Nuclear trafficking of bacterial effector proteins
Funding information: Australian Research Council, Grant/Award Numbers: DP120104911, DP210103881; Faculty of Medicine, Nursing and Health Sciences, Monash University; National Health and Medical Research Council, Grant/Award Number: APP1079904; State Government of Victoria, Grant/Award Number: Operational Infrastructure Support Program
Abstract
Bacterial pathogens can subvert host responses by producing effector proteins that directly target the nucleus of eukaryotic cells in animals and plants. Nuclear-targeting proteins are categorised as either: “nucleomodulins,” which have epigenetic-modulating activities; or “cyclomodulins,” which specifically interfere with the host cell cycle. Bacteria can deliver these effector proteins to eukaryotic cells via a range of strategies. Despite an increasing number of reports describing the effects of bacterial effector proteins on nuclear processes in host cells, the intracellular pathways used by these proteins to traffic to the nucleus have yet to be fully elucidated. This review will describe current knowledge about how nucleomodulins and cyclomodulins enter eukaryotic cells, exploit endocytic pathways and translocate to the nucleus. We will also discuss the secretion of nuclear-targeting proteins or their release in bacterial membrane vesicles and the trafficking pathways employed by each of these forms. Besides their importance for bacterial pathogenesis, some nuclear-targeting proteins have been implicated in the development of chronic diseases and even cancer. A greater understanding of nuclear-targeting proteins and their actions will provide new insights into the pathogenesis of infectious diseases, as well as contribute to advances in the development of novel therapies against bacterial infections and possibly cancer.
1 INTRODUCTION
The secretion of virulence factors represents an important strategy by which bacterial pathogens colonise and survive in the host. The action of these virulence factors on host cells is not limited to surface receptors and cytosolic molecules, but also extend to membrane-bound organelles, such as the mitochondria (Papatheodorou et al., 2006), Golgi apparatus (Pais et al., 2019), endoplasmic reticulum (ER) (Falguières et al., 2001) and the nucleus (Bierne & Cossart, 2012). Based on their biological functions, bacterial effector proteins that directly target the host cell nucleus are as either “nucleomodulins,” which have epigenetic or nuclear-modulating activities (Bierne & Cossart, 2012), or “cyclomodulins,” which specifically interfere with the host cell cycle (Nougayrède, Taieb, De Rycke, & Oswald, 2005).
Well-known paradigms of nucleomodulins include Anaplasma phagocytophilum AnkA, Ehrlichia Chaffeensis TRP47, Legionella pneumophila RomA and Listeria monocytogenes LntA (Bierne & Pourpre, 2020). These proteins use various strategies to modulate immune gene expression, promote efficient intracellular replication and may even reprogram the host genome during infection (Bierne & Pourpre, 2020; Rolando et al., 2013). Examples of cyclomodulins include cytolethal distending toxins (CDTs), colibactin produced by Enterobacteriaceae species and cycle inhibiting factor (Cif) from enteropathogenic E. coli (EPEC). Cyclomodulins have been reported to impair epithelial barrier integrity, prevent cell turnover and inhibit lymphocyte proliferation (Nougayrède et al., 2005). The induction of cell cycle arrest by cyclomodulins may facilitate pathogen invasion and prolong bacterial survival through the inhibition of epithelial cell shedding (Nougayrède et al., 2006).
Recently, it has been proposed that nuclear-targeting factors are not only important for bacterial pathogenesis, but may also mediate the development of chronic diseases and even cancer (Oswald, Nougayrède, Taieb, & Sugai, 2005; Silmon de Monerri & Kim, 2014). These factors induce host cell DNA damage and epigenetic changes, which may contribute to altered gene expression and have been associated with the development of cancer (Basu, 2018; Ilango, Paital, Jayachandran, Padma, & Nirmaladevi, 2020). Examples include cyclomodulins, such as CDTs and colibactin, the production of which has been directly linked to colorectal tumorigenesis in humans (Bossuet-Greif et al., 2018; Dalmasso, Cougnoux, Delmas, Darfeuille-Michaud, & Bonnet, 2014; Dziubańska-Kusibab et al., 2020; Ge et al., 2007; He et al., 2019; Pleguezuelos-Manzano et al., 2020). In contrast, there is currently no direct evidence linking nucleomodulins to chronic diseases, though the epigenetic modulation of host cells by these bacterial factors may be essential to the pathogenesis of such diseases (Silmon de Monerri & Kim, 2014). This review will cover the mechanisms by which nucleomodulins and cyclomodulins enter host cells and traffic to the nucleus. Furthermore, we will discuss the strategies by which these factors can avoid degradation within the host cell before their nuclear translocation. By gaining a greater understanding of the actions of nuclear-targeting factors, it may be possible to develop novel anti-cancer therapies (Bachran et al., 2014; H. J. Lin et al., 2017) or anti-virulence strategies against bacterial infections (Dickey, Cheung, & Otto, 2017). These therapies could be given as stand-alone treatments or in combination with antibiotics (Dickey et al., 2017).
