The role of bacterial corrosion on recolonization of titanium implant surfaces: An in vitro study
Abstract
Background
Inflammation triggered by bacterial biofilms in the surrounding tissue is a major etiological factor for peri-implantitis and subsequent implant failure. However, little is known about the direct effects of bacterial corrosion and recolonization on implant failure
Purpose
To investigate the influence of oral commensals on bacterial corrosion and recolonization of titanium surfaces.
Materials and Methods
Streptococcus sanguinis (S. sanguinis) and Porphyromonas gingivalis (P. gingivalis), which are key bacteria in oral biofilm formation, were cultured on commercially pure titanium and titanium-aluminum-vanadium (Ti6Al4V) plates in artificial saliva/brain heart infusion medium under aerobic or anaerobic conditions. Biofilm formation was examined after 7 and 21 days by crystal violet and live/dead staining. Titanium ions released into culture supernatants were analyzed over a period of 21 days by atomic absorption spectrometry. Visual changes in surface morphology were investigated using scanning electron microscopy. Biofilm formation on sterilized, biocorroded, and recolonized implant surfaces was determined by crystal violet staining.
Results
S. sanguinis and P. gingivalis formed stable biofilms on the titanium samples. Bacterial corrosion led to a significant increase in titanium ion release from these titanium plates (p < 0.01), which was significantly higher under aerobic conditions on pure titanium (p ≤ 0.001). No obvious morphological surface changes, such as pitting and discoloration, were detected in the titanium samples. During early biofilm formation, the addition of titanium ions significantly decreased the number of live cells. In contrast, a significant effect on biofilm mass was only detected with P. gingivalis. Bacterial corrosion had no influence on bacterial recolonization following sterilization of titanium and Ti6Al4V surfaces.
Conclusion
Bacterial corrosion differs between oral commensal bacteria and leads to increased titanium ion release from titanium plates. The titanium ion release did not influence biofilm formation or bacterial recolonization under in vitro conditions
What is known
Few studies have quantified the microbiologically induced corrosion of implant surfaces by periodontal pathogens, and those that have primarily demonstrated morphological surface changes in implant surfaces, as well as occasional titanium ion release into the surrounding medium.
What this study adds
We showed the effects of different oral commensal bacteria on increased titanium ion release from implant surfaces. Interestingly, titanium ions decreased the viability of these pathogens without affecting their biofilm mass. Bacterial corrosion had no effect on the biofilm mass after recolonization.
1 INTRODUCTION
Dental rehabilitation with endosseous implants is an established therapeutic concept in dentistry, and approximately 5.5–6 million implants are placed annually in Europe alone.1 With a steadily increasing number of dental implant placements in recent years and an incidence of 5%–10% for implant failure, the number for clinical complications is ascending.2 Most implant failures are associated with peri-implantitis.3 The importance of bacterial biofilms in peri-implantitis leading to implant failure has been reported in numerous studies.4-8 While the inflammatory response triggered by bacteria has long been considered to be a primary etiological factor for peri-implantitis, recent literature has disputed the existence of peri-implantitis as a distinct disease entity. This is how Albrektsson et al.9 sees marginal bone loss as a “late disturbance of the foreign body equilibrium,” which is related to various factors, such as the implant system, patient characteristics, and complications, such as occlusal overload or cement residues. When this balance is disturbed, macrophages reactivate, which can ultimately lead to bone resorption and mucosal rupture through a complex mechanism.10 Secondary bacterial infection may thus exacerbate bone loss.11
Many studies have investigated electrochemically induced corrosion of titanium or titanium alloys in specific conditions of the oral cavity. However, it should be noted that bacteria in the oral cavity are ubiquitous and can cause microbiologically induced corrosion.12 Specifically, biocorrosion is described as “the accelerated damage to metals due to the presence of biofilms on their surfaces”.13 Nevertheless, only a few studies have addressed implant surface modification due to bacterial colonization. A study published by Rodrigues et al.14 suggested that bacteria may have a negative impact on the corrosion resistance of implant surfaces, allowing for a change in the surface morphology and the release of metal ions.
