A processing product of the Plasmodium falciparum reticulocyte binding protein RH1 shows a close association with AMA1 during junction formation
Karthigayan Gunalan and Xiaohong Gao contributed equally to this study.
Funding information: A*STAR BIOMEDICAL RESEARCH COUNCIL, Grant/Award Numbers: 06/1/22/19/456, 09/1/22/19/613; Singapore Ministry of Education Academic Research Fund Tier 1, Grant/Award Number: RG152/14; Singapore Ministry of Education Academic Research Fund Tier 2, Grant/Award Number: MOE2017-T2-1-034
Abstract
Plasmodium falciparum responsible for the most virulent form of malaria invades human erythrocytes through multiple ligand-receptor interactions. The P. falciparum reticulocyte binding protein homologues (PfRHs) are expressed at the apical end of merozoites and form interactions with distinct erythrocyte surface receptors that are important for invasion. Here using a range of monoclonal antibodies (mAbs) against different regions of PfRH1 we have investigated the role of PfRH processing during merozoite invasion. We show that PfRH1 gets differentially processed during merozoite maturation and invasion and provide evidence that the different PfRH1 processing products have distinct functions during invasion. Using in-situ Proximity Ligation and FRET assays that allow probing of interactions at the nanometre level we show that a subset of PfRH1 products form close association with micronemal proteins Apical Membrane Antigen 1 (AMA1) in the moving junction suggesting a critical role in facilitating junction formation and active invasion. Our data provides evidence that time dependent processing of PfRH proteins is a mechanism by which the parasite is able to regulate distinct functional activities of these large processes. The identification of a specific close association with AMA1 in the junction now may also provide new avenues to target these interactions to prevent merozoite invasion.
1 INTRODUCTION
Malaria is the most deadly parasitic diseases that infect hundreds of millions of people each year and claiming around 500 thousand lives. There is currently no vaccine available that significantly reduces the overall infection rates. The observed clinical symptoms and disease pathology are due to intraerythrocytic life cycle of the parasite making this stage an important vaccine target. Most of the blood stage vaccine research has focused on controlling disease progression by preventing the invasion of erythrocytes by merozoites (Genton et al., 2002; Thera et al., 2011). In the past few decades, numerous studies have uncovered a complex array of proteins in Plasmodium that play important roles during invasion from initial binding to successful penetration of erythrocytes. Two protein families that play an important role in initial recognition of the erythrocyte by the merozoite are Reticulocyte binding protein homologues (RHs) and Erythrocyte binding like proteins (EBLs) (Cowman & Crabb, 2006; Gunalan, Gao, Yap, Huang, & Preiser, 2013; Iyer, Gruner, Renia, Snounou, & Preiser, 2007).
In P. falciparum, there are five members of RH proteins and they play a crucial role in host cell selection by interacting with cognate erythrocyte receptors. They are high molecular weight proteins (>200 kDa) with a signal peptide, an N-terminal conserved domain and a C-terminal transmembrane domain (Duraisingh et al., 2003; Gao et al., 2008; Gunalan, Gao, Liew, & Preiser, 2011; Rayner, Galinski, Ingravallo, & Barnwell, 2000; Rayner, Vargas-Serrato, Huber, Galinski, & Barnwell, 2001; Stubbs et al., 2005). The only exception is the PfRH5 protein, which is 63 kDa without a transmembrane domain (Baum et al., 2009; Crosnier et al., 2011). We have identified PfRH1 erythrocyte binding domain (EBD) (also named “RII-3” in Gao et al., 2008), which is located at the N-terminus. Our previous study has been shown that the mAbs against the EBD is able to inhibit the invasion by blocking the Ca2+ signalling in the merozoite, suggesting that RH1 triggers a signalling pathway through its N-terminus following receptor-binding leading to release of cytosolic Ca2+ during invasion. (Gao, Gunalan, Yap, & Preiser, 2013). Recent work has shown that the interaction of PfRH2b with the erythrocyte triggers a calcium signal within the merozoite while the interaction of PfRH5 with its receptor basigin mediates a Ca2+ signal inside the erythrocyte that appears to be critical for merozoite invasion (Aniweh et al., 2017; Aniweh, Gao, Gunalan, & Preiser, 2016; Gao et al., 2013).
Proteolytic processing of parasite ligands appears to be an important process that unleashes functional domains at appropriate time during invasion. A well-studied example is the Merozoite Surface Protein 1 (MSP1) and Apical Membrane Antigen 1 (AMA1), which are sequentially processed during merozoite maturation and invasion (Blackman, Scott-Finnigan, Shai, & Holder, 1994; Boyle et al., 2014; Howell, Withers-Martinez, Kocken, Thomas, & Blackman, 2001). The different processing events are tightly regulated, and the parasite utilises several different proteases to achieve this. It has been shown that PfRHs are cleaved near the transmembrane domain and released from the merozoite surface by rhomboid proteases (Triglia, Tham, Hodder, & Cowman, 2009). Similarly, novel classes of proteases are thought to cleave and release small functional fragments of PfRH1, PfRH2a and RH4 (Gunalan et al., 2011; Sahar et al., 2011; Triglia et al., 2009; Triglia et al., 2011). Interestingly a study focusing on PfRH2a indicates that the different proteolytic products have different erythrocyte binding properties to aid successful invasion (Gunalan et al., 2011). Based on these studies, proteolytic processing appears to be a common phenomenon evident in many of the invasion proteins, and therefore is likely to have functional significance.