2 MECHANISMS OF HOST CELL ENTRY BY NUCLEAR-TARGETING PROTEINS
Similar to bacterial proteins that do not traffic to the nucleus, those that target the nucleus can either be transported into cells by specialised secretion systems (M. Lin, Den Dulk-Ras, Hooykaas, & Rikihisa, 2007), endocytosed following their release or secretion into the extracellular medium (Norkowski et al., 2018), or internalised as part of bacterial extracellular vesicles (EVs) (Khairalla et al., 2015; Rompikuntal et al., 2012). Receptor-mediated endocytosis of these proteins involves host receptor molecules and/or cholesterol-enriched microdomains, commonly known as lipid rafts (Khairalla et al., 2015; Rosenberger, Brumell, & Finlay, 2000). Pathogens often target lipid rafts because cellular receptors are concentrated at these sites, thus allowing effector proteins to access endocytic pathways and traffic to different intracellular compartments, including the nucleus (Ewers & Helenius, 2011; Sviridov & Bukrinsky, 2014).
2.1 Delivery of nuclear-targeting proteins by bacterial secretion systems
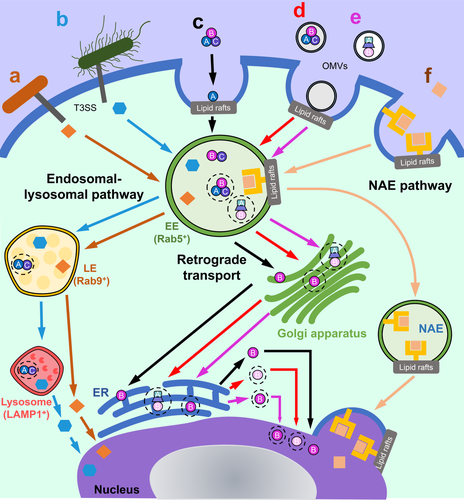
Many nucleomodulins are known to be delivered directly to eukaryotic cells by appendages on the outer surfaces of Gram-negative bacteria, known as type III or type IV secretion systems (T3SS and T4SS, respectively). Anaplasma phagocytophilum translocates the nucleomodulin ankyrin repeat-containing protein (AnkA) into granulocyte cells via a T4SS (M. Lin et al., 2007), whereas Shigella flexneri uses its T3SS to translocate the nuclear-targeting proteins outer Shigella protein F (OspF), invasion plasmid antigen H9.8 (IpaH9.8) and IpaB (Zurawski, Mumy, Faherty, McCormick, & Maurelli, 2009). Likewise, Yersinia outer protein M (YopM) and the EPEC effector protein NleC are delivered into cells via T3SS-dependent mechanisms (Stolle et al., 2017; Wei et al., 2016) (Figure 1a,b, respectively).
2.2 Autonomous cell entry by nuclear-targeting proteins
Recently, it has become apparent that T3SS effector proteins may also enter cells independently of their cognate secretion systems (Norkowski et al., 2018). This was demonstrated for recombinant YopM (rYopM) and NleC (rNleC), which are substrates for Yersinia and EPEC T3SSs, respectively (Scharnert et al., 2013; Stolle et al., 2017). Yersinia YopM was detected in tissue culture media suggesting that the protein is secreted, however, the mechanism for protein release has yet to be determined (Skrzypek, Cowan, & Straley, 1998). rYopM enters host cells in a manner analogous to the family of cell permeable proteins, known as cell-penetrating peptides (CPPs) (Norkowski et al., 2018; Rüter, Buss, Scharnert, Heusipp, & Schmidt, 2010). This autonomous form of cell entry by YopM is mediated by its N-terminal α-helix structure which is absent in other classical Yersinia T3SS effectors, such as YopE (Rüter et al., 2010; Von Pawel-Rammingen et al., 2000). rYopM entry into cells occurs mainly by clathrin-dependent endocytosis, involving lipid rafts (Rüter et al., 2010; Von Pawel-Rammingen et al., 2000). Following proteinase-K-mediated removal of surface proteins on HeLa cells, rYopM could still enter cells, albeit less effectively, suggesting that a surface protein may facilitate its initial association with the plasma membrane (Scharnert et al., 2013). rYopM was reported to interact with two kinases, ribosomal S6 protein kinase (RSK) and protein kinase C-related kinase (PRK) (Hentschke et al., 2010; Höfling et al., 2014). Interactions of recombinant and T3SS-delivered YopM with these kinases were shown to down-regulate pro-inflammatory responses in host cells (Chung et al., 2016; Höfling et al., 2014).
Similar to rYopM, rNleC from EPEC enters cells via lipid raft-mediated endocytosis (Stolle et al., 2017). The ability of NleC to autonomously enter host cells may reflect its evolutionary link with the AB toxin AIP56 of the fish pathogen, Photobacterium dameselae piscicida (Stolle et al., 2017). Two potential protein transduction domains (PTDs), characteristic of CPPs, were identified in NleC, however, these are non-functional and therefore NleC is not considered to be a true CPP (Stolle et al., 2017). NleC is a member of a new family of cell penetrating effector proteins (CPEs) that includes Yersinia YopM, as well as a range of other proteins for example, Salmonella secreted protein H1 (SspH1) from Salmonella enterica serovar Typhi, Shigella IpaH9.8 and Toll/interleukin-1 receptor domain containing-protein C (TcpC) from uropathogenic E. coli (Norkowski et al., 2018). CPEs are thought to enter eukaryotic cells via endocytosis, involving different uptake mechanisms depending upon the target cell type (Norkowski et al., 2018). The concentration of the CPEs and their associated cargo was also suggested to influence uptake (Norkowski et al., 2018).
