy followed by two major operations. It therefore follows that liver-first patients in the present cohort were younger and had fewer comorbidities than those in the bowel-first cohort. There may also be more treatment directed at attempting to cure a younger fitter patient. Synchronous resection increases the complexity of the surgical procedure, and it is therefore notable that patients selected for a simultaneous approach tended to be older and have a higher ASA grade than patients undergoing alternative approaches. The safety of simultaneous resection when involving a minor resection in combination with high- or low-risk CRC resection has been demonstrated 33, 34 and it may be considered clinically appropriate to offer this strategy to higher-risk patients with low-volume liver disease to avoid the cumulative morbidity and mortality of separate interventions. Differences in the extent of liver metastases, age and comorbidities between patients undergoing each surgical strategy may act to cancel each other out, resulting in similar survival between the unmatched groups. For example, patients in the simultaneous group had less extensive liver metastases, yet they were more elderly with higher ASA than patients in the bowel-first cohort.
The landmark survival analysis of the matched cohorts in the present study also showed no evidence of a difference in long-term survival between patients undergoing an alternative strategy and those undergoing a bowel-first approach. The limited studies comparing survival between the three strategies 27, 28 are hampered by direct comparison of strategies without accounting for selection bias in patients planned to have a staged approach. Our study demonstrates that the characteristics of the bowel-first patients matched to the simultaneous group were widely different from those of the bowel-first patients matched to the liver-first group. Furthermore, the time point at which to begin the comparison of the three groups varies throughout the literature. In the analysis of 1004 patients who completed one of the three treatment strategies, Mayo and co-authors (2013) analysed long-term survival from 90-days after the date of the liver-directed operation 28. This analysis therefore included the post-operative mortality associated with CRC resection for patients in the liver-first group, yet no post-operatively mortality for patients in the liver-first and simultaneous cohort. Broquet and co-authors (2010) analysed survival in 142 patients from the date of the last surgery, therefore comparing postoperative mortality of a combined liver and CRC resection in simultaneous patients with a single procedure in staged patients. In both of the aforementioned methods, the start date of survival analysis for each patient varied from the date of diagnosis. In this study, survival in each cohort was estimated conditional on a patient's survival to the landmark time to reduce such time bias.
This study suggests that a liver-first or a synchronous approach can achieve similar survival rates in selected cases to the conventional bowel-first approach for CRC patients with synchronous liver-limited metastases. This study has also indicated that there is substantial variation in how patients with synchronous disease are managed. There may be scope for increased resection rates of liver metastases in either the liver-first or the simultaneous settings. Patients with synchronous disease should be discussed in a hepatobiliary MDT at the earliest point in their journey.
Acknowledgements
HES data were made available by NHS Digital. This study was based on data collected by the NBOCA and funded by the Healthcare Quality Improvement Partnership.
Conflicts of interests
No conflicts of interests.