In the current study using three different mAbs targeting different regions of PfRH1, we show that PfRH1 is processed to release at least eight fragments following schizont rupture. We furthermore show that the N-terminus fragment of PfRH1 specifically in close contact with the other invasion molecule AMA1 at the merozoite-erythrocyte junction indicating that the released N-terminal fragment is directly involved in formation of merozoite-erythrocyte tight junction. The demonstration that PfRH proteins are also part of the moving junction further expands the functional role of this protein family, from initial host cell recognition and merozoite signalling to now early junction formation.
2 RESULTS
2.1 PfRH1 undergoes processing during invasion
To more fully characterise the extend of PfRH1 processing we generated mAbs against the MD of PfRH1, named as αMD and the region at the C-terminus end (RVIII), named as αRVIII (Gao et al., 2008) as well as utilised the previously described mAbs C41 and C49 targeting the EBD, named as αEBD (Gao et al., 2008; Gao et al., 2013) (Figure S1a). The specificity of the mAbs was confirmed in Western blot analysis using recombinant proteins of these three different domains (Gao et al., 2008) as well as in Immunofluorescence assay (IFA) of T994 and T994ΔRH1 parasites. The mAbs C41 or C49 was able to specifically recognise the EBD which was previously described as PfRH1-RII-3 (Gao et al., 2008; Gao et al., 2013) but not the other two domains, MD and RVIII (Figure S1b). The similar profile was found in the recombinant MD and RVIII recognised by its own specific mAbs (Figure S1c). No cross-reaction was found in all four mAbs. αHis antibody was used as a loading control to prove the presence of the protein in each lane (Figure S1c). Native PfRH1 was detected by IFA as punctuated dot like pattern at the apical tip of segmented merozoites in late schizont stage T994 parasite using αMD and αRVIII that completely co-localised with the previously validated αEBD staining (Figure S1d). None of these antibodies detected any signal in the T994ΔRH1 parasites, further confirming the specificity of these mAbs (Figure S1e). Similarly none of three mAbs detected PfRH1 in schizonts and merozoite extracts as well as culture supernatant in Western blot analysis in T994ΔRH1 parasite (right panel blots in Figure 1a–c).
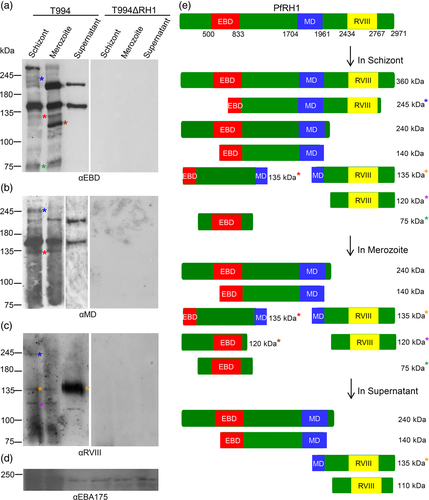
The specific mAbs provided us now with a powerful tool to examine the processing of PfRH1 during merozoite maturation and invasion. In T994 schizont extract, both αEBD and αMD detected approximately 360, 240 and 140 kDa fragments similar to the previous report although the amount of the 240 kDa fragment is significantly lower. A 245 and 135 kDa fragment were also detected by both with much lower expression by αEBD, while a 75 kDa fragment was detected by αEBD only (right panel blots in Figure 1a–b). In contrast αRVIII detected a 245, 135 and 120 kDa products in size (right panel blots in Figure 1c). Furthermore, αRVIII recognised a 360 kDa in schizonts shown in the longer exposure Western blot (Figure S2). Two major PfRH1 fragments of approximately 240 and 140 kDa were detected in merozoite extract and culture supernatant by both αEBD and αMD (Figure 1a and b). A 135 kDa fragment was also detected in the merozoites by both αEBD and αMD, which could be carried over from the schizont stage (Figure 1a-b). In addition, αEBD also detected a 120 and 75 kDa N-terminal processed fragments in merozoite extract (Figure 1a). Apparently αRVIII detected a different 135 kDa fragment from C- terminus of PfRH1 in both schizont and merozoite samples (Figure 1c). The αRVIII also appears to recognise an additional different120 kDa C-terminal fragment in both schizont and merozoite fractions (Figure 1c). αRVIII recognised the full-length 360 kDa in schizonts and a processing product of 120 kDa in schizonts and merozoites which is consistent with previous results (Triglia et al., 2009). Interestingly, only 135 and 120 kDa fragments were detected in merozoite extract by αRVIII at a similar expression level to that seen in schizonts (Figure 1c). The αRVIII detected a 110 kDa processed product in the culture supernatant along with high amounts of a 135 kDa fragments. Taken together, the Western blot data is consistent with the sequential processing of different PfRH1 products during merozoite maturation in the schizont, merozoite release and finally release to the supernatant during invasion (Figure 1e).