2.3 Cell entry by nuclear-targeting proteins that are secreted extracellularly
Several nuclear-targeting proteins are transported by secretion systems situated in Gram-negative cell membranes that translocate proteins from the cytoplasm, across the cell envelope and out into the extracellular milieu. Examples of these proteins include nucleomodulins, such as Neisseria meningitidis adhesion and penetration protein (App), meningococcal serine protease A (MspA), Pseudomonas aeruginosa ExoA and bacterial CDTs.
Neisseria meningitidis App and MspA are autotransporter proteins with highly adhesive properties that are released as different size fragments from bacterial surfaces via the actions of T5SSs (D. P. J. Turner et al., 2006; van Ulsen et al., 2003). The auto-proteolysis of App and cleavage by a surface protease, NaIP, releases 100 and 140 kDa fragments (van Ulsen et al., 2003). Interestingly, only the large fragment of App can enter the nucleus. This is due to the presence of a nuclear localization sequence (NLS) located in the α-peptide region of the 140 kDa fragment, which is absent in the shorter 100 kDa fragment (Khairalla et al., 2015; van Ulsen et al., 2003). MspA is also secreted as two different size fragments (of 95 and 125 kDa), however, an NLS has yet to be identified in this protein (Khairalla et al., 2015; D. P. J. Turner et al., 2006). The large fragments of App and MspA enter dendritic cells via mannose and transferrin receptors, which have been reported to mediate the endocytosis of various substrates, bacteria and viruses (Khairalla et al., 2015).
Pseudomonas aeruginosa exotoxin A (PE) is secreted by a membrane-bound protein complex, known as a T2SS. PE is classified as an AB toxin, composed of an enzymatically “active” (A) subunit, and a “binding” (B) subunit, which specifically binds to the glycoprotein receptor, CD91 (also known as α-2-macroglobulin receptor/low density lipoprotein receptor-related protein, LRPAP1) (Kounnas et al., 1992). The PE-CD91 complex is internalised via clathrin-coated pits, prior to PE trafficking to the ER (Manhart, Morris, Bonventre, Leppla, & Saelinger, 1984; Michalska & Wolf, 2015). Additionally, PE can traffic to the ER via detergent-resistant microdomains, consistent with lipid rafts (Smith et al., 2006). This PE is bound to CD91 in lipid rafts and undergoes endocytosis that is caveolin-dependent but clathrin-independent (Michalska & Wolf, 2015). PE binding to lipid rafts was completely abolished by treatment with the cholesterol-depleting agent, methyl-β-cyclodextrin (Smith et al., 2006). Conversely, chemical disruption of lipid rafts did not greatly affect PE internalisation or cytotoxicity, thus showing that these membrane microdomains are not essential for PE toxicity (Smith et al., 2006).
In contrast to the cell entry mechanisms of PE, internalisation of CDTs is mostly dependent on lipid rafts. These AB2 toxins are produced by a range of Gram-negative pathogens for example, Aggregatibacter actinomycetemcomitans, Campylobacter jejuni, E. coli, Haemophilus ducreyi and Helicobacter hepaticus (Scuron, Boesze-Battaglia, Dlakić, & Shenker, 2016). CDTs share a tripartite structure in which CdtA and CdtC are the binding moieties that together facilitate the entry of the active subunit CdtB, which traffics to the nucleus to induce cell cycle arrest and apoptosis in sensitive cells. The effect of CdtB on host cells appears to be cell type-specific, however, as the protein induces proinflammatory responses in macrophages (Shenker, Walker, Zekavat, Dlakić, & Boesze-Battaglia, 2014). In these cells, CdtB functions as a phosphatidylinositol-3,4,5 triphosphate (PIP3) phosphatase, which disrupts PI-3 K signalling in the cytoplasm leading to increased expression and production of interleukin-1β, tumour necrosis factor and interleukin-6 (Shenker et al., 2014).
Several families of CDTs have reported the requirement of lipid rafts to enter host cells (Frisan, 2016). Interestingly, the cellular receptors required for their internalisation differ, as do the intracellular trafficking routes (Frisan, 2016). This review will focus on A. actinomycetemcomitans CDT (AaCDT) as a paradigm for CDT family toxins. All three subunits of AaCDT localise to ganglioside GM1-enriched membranes, characteristic of lipid rafts (Boesze-Battaglia et al., 2006, 2009) (Figure 1c). CdtB and CdtC have cholesterol recognition/interaction amino acid consensus (CRAC) regions that allow their binding to the cholesterol in lipid rafts (Boesze-Battaglia et al., 2009, 2015). Upon cell binding by AaCDT, the CdtA subunit remains on the cell surface, whereas CdtB and CdtC are internalised into the cell (Boesze-Battaglia et al., 2017; Damek-Poprawa, Jang, Volgina, Korostoff, & DiRienzo, 2012). Although AaCDT was clearly shown to enter host cells via lipid rafts, cholesterol depletion did not abolish CdtB-CdtC internalisation into cells, nor did it affect CdtA association with cell membranes (Damek-Poprawa et al., 2012). This indicates that besides cholesterol, a receptor may exist in lipid rafts to facilitate the cellular binding and internalisation of CdtB-CdtC (Damek-Poprawa et al., 2012). Indeed, a loss-of-function genetic screen revealed that E. coli CDT binds to a G-protein coupled receptor in lipid rafts, TMEM181 (Carette et al., 2009). The uptake of AaCDT into cells may similarly require a protein receptor located in lipid rafts (Damek-Poprawa et al., 2012). As many cell types were shown to be sensitive to CDT-induced intoxication, it is reasoned that such receptors should be ubiquitously expressed (DiRienzo, 2014). Further studies are required to identify the protein receptors for bacterial CDTs.