2.2 Changes in the location of PfRH1-EBD, MD and RVIII during invasion
To understand whether the different processing products of PfRH1 have different functions during merozoite maturation and invasion we studied the location of the fragments recognised by the three mAbs available to us in schizonts, free merozoites and junction arrested merozoites using airyscan super-resolution microscopy. In late stage schizonts both MD and RVIII completely co-localise with EBD at the apical end of the merozoite (Figure 2a) with both MD and RVIII either partially or not co-localising with micronemal proteins AMA1 and EBA175 (Figure 2b,c). The same pattern was also seen in free merozoites after schizonts ruptured (Figure S3), indicating that the different PfRH1 processing products are all located closely together in the rhoptry neck in schizont as well as free merozoites.
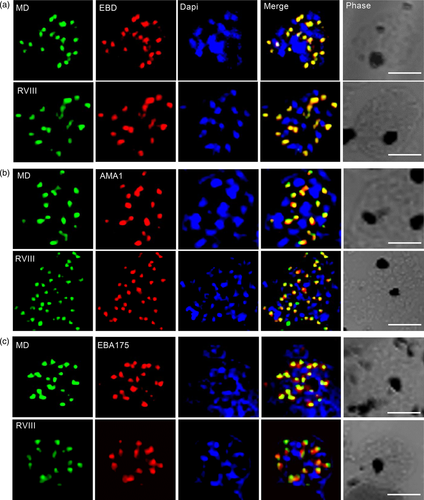
Rhoptry and microneme proteins are discharged during merozoite invasion, with AMA1 being a key player in the formation of the moving junction (Riglar et al., 2011). To investigate whether the different fragments of PfRH1 are involved in the early junction formation, invading merozoites were arrested using Cytochalasin D (Cyto D) followed by airyscan super-resolution microscopy (Figure 3). This showed that in junction arrested merozoites MD was still completely co-localised with EBD (top panel in Figure 3a) while this was not the case for RVIII and EBD (bottom panel in Figure 3a) indication potential functional differences between the fragments at this step of invasion. Interestingly, MD but not RVIII completely co-localised with AMA1 (Figure 3b) in the junction, suggesting that EBD and MD may form complex with AMA1 at this stage. However, neither MD nor RVIII co-localised with EBA175, another micronemes protein that has been suggested to be involved in junction formation (Figure 3c). Taken together these data indicate that the processed products of PfRH1 localised differently during invasion, in which the cleaved RVIII is not carried into the junction whereas EBD and MD are completely co-localised with the junction marker AMA1. However, considering the limitation in resolution of even light microscopy it was important to utilise alternative strategies to substantiate this data.
2.3 Validation of PfRH1 interactions in the schizont
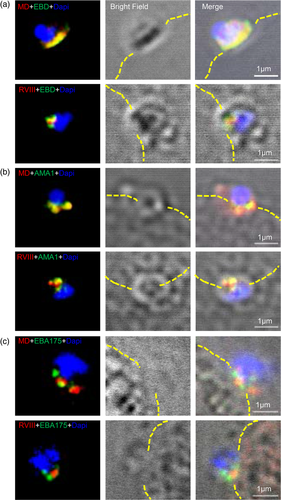
The data reported here identifies a number of distinct processing products of PfRH1 in both late stage schizonts and free merozoites. Moreover, fragments containing EBD, MD or RVIII respectively were completely co-localised with each other in the schizonts before invasion but not at the junction. To investigate this further we performed in-situ PLA as well as FRET by acceptor photobleaching with different combination of αEBD, αMD and αRVIII and antibodies targeting AMA1 and EBA175. These two techniques allow us to investigate the dynamic processing events and possible close contact or interactions within PfRH1 and related invasion proteins at a much higher spatial resolution, with PLA identifying protein–protein interactions at the distance of <30–40 nm in fixed cells (Bellucci, Fiorentini, Zaltieri, Missale, & Spano, 2014; Soderberg et al., 2006) while a FRET signal will only be observed at distances between 1 and 10 nm (Goedhart, Hink, & Jalink, 2014; Sekar & Periasamy, 2003). Consistent with the IFA data, PLA (Figure 4a–c) and FRET (Figure 4g) confirmed the spatial co-localization among EBD, MD and RVIII at the late stage of schizonts suggesting that the different processing products of PfRH1 stay in close association in the rhoptries of schizont stage merozoites. Consistent with the super-resolution microscopy data that indicated that none of the PfRH1 fragments completely co-localise with micronemal invasion proteins AMA1 or EBA175 in schizonts, PLA as well as FRET also showed no close contact between RH1 and AMA1 (Figure 4d–f,h) or RH1 and EBA175 (Figure S4a–c,e). This is in line with the fact that PfRH1 and AMA1/EBA175 are localised in two different organelles (rhoptry and microneme) which are 100 nm apart, depending on the numbers of micronemes and the sizes and shapes of the rhoptries in the merozoite (Bannister et al., 2003; Bannister, Hopkins, Fowler, Krishna, & Mitchell, 2000; Kats, Black, Proellocks, & Coppel, 2006). In line with this, PLA (Figure S4d) but not FRET (Figure S4e) showed certain contact between AMA1 and EBA175 (both localise in the microneme) in some but not all schizonts suggesting that while AMA1 are located within the same organelles they do not form close association. Together the data supports that RH1 does not interact with the mirconeme invasion proteins AMA1 and EBA175 in late stage of schizonts.