The CDT produced by S. enterica serovar Typhi, typhoid toxin, is an A2B5 toxin that comprises a pentameric B subunit, PltB, linked to two active subunits: PltA, which has ADP-ribosyltransferase activity; and CltB, which shares the nuclease activity of other CdtB subunits (Haghjoo & Galán, 2004). Salmonella bacteria replicate in Salmonella-containing vacuoles (SCVs) and secrete typhoid toxin, which is carried to the cell surface via vesicle carrier intermediates (S.-J. Chang, Song, & Galán, 2016). Typhoid toxin is secreted into the extracellular environment before binding to and inducing cell cycle arrest in neighbouring cells (Fowler et al., 2017). Unlike other CDTs, which require lipid rafts to enter cells, typhoid toxin binds to glycosylated receptors, podocalyxin 1 and CD45, located on the surface of epithelial cells and leukocytes, respectively (Song, Gao, & Galán, 2013). Glycosidase-based removal of cell surface glycans significantly reduced the binding of typhoid toxin to Henle-407 cells (Song et al., 2013). Thus, typhoid toxin appears to preferentially bind carbohydrate moieties rather than specific receptors on cells (Fowler et al., 2017; Song et al., 2013), allowing this CDT to intoxicate a broad range of cells (Fowler et al., 2017). Taken together, these data underline the fact that extracellularly released bacterial proteins which traffic to the host nucleus may have different cell entry requirements.
2.4 Cell entry by nuclear-targeting proteins in bacterial EVs
It is now recognised that EVs represent an important mechanism whereby bacteria can efficiently deliver their products inside eukaryotic cells. Bacterial EVs are spherical, membrane-derived nanostructures (20–250 nm) that are enriched in virulence factors, proteins, toxins and even nucleic acids (Kaparakis-Liaskos & Ferrero, 2015). As Gram-negative bacteria have an outer membrane, EVs produced by these organisms are referred to as outer membrane vesicles (OMVs). The delivery of nuclear-targeting proteins by OMVs is likely to offer certain advantages over extracellular secretion, notably: the protection of proteins from external proteases; delivery of higher concentrations of effector proteins to distant target sites and simultaneous delivery of bacterial factors (Bonnington & Kuehn, 2014). Together, this would ensure that reduced amounts of proteins are needed for cell intoxication.
The OMVs from several bacterial species were shown to deliver CDTs (Kieselbach, Zijnge, Granström, & Oscarsson, 2015; Rompikuntal et al., 2012) and T3SS effectors (Sirisaengtaksin, O'Donoghue, Jabbari, Roe, & Krachler, 2018) into eukaryotic cells. Similarly, Helicobacter pylori OMVs were found to contain putative nucleus-targeting proteins for example, urease subunit A, peptidoglycan-associated lipoprotein (also known as Omp18) (Lee, Jun, Kim, Baik, & Lee, 2015; Suganuma et al., 2008; L. Turner et al., 2015). As reported previously, CDT-harbouring OMVs from enterohemorrhagic E. coli 0157 are internalised into host cells by interactions with lipid raft domains on the plasma membrane (Bielaszewska et al., 2017) (Figure 1d). The CdtB associated with these OMVs targets the nucleus and causes cell death (Bielaszewska et al., 2017). In contrast to the soluble form of CDT, OMV-associated CDT enters cells independently of CdtA/C, suggesting that the binding subunits are redundant. Both binding subunits were shown to remain associated with OMVs, prior to trafficking to the lysosome for degradation (Bielaszewska et al., 2017). Whether OMV-associated CdtA and CdtC have additional functions inside the cell is not known. It is also unclear whether the secreted forms of CDTs have a different impact on host cells than OMV-associated CDT.
A similar question arises for vacuolating cytotoxin A (VacA) in H. pylori OMVs (L. L. Turner et al., 2015). Although H. pylori VacA is not considered a nuclear-targeting protein, it also induces cell death in host cells (Ricci et al., 2005). Of the VacA produced by H. pylori bacteria, only 25% is released in OMVs, with the remainder secreted in a soluble form by a T5SS (Ricci et al., 2005). Secreted VacA is responsible for most of the cytotoxic activity, suggesting that cell vacuolation may not be the main function of OMV-associated VacA (Ricci et al., 2005). Indeed, H. pylori OMVs containing VacA induced the formation of micronuclei in cells (Chitcholtan, Hampton, & Keenan, 2008), indicative of genotoxicity and chromosomal instability (Luzhna, Kathiria, & Kovalchuk, 2013). This is similar to the DNA damage caused by cyclomodulins (Cañas et al., 2016; Tyrer, Frizelle, & Keenan, 2014). We propose that secreted and OMV-associated VacA may target different receptors, thereby influencing both their intracellular trafficking and effects on host cells. This may also be true for other OMV-associated toxins that target the nucleus.
Among the most potent nuclear-targeting toxins released in OMVs are those produced by Enterobacteriaceae species (Cañas et al., 2016; Tyrer et al., 2014). One example is typhoid toxin, which is secreted in the SCVs and can be extracellularly released or contained within OMVs that bud off the SCVs as packaged cargoes (Guidi et al., 2013). These toxin-loaded OMVs travel via anterograde transport to the host cell membrane and are exported to the extracellular environment (Guidi et al., 2013). The exported OMVs then enter bystander cells via dynamin-dependent endocytosis (Figure 1e), resulting in the induction of DNA damage in these cells (Guidi et al., 2013). In a similar fashion, colibactin was detected within OMVs and shown to enter host cells whereupon it causes DNA damage (Cañas et al., 2016; Tyrer et al., 2014). The mutagenic effects induced by colibactin promote colon carcinogenesis (Bossuet-Greif et al., 2018; Dziubańska-Kusibab et al., 2020; Ge et al., 2007; Pleguezuelos-Manzano et al., 2020). Whether OMVs play a role in colibactin delivery to and its genotoxic effects on host cells needs to be established experimentally.
3 HITCHING A RIDE TO THE NUCLEUS VIA ENDOSOMES
Endocytic pathways are normally used as a method of communication between cells and the environment. Macromolecules are internalised from the plasma membrane into endosomes, before being sorted and disseminated to their final destinations (Elkin, Lakoduk, & Schmid, 2016). The trafficking and sorting of cargoes are strictly regulated by numerous regulatory proteins, such as the Rab family guanosine triphosphatases (GTPases) (Elkin et al., 2016). Rab4, Rab5 and Rab11 regulate the formation of early endosomes, whereas Rab7 and Rab9 allow the formation of the late endosome before sorting of these organelles to the lysosome or Golgi apparatus, respectively (Wandinger-Ness & Zerial, 2014). Internalised cargoes can either be: (1) recycled back to the plasma membrane; (2) undergo retrograde transport from the trans-Golgi network (TGN) to the ER; or (3) traffic to the lysosome for degradation. Recently, a new endosomal route was described, in which nuclear envelope-associated endosomes (NAEs) mediate the direct trafficking of macromolecules from the cell surface to the nucleus (Chaumet et al., 2015) (Figure 1).
3.1 Trafficking of nuclear-targeting proteins via the endosomal–lysosomal pathway
Yersinia YopM traffics to the nucleus from late endosomes (Rüter et al., 2010; Scharnert et al., 2013; Skrzypek et al., 1998) (Figure 1a). This protein avoids lysosomal degradation by escaping to the cytosol (Scharnert et al., 2013). Using ultra-cryoelectron microscopy and particle labelling, YopM was detected in both early (12% of total protein) and late endosomes (18% of total protein) (Scharnert et al., 2013). The presence of YopM in the cytoplasm (24% of total protein) 1 hr after incubation with HeLa cells, suggests that this protein can avoid lysosomal degradation by escaping to the cytoplasm before entering the nucleus (18.7% of total protein) (Scharnert et al., 2013) (Figure 1a). rNleC from EPEC also traffics to early and late endosomes, however, it is partially degraded in lysosomes (Stolle et al., 2017) (Figure 1b). The endosomal escape of YopM and rNleC to the cytoplasm appears to be independent of retrograde trafficking via the ER (Scharnert et al., 2013; Stolle et al., 2017). It has been suggested that rNleC escapes acidified endosomes in a manner similar to that of the related AB toxin, AIP56 (Pereira et al., 2014; Stolle et al., 2017). Thus, despite the utilisation of similar pathways, there are differences in the trafficking routes used by YopM and NleC. These differences may be attributed to the intrinsic properties of the two proteins.
3.2 Retrograde transport of bacterial toxins
A range of toxins have been shown to traffic to the nucleus via retrograde transport for example, CDTs, PE, ricin, cholera toxin (Smith et al., 2006) (Figure 1c). Such toxins are sorted to the TGN before undergoing retrograde transport to the ER. The retrograde trafficking pathways undertaken by these toxins are highly complex, involving intertwined pathways that converge and diverge at multiple points (Moreau et al., 2011). This means that although toxins may follow similar intracellular routes, there are also likely to be toxin-specific requirements for trafficking (Moreau et al., 2011).
Once within the ER, the enzymatic part of the toxins is released and then transported into the cytosol, exploiting components of the ER-associated degradation (ERAD) pathway (Eshraghi et al., 2014). The ERAD pathway is normally involved in the translocation of misfolded secretory proteins to the cytosol for proteasomal degradation (Eshraghi et al., 2014; Vembar & Brodsky, 2008). CDT exit from the ER and induction of cell intoxication was shown to depend on two components of the ERAD machinery: Derlin-2, an E3 ubiquitin-protein ligase, HRD-1, and the ATPase, p97 (Eshraghi et al., 2014). Conversely, one study found that cell intoxication by Haemophilus ducreyi CdtB was not dependent on the ERAD component, Derlin-1 (Guerra et al., 2005). It was suggested that this may be related to the ability of this toxin to exit the ER in an unfolded state (Guerra et al., 2009). Another report, however, showed that expression of Derlin-1 could not restore the sensitivity of Derlin-2-deficient cells to CDT toxicity (Eshraghi et al., 2014), suggesting that Derlin-1 is not required for CDT trafficking to the nucleus.