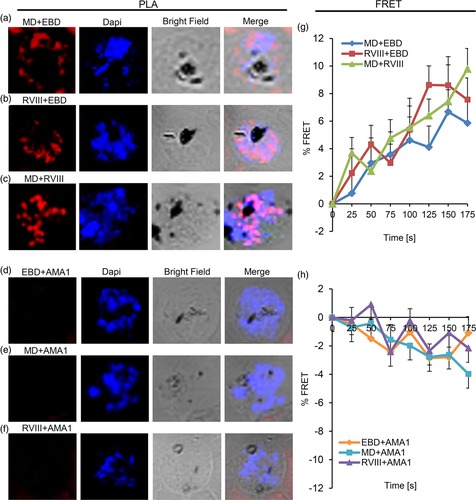
2.4 PfRH1 interactions in the junction
Super-resolution microscopy indicated that there are changes in the spatial relation between the processed PfRH1 fragments as well as AMA1 and EBA175. To further explore these changes we carried out PLA as well as FRET on junction-arrested merozoites. PLA confirmed the close distance between EBD-MD (Figure 5a) but indicated the neither EBD-RVIII (Figure 5b) nor MD-RVIII (Figure 5c) in contrast to the schizont stage are in close proximity to each other in junction arrested merozoites. These findings with the exception of the RVIII + EDB were also confirmed by FRET which showed strong close association for EBD and MD, a much-reduced close contact between RVIII and EBD (Figure S6) and no close association between MD and RVIII (Figure 5g). It is interesting to note that while the interaction between RVIII and EDB was confirmed by PLA and FRET in schizonts, only FRET provided evidence of association between these two fragments in the junction (Figure 5g). At this stage the data is therefore not conclusive though based on the average FRET intensities the RVIII+EDB interaction appears to be much weaker than any of the other interactions measured here (Figure S6). Taken together the data suggests that the C-terminal processed fragment containing RVIII is disassociated from EBD and MD and does not interact with EBD and MD in the junction, which is in line with the previous findings (Triglia et al., 2009).
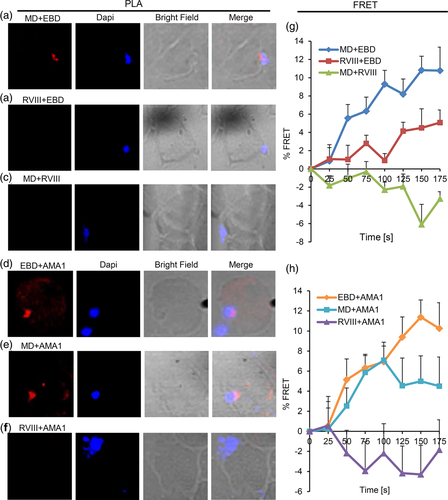
The micronemal proteins AMA1 and EBA175 play a critical role in junction formation and AMA1 is a validated protein of the junction complex (Chen, Paing, Salinas, Sim, & Tolia, 2013; Riglar et al., 2011; Tonkin et al., 2011; Triglia et al., 2009). We therefore explored the close proximity and/or close association of the PfRH1 processed fragments with AMA1 and EBA175 in junction arrested proteins using PLA and FRET. These approaches showed that the N-terminal region of PfRH1 containing EBD and MD closely associate with AMA1 as measured by PLA (Figure 5d,e) as well as by FRET (Figure 5h). However, no association was observed between RVIII and AMA1 by either PLA or FRET (Figure 5f,h). In contrast, although EBA175 has been shown by our super-resolution microscopy (Figure 3) as well as previous publications (Gunalan et al., 2011; Triglia et al., 2009) to be in the junction none of the processed PfRH1 fragments interact with EBA175 by either PLA (Figure S5a–c) or FRET (Figure S5e). Moreover, AMA1 was in close proximity to EBA175 in the junction measured by PLA with the spatial resolution <40 nm (Figure S5d). Yet there was no close contact between AMA1 and EBA175 in the junction based on FRET analysis with the spatial resolution <10 nm (Figure S5e), indicating that FRET is more sensitive and accurate to detect a true protein–protein association even within the same organelle. FRET data also suggests that AMA1 and EBA175 in the microneme may function independently during invasion. Together the data shows that the N-terminal but not the C-terminal processed fragments of PfRH1 specifically associate with AMA1 in the junction possibly playing a prominent role during the invasion process.
3 DISCUSSION
The molecular crosstalk between the parasite and the erythrocyte is a complex process and highly regulated. This crosstalk is mediated by a direct interaction or in close association between proteins secreted from the apical end of the merozoite and the suitable receptors on the erythrocyte. These not only determine reticulocyte or normocyte preference of a parasite species but would also directly impact on overall parasite virulence. PfRHs are important for host cell selection and evasion. It was postulated that PfRHs have a sensing function, which initially identifies a suitable erythrocyte and then recruits EBLs (Duraisingh et al., 2003; Rayner et al., 2000). Subsequently a tight junction is formed between the merozoite and erythrocyte leading to irreversible commitment to invasion. Processing of different invasion proteins has been widely reported including the PfRHs (Aniweh et al., 2016; Baum et al., 2009; Gunalan et al., 2011; Rodriguez, Lustigman, Montero, Oksov, & Lobo, 2008) as well as AMA1 and MSPs (Blackman et al., 1994; Boyle et al., 2014; Howell et al., 2001) suggesting that proteolytic processing of invasion proteins is one of the key-signalling/regulation events important for parasite invasion. Here we investigated how processing of PfRH1 could play a role in mediating additional functions during the merozoite invasion process.
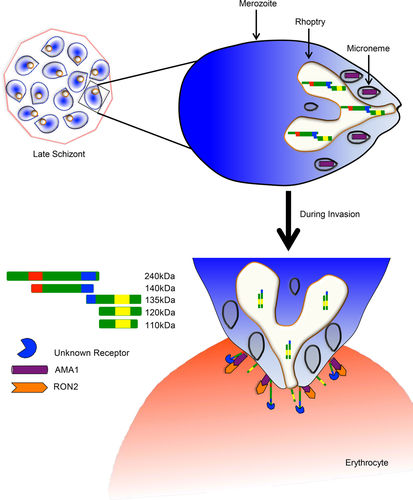
The work presented here has significantly extended our understanding of the complexity of processing events of PfRH1 during maturation and invasion beyond the previous data (Triglia et al., 2009) identifying a range of new processing products. The timing and quantitative differences between the different processing products suggests a tightly regulated cascade of processing events during merozoite maturation and subsequent invasion and suggests key functional differences between the different products, with for example different products having different binding properties as has been observed for PfRH2a (Gunalan et al., 2011). However at this step we do not know about the epitopes of αMD, based on previous work on the epitope mapping of αEBD (Gao et al., 2013) as well as the online software of predicting amino acid sequence to kDa, a 135 kDa detected by both αEBD and αMD (EBD/MD-135 kDa) in schizont and merozoite but not in the supernatant is different from that detected by αRVIII (RVIII-135 kDa) in schizont, merozoite and a significant increase in the supernatant shown in Figure 1a–c. The RVIII-135 kDa fragment was most likely generated by a cleavage near the C-terminus as well as another cleavage within MD, This second cleavage removes the epitopes recognised by the αMD monoclonal antibody. This is in contracts to the EBD/MD-135 kDa fragment which is the likely processing product of the 240 or 140 kDa including part of EBD and MD which is retains the epitopes recognised by the two mAbs. In this study we have identified a new series of processed PfRH1 fragments including a 245 kDa C-terminal and a number of N-terminal fragments of 120 and 75 kDa, suggesting a tightly coordinated process combining cleavage and spatial rearrangement during the pre-invasion phase. Our data suggests that the fragments which are not released into the supernatant remain in the rhoptry and are carried into the erythrocyte as was indicated by Triglia et al., 2009. At this stage it is not clear how many different proteases are required to carry out all the different processing steps and how these are regulated nor is it at this stage possible to assign clear biological functions to each of the fragments though it is attractive to speculate that the precision of processing and timing is in line with each step being crucial for invasion.
Our data to our knowledge for the first time is able to link different PfRH1 processing products to different functional invasion complexes. In schizonts and free merozoites, processed RVIII is completely co-localised with EBD and MD in the rhoptry but not with either AMA1 or EBA175, in line with these key invasion proteins staying in their subcellular organelles before invasion. This is consistent with the findings in Triglia et al., 2009. The remarkable change of location of the PfRH1 processed products in the junction arrested merozoites with EBD and MD completely co-localising with AMA1 while RVIII is not, indicates distinct functional roles of processed PfRH1 in invasion. It is possible that the RVIII fragment remains associated with the merozoite surface after rhoptry secretion while the EBD/MD fragments are recruited into the junction along with the rhoptry neck proteins RON2 and RON4 (Besteiro, Michelin, Poncet, Dubremetz, & Lebrun, 2009; Proellocks, Coppel, & Waller, 2010; Riglar et al., 2011; Tonkin et al., 2011). The fact that rhoptry and microneme components form functional relevant complexes after microneme discharge has already been demonstrated for PfRH5/PfRipr/CyRPA (Volz et al., 2016). At the same time the observation that EBA175 does not form a complex with AMA1 and EDB/MD in the junction suggests that EBA175 mediates its function ahead of junction formation and may be solely act as a critical merozoite—erythrocyte receptor interacting protein. The work reported here demonstrates the importance of complementing super-resolution light microscopy with alternative techniques that provide even better spatial resolution to gain a more complete understanding of the complex invasion process. Our study provides the first insight on how different processed products of PfRH1 can differentially associate with other invasion proteins and supports the hypothesis that these associations mediate different time dependent functions during invasion.