A detailed investigation of the intracellular trafficking of E. coli CdtB in OMVs concluded that this toxin is carried by retrograde transport to the ER, before translocating to the nucleus and causing cell cycle arrest (Bielaszewska et al., 2017) (Figure 1d). Conversely, another study reported that pre-treatment of cells with a retrograde transport inhibitor, brefeldin A, had no effect on the trafficking of OMVs harbouring AaCDT (Rompikuntal et al., 2012). This finding was similar to that for P. aeruginosa OMVs, which were able to deliver their protein cargo independently of retrograde trafficking (Bomberger et al., 2009). Further work is required to clarify whether retrograde trafficking is important for the delivery of OMV-associated CDTs. These contradictory findings may reflect differences between the various CDTs. Likewise, it is possible that the mechanism of cell entry, via either extracellular secretion or OMVs, influences the type of intracellular trafficking route of these toxins.
The intracellular trafficking of typhoid toxin has similarities but also features that are unique with respect to that of other CDTs (S. Chang, Jin, Jiao, & Galán, 2019). In common with CDTs, typhoid toxin is released in OMVs (Guidi et al., 2013) and can also be secreted from SCVs, however, the mechanism has yet to be fully elucidated (S. Chang et al., 2019). The active CdtB subunit in both OMV-associated and secreted forms of typhoid toxin undergo retrograde transport to the Golgi, before trafficking to the ER, then to the nucleus (Guidi et al., 2013) (Figure 1e). The trafficking of secreted typhoid toxin depends on the ERAD, as it was incapable of inducing toxic effects in cells that were deficient in HRD-1, or its adaptor protein Suppressor/Enhancer of Lin-12-like (SEL1) (S. Chang et al., 2019). Conversely, unlike CDTs, secreted typhoid toxin does not disassemble at the cell surface, but rather traffics to the ER before disassembling into its subunits due to the reduction of disulfide bonds between PltA and CdtB (S. Chang et al., 2019). These bonds are cleaved by the actions of a disulfide reductase in the ER (S. Chang et al., 2019). Also, whereas intoxication of host cells by secreted typhoid toxin depends on the conserved oligomeric Golgi (COG) components, COG1 and COG5, these proteins are dispensable for CDT-mediated intoxication (S. Chang et al., 2019). This highlights the fact that even though the same pathways may be utilised by two toxins, subtle differences exist.
3.3 Trafficking of bacterial nuclear-targeting proteins via the NAE pathway
PE can exploit multiple retrograde trafficking pathways to reach the TGN and ER (Smith et al., 2006). Retrograde transport of PE is important, with furin in the TGN cleaving the toxin before its release into the cytosol (Chiron, Fryling, & FitzGerald, 1997). Interestingly, however, unprocessed forms of PE were found within the nucleus, highlighting the existence of another route that may be exploited by PE to mediate its trafficking to the nucleus (Chaumet et al., 2015). Indeed, PE was reported to exploit a novel intracellular trafficking route mediated by NAEs (Figure 1f). This route facilitates the direct nuclear transport of extracellular proteins and surface-bound receptors, including CD91, the cellular receptor for PE (Chaumet et al., 2015). NAE-mediated transport of PE to the nucleus occurs within 1 hr, suggesting a direct route from the cell surface to the nucleus. Co-localization of NAE-containing PE with early endosome antigen 1 (EEA1), but not late endosomal markers Rab7 or lysosomal marker Lamp-1, suggests that these NAEs are derived from early endosomes and that PE does not transit via the endosomal–lysosomal pathway (Chaumet et al., 2015) (Figure 1f). Likewise, brefeldin A pretreatment of cells did not interfere with the association of PE with the nuclear envelope, indicating that this toxin does not transit the Golgi apparatus either (Chaumet et al., 2015). Further work is required to determine whether other bacterial toxins may exploit the NAE pathway to traffic to the nucleus.
4 NUCLEAR TARGETING BY BACTERIAL PROTEINS
4.1 Transport of nuclear-targeting proteins with NLSs
The transport of macromolecules from the cytoplasm into the nucleus is regulated by nuclear pore complexes (NPCs) (Wu, Corbett, & Berland, 2009). Small molecules pass through the NPC by passive diffusion, whereas the transport of larger molecules (>40 kDa) is mediated by a family of carrier proteins, known as karyopherins (Lange et al., 2007). Karyopherins recognise and bind to protein cargoes in the cytoplasm that have an NLS. Additional adaptor proteins are also sometimes required to transport proteins to the nucleus, by acting as a “bridge” between the transport receptors and the NLS on the cargo protein (Cautain, Hill, de Pedro, & Link, 2015).
NLSs usually consist of short amino acid sequences enriched in lysine and arginine (Cautain et al., 2015). The exact sequences, however, vary between different nuclear proteins. The best characterised NLS for nuclear transport is termed the classical NLS (cNLS), which consists of either mono- or bi-partite short sequences of basic amino acids (Lange et al., 2007). Proteins with a cNLS are imported into the nucleus by the karyopherin transport receptor, importin-β, as well as an adaptor protein, importin-α (Figure 2a). A range of other NLSs have been identified, but are less well characterised (Lange et al., 2007).