Collectively our data provides clear evidence that PfRH1 undergoes processing to release multiple different sized fragments post schizont rupture and merozoite-erythrocyte junction formation (Figure 6). Merozoite invasion is rapid and tightly regulated, (Gilson & Crabb, 2009) requiring a precise and coordinated ligand-receptor binding cascade that regulates downstream events. We have previously shown that PfRH1 binding to its erythrocyte receptor is important for Ca2+ signalling in the merozoite (Gao et al., 2013), leading to microneme discharge and junction formation. The data obtained here now suggests that once the Ca2+ signal has been initiated in the merozoite, PfRH1 is further processed and now closely associated with AMA1 to move into the merozoite junction. It is interesting to note that all members of the PfRH family are processed (Aniweh et al., 2016; Baum et al., 2009; Gunalan et al., 2011; Rodriguez et al., 2008; Sahar et al., 2011; Triglia et al., 2009; Triglia et al., 2011) and at PfRH2b has also been implicated in merozoite Ca2+ signalling (Aniweh et al., 2016) suggesting a broadly conserved role of the members during merozoite invasion. We would hypothesize that like PfRH1 the other large members of this family PfRH2a/b and PfRH4 in addition to their initial binding to an erythrocyte receptor, and their role in merozoite Ca2+ signalling have another important role in the moving junction. Analogous to what we have demonstrated for PfRH1 we would suspect that processing products of PfRH2a/b and PfRH4 also closely associate with AMA1 in the junction and perform an important role during the invasion process. It will be now interesting to evaluate how and whether the different processing steps are indeed critical for regulating downstream invasion events making it important to establish the exact sites of processing and the specific proteases involved as they may provide new insights on the regulation of the invasion process.
4 EXPERIMENTAL PROCEDURES
4.1 Ethics statement
The whole blood was donated by healthy non-malarial immune adult volunteers at the National University Hospital, Singapore. Informed consent given was written. Informed consents were obtained from all donors in accordance with protocols approved by Institutional Review Board of Nanyang Technological University, Singapore (IRB-2016-01-045 and IRB-2018-02-031).
4.2 Plasmodium falciparum parasite culture
Parasite strains (W2mef, T994 and T994 knockout) were cultured in fresh erythrocytes at 4% haematocrit in RPMI 1640 supplemented with 5% albumax with 3% O2, 5% CO2, and balance with N2 gas and incubated at 37°C as previously described (Trager & Jensen, 1976). Growth media was replaced every day with fresh complete RPMI. Smears were made on microscope slide to check the parasitemia. Isolation of late schizont stage parasites were achieved by performing percoll centrifugation as described (Kutner, Breuer, Ginsburg, Aley, & Cabantchik, 1985). Merozoites were purified from schizont extract by filtration under nitrogen cavitation (Blackman, 1994; Heidrich, 1988; Shimizu, Shimura, Ito, & Minami, 1992). Schizonts and merozoite extracts were washed with 1X PBS for twice and lysed directly into sample buffer and stored at −20°C. Parasite culture supernatant was prepared by allowing the percoll purified schizont parasites to grow in complete RPMI medium in the absence of erythrocytes for 14–16 h at 37°C with constant agitation. After 16 h, the culture was centrifuged for 3 min at 1,500 RPM at 4°C. The supernatant is collected and further centrifuged at 35,000 RPM for 30 min at 4°C. The supernatant was stored at −80°C.
4.3 Cloning, expression and purification of MD of PfRH1
PCR forward primer (5′-GTGAGTccatggGAAAACAAGTACAA GAAAATGTTGA GAA-3′) and reverse primer (5′-AATTACgagctcTTACTTAAAAACATCGCTCAG TGTATTT-3′) were used to amplify the region of middle region (MD) from P. falciparum 3D7 genomic DNA (gene ID PF3D7_0402300). The PCR product was cloned into a modified expression vector pET-9d (Novagen) in which an additional SacI restriction digestion site has been introduced to produce a N-terminal 6X His-tag. The construct was chemically transformed into BL21(DE3) competent cells for recombinant protein expression. The MD protein expression and purification were carried out by using standard purification procedures (GE Healthcare).
4.4 PfRH1 rat monoclonal antibodies (mAbs) generation and purification
Purified MD and RVIII (Gao et al., 2008) were used to immunise rats. One hundred microgram of protein was used in primary injection with complete Freud's adjuvant (Sigma) and 50 μg of protein with incomplete Freud's adjuvant (Sigma) in each boosting subsequently. The boosting was performed every 2 weeks in the total of three times. Isolation of B-cells was done by squashing the rat lymph nodes in a petri dish in Iscove's Modified Dulbecco's Media (IMDM) with 5% Fetal Bovine Serum (FBS) (Gibco). Fusion of B-cells and Sp2/0 cells was carried out by adding 1.5 ml of Polyethylene glycol (PEG) (Sigma) drop by drop to the mixed cells at 37°C.
Viable hybridoma clones were identified 10–12 days after fusion and screened by ELISA. Positive clones were further subcloned obtain single clone that was again validated by ELISA. ELISA screening was performed based on the standard procedures by using purified MD or RVIII (0.2 μg/well) coated in 96-well plates. The plate was blocked with 1% BSA. Five microliter of supernatant from each hybridoma clone was tested.
Validated hybridoma clone culture was expanded to produce IgG in large scale. Purification of MD or RVIII IgG from cell culture medium was carried out by using standard purification procedures (GE healthcare). Purified IgG was later buffer-exchanged to 1XPBS for all assays.
4.5 Western blot analysis
Purified recombinant proteins EBD, MD and RVIII (Gao et al., 2008) were loaded on 12% SDS-PAGE and transferred to the nitrocellulose membrane using semi-dry transfer for 45 min at 18 V at RT. After the membrane was blocked with 5% skimmed milk/PBST, membranes were sliced into strips followed by incubation with mAbs αEBD (C41 or C49) (Gao et al., 2013), αMD, αRVIII and αHis respectively. This would ensure that samples are from the same batch and loaded equally. In order to avoid the sample variation, we have scaled up the parasite culture and applied the same batch of parasite schizont and merozoite exaction and culture supernatant from T994 and T994ΔRH1 respectively in the western blot analysis. Parasite schizont extraction was obtained by saponin lysis in the presence of protease inhibitor cocktail (Nacalai Tesque, Japan) to prevent the degradation. Merozoites were purified by filtration after late stage schizonts were treated with E64 (Boyle et al., 2010). Purified merozoites were lysed immediately in SDS sample loading buffer to prevent any degradation and tested by Western blot. All samples were loaded onto the 6% SDS-PAGE gel in a schizont-merozoite-supernatant manner with fixed loading amount in each gel (for example, schizont in 5 μl, merozoite in 5 μl and supernatant in 10 μl) and transformed to the nitrocellulose membranes using wet transfer for 2 h at 100 V at 4°C followed by probed with different mAbs. Due to the limited loading wells per SDS-PAGE gel, samples have to be loaded onto different gels and transferred to different membranes. As mentioned above, we have lowered the variation as much as possible by applying the same batch of sample preparation for all western blots in this study. After the wet transfer, the membrane was blocked with 5% skimmed milk/PBST for 1 h followed by incubation with mAbs αEBD (C41) (Gao et al., 2013), αMD, αRVIII overnight at 4°C. The same batch of schizont, merozoite and supernatant with the same amount and loaded in the same blot from T994 and T994ΔRH1 were also probed with EBA175 mAbs (EBI-MR4) as a loading control. A white line is present in the Figures if the samples are not loaded next to each other or from different membranes. The membrane was washed three times for 5 min with PBST, followed by 1 h at RT incubation with the respective horseradish peroxidase (HRP) conjugated secondary antibody. The membrane was again washed three times for 10 min with PBST. Protein recognised by antibodies will be visualised using ECL plus Western blotting detection system or Super signal-west pico chemiluminescent substrate according to manufacturer's instruction.
4.6 Immunofluorescence assay and microscopy
Thin blood smears containing the late schizont and free merozoites were prepared. Junction arrested merozoites were prepared by incubating the late schizont stage parasites with cytochalasin D (0.1 μm) for 6–8 hrs (Gao et al., 2013; Gunalan et al., 2011; Miller, Aikawa, Johnson, & Shiroishi, 1979). Smears were fixed with 100% acetone for 5 min. The slides were blocked in 3% BSA in 1x PBS for 30 min and incubated in a humidity chamber at 37°C with primary antibodies of mAbs αEBD, αMD, αRVIII, αEBA175 and αAMA1 (EBI-MR4) for 1 h. After incubation, the slides were washed three times for 5 min with 1x PBS. The slides were incubated with the secondary antibodies: Alexa flour 488 or 594 conjugated goat anti-rat or goat anti-mouse antibodies (Molecular Probes) for 1 h at 37°C. The slides were air-dried at room temperature and sealed using mounting medium containing DAPI. About average of 6–10 images were taken for each pair of mAbs combination. Fluorescent images were captured using laser scanning confocal microscopy or fluorescent microscopy.
4.7 Super-resolution microscopy and analysis
Thin blood smears were fixed with glutaraldehyde (Sigma) and permeabilised with 0.1% Triton X-100 (Sigma). Smears were prepared for confocal microscopy as described previously. Slides were mounted in ProLong Gold Antifade Mountant with DAPI (Life Technologies) and images were captured with a Zeiss LSM710 confocal microscope equipped with an Airyscan detector using a Plan-Apochromat 100x/1.46 oil objective. About average of 6–10 images were taken for each pair of mAbs combination. Image processing was carried out by using the Zen software (Carl Zeiss).