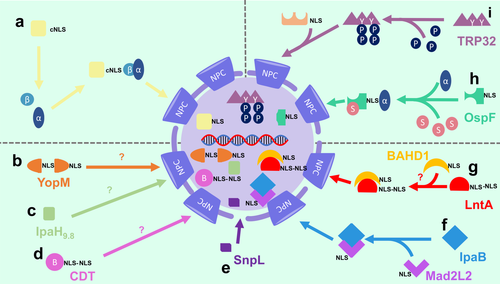
Several nucleomodulins and cyclomodulins have been found to contain novel NLSs that play critical roles in their nuclear transport (Hicks & Galán, 2013). Indeed, deletion of the NLS resulted in the cytoplasmic localization of these nuclear-targeting proteins (Lee et al., 2012). The nuclear-targeting protein from S. flexneri, OspF, has a non-classical NLS in its C-terminus, consisting of amino acids 209–239 (Zhao, Zhang, Wu, Wang, & Li, 2019) that are essential for its binding to the adaptor, importin-α1 (Zhao et al., 2019). Yersinia YopM was found to have a novel bipartite NLS comprising three leucine-rich repeat (LRR) motifs within the central parts of the protein and a 32-residue region in the C-terminus (Benabdillah, Jaime Mota, Lützelschwab, Demoinet, & Cornelis, 2004). YopM is part of a family of bacterial LPX effector proteins have E3 ubiquitin ligase activities and are characterised by N-terminal α-helical domains, followed by a variable number of LRRs (Norkowski et al., 2018). This family includes Shigella IpaH, Salmonella SspH1 and SspH2 (Norkowski et al., 2018). Interestingly, the YopM C-terminus contains an 8-amino acid sequence that is 62% identical to an NLS present in influenza virus and a 100-residue stretch with 32% identity to a 35-amino acid sequence, QCTDVHMVSD SDGDDFEDAT E-FGWDDGEM FG, which is critical for the nuclear localization of FAS-associated factor 1 (Benabdillah et al., 2004; Davey, Dimmock, & Colman, 1985; Frohlich, Risau, & Flamme, 1998; Skrzypek, Myers-Morales, Whiteheart, & Straley, 2003).
The N-terminus of AaCdtB contains an amino acid region that was able to mediate the nuclear transport of a fusion protein composed of CdtB and green fluorescent protein (GFP) (Nishikubo et al., 2003). This 48–124 amino acid region is highly conserved among CdtB proteins from a range of bacteria, including E. coli (Nishikubo et al., 2003). In another study, however, two bipartite NLSs were identified in E. coli CdtB, comprising residues 77–100 and 98–126, each of which was able to mediate its nuclear transport (McSweeney & Dreyfus, 2004). These discordant findings may be attributed to the fact that the 48–124 amino acid region in CdtB consists of a complete bipartite NLS, comprising residues 77–100, as well as most of a second bipartite NLS, comprising residues 98–126 (McSweeney & Dreyfus, 2004).
Based on a simple hidden Markov model, researchers identified and validated a putative NLS at amino acids 56–82 in the N-terminus of RomA from Legionella pneumophila (Paris strain) (Rolando et al., 2013). Furthermore, it was shown that the L. pneumophila Philadelphia strain has a RomA homologue, LegAS4, with an NLS at amino acids 21–60 (Schuelein et al., 2018). Comparison of the NLSs from these two effector proteins found similar stretches of basic amino acids, however, the sequences differed by three amino acids. Interestingly, RomA localises to the nucleus, whereas LegAS4 associates with the nucleolus (Schuelein et al., 2018). It was suggested that the nucleolar localization of LegAS4 may be attributed to the three amino acid difference between the NLSs of these two Legionella effector proteins (Schuelein et al., 2018).
4.2 Transport of bacterial proteins independently of karyopherins
Deletions of karyopherin genes did not interfere with the nuclear localization of different GTP-YopM domains (Benabdillah et al., 2004), suggesting that Yersinia YopM does not require karyopherins for nuclear translocation (Figure 2b). The S. flexneri T3SS effector IpaH9.8 was also reported to be transported across the NPC independently of importins, but the mechanism was not elucidated (Toyotome et al., 2001) (Figure 2c). Similarly, the absence of an association between CdtB and importins-α or -β suggested that CdtB may not require karyopherins to enter the nucleus (Nishikubo et al., 2003) (Figure 2d). CdtB import was shown to be mediated by active transport, involving residues 28–124 in the N-terminal domain of the protein (McSweeney & Dreyfus, 2004; Nishikubo et al., 2003). It is possible that the nuclear transport of CdtB is dependent on a novel karyopherin, or on other unknown host or bacterial factors.
Several potential strategies have been described by which bacterial proteins may undergo nuclear transport independently of karyopherins. One such strategy is provided by the eukaryotic protein, β-catenin. This protein contains specific armadillo repeat sequences, which are common to karyopherins, thus allowing direct binding of β-catenin to the nucleoporins within the NPC (Sharma, Jamieson, Johnson, Molloy, & Henderson, 2012). Many bacterial proteins contain armadillo repeat sequences (Jernigan & Bordenstein, 2015), but whether these sequences promote the transport of bacterial effectors to the nucleus is currently unknown.