4.8 Monoclonal antibody labelling
PfRH1 (EBD, MD and RVIII) and EBA175 mAbs were labelled using Alexa Fluor 488 or 546 monoclonal antibody labeling kit (Life tech) respectively. To 1 mg/ml of PfRH1 (EBD, MD and RVIII) and EBA175 mAbs, one-tenth volume of 1 M sodium bicarbonate buffer (pH 8.3) was added. One hundred microliter solution-containing 1 mg of mAbs were transferred to a vial containing reactive dye (Alexa Fluor 488 or 546). The vial was gently inverted for few times to fully dissolve the dye. The antibody-reactive dye solution was incubated for 1 h at RT and followed by 4°C overnight. The solution was then loaded drop wise onto the centre of the spin column containing the Protein A resin. The solution was allowed to absorb into the gel bed for at least 30–40 min, followed by centrifugation of the spin column for 5 min at 1,100 g to elute the labelled antibodies.
4.9 Proximity ligation assay
Proximity Ligation Assay (PLA) is to investigate the interaction of proteins (Soderberg et al., 2006). The assay was performed according to manufactures protocol (Olink Bioscience). The glass slides with the blood smear were incubated with mAbs EBD, AMA1 or EBA175 and directly labelled mAbs MD and RVIII (plus or minus probe). The slide was then incubated with corresponding secondary antibodies conjugated with either plus or minus PLA probes. The staining was performed following manufacturer's protocol. Fluorescent images were captured using laser scanning confocal microscopy. The PLA signal was visualised as individual fluorescent dots.
4.10 FRET analysis
FRET by acceptor photobleaching was performed to analyse the interaction between invasion proteins at different stages of the parasites. Late schizont stage parasites were prepared by Percoll centrifugation. Junction arrested merozoites were prepared by incubating the late schizont stage parasites with cytochalasin D (0.1 μm) for 6–8 hrs (Gao et al., 2013; Gunalan et al., 2011; Miller et al., 1979). Parasite smears were made on the glass slides and fixed with acetone for 5 min. mAbs against PfRH1 (EBD, MD and RVIII), EBA175 and AMA1 were labelled directly using alexa fluor 488/546 labeling kit or indirectly using alexa fluor 488/594-labelled secondary antibodies. FRET analysis was performed by acceptor photobleaching as described (Guillaud, Wong, & Hirokawa, 2008; Raimondi, Chikh, Wheeler, Maffucci, & Falasca, 2012). The analysis was performed on the selected region of interest. Acceptor photobleaching measures FRET by irreversibly photobleaching the acceptor by shining 543 nm LASER for seven consecutive times on the selected area, and thereby abrogating the energy transfer. If any interactions leading to energy transfer were present in the cell, photobleaching of the acceptor will lead to an increase of donor fluorescence, as it is no longer quenched by the acceptor. This increase of donor fluorescence after photobleaching can therefore be used to quantitatively determine FRET efficiency (Dinant, van Royen, Vermeulen, & Houtsmuller, 2008). Three control images were taken before photobleaching. FRET efficiency was measured by increase in the donor fluorescence after photobleaching the acceptor fluorophore and percentage FRET efficiency was calculated by comparing the value of pre and post bleached region of interest. The time (in s) used for plotting is normalised to 0, 25, 50, 75,100, 125, 150 and 175 s for all the FRET graphs for consistency. For each combination of FRET pairs, the average values for each time points were calculated based on at least 3–6 FRET interaction images. Experimental data were shown as the mean ± s.e.m. Carl Zeiss laser scanning microscopy 710 was used for FRET experiment and Zen software was used for all the fluorescence measurements.
ACKNOWLEDGMENTS
We are grateful to all the blood donors. We thank EBI-MR4 for providing us with AMA1 and EBA175 monoclonal antibody. This research is supported by A*Star BMRC (06/1/22/19/456 and 09/1/22/19/613), Singapore Ministry of Education Academic Research Fund Tier 1 (RG152/14) and Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2017-T2-1-034). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Karthigayan Gunalan and Xiaohong Gao contributed extensively and equally to the experiments, analysed the data and wrote the manuscript. Karthigayan Gunalan, Xiaohong Gao and Peter R. Preiser conceived the experiments. Xiaohong Gao performed all parasite culture, harvest the samples, carry out the western blots analysis and analysed the FRET data. Karthigayan Gunalan performed the PLA and FRET experiments and analysed the PLA data. Karthigayan Gunalan and Sundar Ganesan analysed the FRET data. Xiaohong Gao, Soak Kuan Lai and Hoi Yeung Li performed confocal and super-resolution microscopy and analysed the data. Xiaohong Gao and Sally Shu Lin Yap performed all the recombinant protein purifications. Sally Shu Lin Yap and Andrea Ravasio generated the rat monoclonal antibodies against MD and RVIII. Peter R. Preiser supervised the project, analysed the data, and wrote the manuscript.