Proteins may also undergo nuclear transport by passive diffusion, as was shown for small proteins, such as GFP1 (Wang & Brattain, 2007). The L. pneumophila effector SnpL does not have an NLS yet may be small enough (26 kDa) to enter the nucleus via passive diffusion (Schuelein et al., 2018) (Figure 2e). This may also be the case for YopM, which is 41–55 kDa in size (Grabowski, Schmidt, & Rüter, 2017). The Shigella effector IpaB does not appear to have an NLS and is too large to undergo passive diffusion (Iwai et al., 2007). Instead, it has been proposed that IpaB may “piggyback” its way into the nucleus by targeting the NLS-containing protein, Mad2L2, resulting in the induction of cell cycle arrest (Iwai et al., 2007) (Figure 2f). A similar strategy may be used by L. monocytogenes LntA to enter the nucleus as it binds to the chromatin repressor BAHD1, which also has an NLS (Lebreton et al., 2011; Lebreton et al., 2014) (Figure 2g). Additionally, LntA has a putative bipartite NLS (Lebreton et al., 2014), so it is possible that it may also undergo nuclear import via the actions of karyopherins.
4.3 The role of post-translocation modifications in the nuclear trafficking of bacterial proteins
An emerging theme in the field of bacterial effector proteins has been the role of post-translational modifications (PTMs) in their nuclear transport. It was reported that the PTM of S. flexneri OspF by small ubiquitin-related modifier (SUMO) promoted its nuclear localization (Jo, Kim, Yu, Yun, & Kim, 2017) (Figure 2h). Two putative SUMO modification consensus sequences were identified within this protein, with one demonstrated to be targeted for SUMOylation (Jo et al., 2017). The mechanism by which this PTM promotes the nuclear transport of OspF has yet to be elucidated. It is also unclear whether other bacterial factors can be SUMOylated, and whether this may facilitate the nuclear transport of bacterial nucleomodulins.
The tick-borne bacterium Ehrichia chaffeensis produces a nucleomodulin, TRP32, which is also reported to require a PTM to be translocated into the nucleus (Figure 2i). The C-terminus of TRP32 contains two tri-tyrosine motifs, with residues Y168 and Y179 found to be crucial for its nuclear localization. Immunoprecipitation experiments confirmed that the phosphorylation of Y179 and Y168 is required for its nuclear localization (Farris et al., 2016). As TRP32 lacks a cNLS, it was suggested that phosphorylation may be crucial for its association with another protein that harbours an NLS (Farris et al., 2016) (Figure 2i). A similar mechanism was observed for signal transducer and activator of transcription 1 (STAT1) (Fagerlund, Mélen, Kinnunen, & Julkunen, 2002; Farris et al., 2016). This protein homodimerises in a phosphorylation-dependent manner to create a dimer-dependent NLS, thus allowing the binding of importin-α5 (Fagerlund et al., 2002; Farris et al., 2016).
5 QUESTIONS AND FUTURE DIRECTIONS
Bacterial pathogens target the nucleus as a strategy to subvert and infect plant cells (Silmon de Monerri & Kim, 2014). In contrast, the roles of nuclear-targeting proteins in bacteria that infect animal hosts have yet to be extensively investigated. Until now, the focus has been on the nuclear targets of these proteins and their effects on host cell responses (Silmon de Monerri & Kim, 2014). Many questions in the field therefore remain unanswered, not least whether host receptors may be involved in the uptake of nuclear-targeting proteins, as well as the specific intracellular pathways required for their trafficking to the nucleus. It is also unclear whether nuclear-targeting proteins express non-classical NLSs and if host proteins are required for their entry into the nucleus.
Some nuclear-targeting proteins, such as CDTs, are released from bacterial cells within OMVs. Moreover, OMVs have been reported to traffic to the nucleus (Rompikuntal et al., 2012; Rosenberger et al., 2000; Rüter et al., 2010; Sirisaengtaksin et al., 2018). Together, these observations raise the question as to whether OMVs may serve as an important mechanism whereby nuclear-targeting proteins and other bacterial components are delivered to the nucleus and subvert host cell functions during bacterial infection. A greater understanding of the mechanisms by which bacterial effector proteins access the nuclear compartment is expected to provide critical new insights into the bacterial pathogenesis of infectious diseases, particularly those associated with chronic infections. It may be possible to use this information to develop anti-virulence therapies against bacterial infection (Dickey et al., 2017). As nucleomodulins have been shown to cause epigenetic changes affecting the immune responses of host cells, it will also be interesting to determine the potential roles of these proteins in the development of innate immune memory (Bierne, Hamon, & Cossart, 2012). Finally, the potent nuclear modulation and efficient trafficking abilities of these proteins may be exploited in therapeutic applications. An example of this has been the use of modified CDT to treat radio- and drug-resistant cancer cells (Bachran et al., 2014; H. J. Lin et al., 2017).
ACKNOWLEDGMENTS
The authors would like to thank Dr Frances Cribbin for critical reading of the initial draft of this manuscript. Richard L. Ferrero was a Senior Research Fellow of the National Health and Medical Research Council of Australia (APP1079904). Research related to this article is funded by the Australian Research Council (DP120104911, DP210103881). Lena Hoang My Le is supported by an Australian Postgraduate scholarship (Monash University Faculty of Medicine, Nursing and Health Sciences). Research at the Hudson Institute of Medical Research is supported by the State Government of Victoria's Operational Infrastructure Support Program.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.