In dentistry and orthopedics, pure titanium and titanium alloys (e.g., Ti6Al4V) are widely used owing to their corrosion resistance, mechanical properties, and biocompatibility.15-19 In particular, titanium's ability to form a spontaneous passive oxide layer makes it the most widely used material for dental implants, as this amorphous layer promotes osseointegration by absorbing calcium ions and phosphate.20, 21 Bacterial biofilms may result in the development of an acidic environment, leading to degradation of the titanium oxide layer and the release of metal ions.14 This hypothesis is supported by clinical studies of foreign bodies found in soft tissue samples of peri-implant tissues in patients with peri-implantitis. The examined foreign bodies revealed the presence of titanium particles predominantly surrounded by inflammatory cells.22
Numerous studies have been conducted on bacterial adhesion to titanium both in vivo and in vitro.23-26 Complex interactions between enamel/implant surfaces and salivary proteins lead to the formation of a thin homogeneous film of salivary glycoproteins, called the pellicle, immediately after surface cleaning.27-30 The initial attachment of gram-positive early colonizers, such as Streptococcus sanguis, Streptococcus oralis, and Streptococcus mitis, leads to further bacterial adhesion and dental plaque formation, fulfilling all the characteristics of a typical biofilm.31-33 The host immune cascade triggered by a similar bacterial spectrum leads to gingivitis or periodontitis, as well as mucositis or peri-implantitis, in a fundamentally similar mechanism.34-36 The mainstay of treatment for peri-implantitis is the mechanical and/or antimicrobial reduction or elimination of pathogenic biofilms.37, 38
The main objective of this study was to investigate the effect of biocorrosion on titanium release and bacterial recolonization on dental implant surfaces. In particular, we focused on the evidence of microbiologically induced corrosion and the difference between aerobic and anaerobic bacteria.
2 MATERIAL AND METHODS
2.1 Samples, bacterial strains, and growth conditions
Thin, disc-shaped implant samples (diameter, 11 mm × 2 mm) that were unilaterally polished (DOT GmbH, Rostock, Germany) and comprised commercial pure titanium (cp-Ti, grade 2) and titanium-aluminum-vanadium (Ti6Al4V, grade 5) were used. The samples were sterilized by autoclaving prior to biofilm formation. Streptococcus sanguinis ATCC 10556 as a facultative anaerobic species and Porphyromonas gingivalis W83 as an anaerobic species were used for all experiments. The bacterial strains were commercially purchased (Leibniz Institute DSMZ, German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) and available for long-term storage in the MicrobankTM system of Pro-Lab Diagnostics (Neston, England), according to the manufacturer's instructions. Streptococcus sanguinis (S. sanguinis) was grown under aerobic conditions (5% CO2, 20% O2), and Porphyromonas gingivalis (P. gingivalis) was grown under anaerobic conditions (80% N2, 10% CO2, 10% H2) at 37°C for at least 48 h on Columbia blood agar plates (BD, Franklin Lakes, USA).
2.2 Biofilm formation
A liquid culture was prepared by inoculating the samples in brain heart infusion (BHI) medium (Oxoid, Basingstoke, Hampshire, England) under aerobic or anaerobic conditions in 50 ml culture tubes (Greiner Bio-One GmbH, Kremsmünster, Austria). After approximately 24 h of incubation, the bacteria were harvested by centrifugation (Heraeus varifuge 3.0 R, 4000 rpm, 10 min, 4°C) and washed once with phosphate buffer solution (PBS, pH 7.4). Subsequently, the bacterial pellets were resuspended, and the respective specific optical density was photometrically determined at 600 nm (OD600, Bio-Rad Laboratories, Hercules, CA, USA) in PBS, corresponding to approximately 1 × 108 CFU/ml. Bacterial suspensions were diluted to 1 × 105 CFU/ml and seeded in 24-well microtiter plates (Greiner Bio-One GmbH, Frickenhausen, Germany) on cp-Ti and Ti6Al4V samples and on plastic cover slips (Nunc, Wiesbaden, Germany). Bacteria were cultivated for 7 and 21 days, respectively, in a medium comprising 1/3 BHI plus 2/3 artificial saliva (modified from Pratten et al.39 and were cultured at 37°C under aerobic and anaerobic conditions. The medium was changed during this period, and the supernatants obtained were frozen at −20°C until further use. The corresponding medium without bacteria served as the reference.
After 7 and 21 days of biofilm formation, the samples were transferred to new well plates and washed twice with PBS. The biofilms were stained with 0.1% crystal violet solution (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) for 15 min and rinsed thrice with distilled water to remove unbound crystal violet. Elution with 1% sodium dodecyl sulfate (Serva, Heidelberg, Germany) for 15 min with constant shaking was performed, and 200 μl of the eluted dye was transferred to a 96-well plate for quantitative spectrophotometric evaluation at 570 nm (SpectraMax M2, Molecular Devices, Ismaning, Germany). This protocol was modified from that reported by Shen et al.40
For viability detection and visualization of biofilm formation, the samples were stained with a live/dead dye (Live/Dead BacLight Bacterial Viability Kit, Invitrogen, Darmstadt, Germany). The dye (Syto9/propidium iodide 1:1) was diluted 1:1000 in PBS and added to the samples for 10–15 min in the dark. Microscopic analyses were performed with a BX60 fluorescence microscope (Olympus, Hamburg, Germany) using U-MWB (Ex 470–490, Em 520 nm), U-MWU (Ex 330–385 nm, Em 420 nm), and U-MWG (Ex 510–550 nm, Em 590 nm) filters (Olympus, Hamburg, Germany). Images were acquired using an attached digital camera (Leica, Wetzlar, Germany).
2.3 Titanium release and surface changes
The concentration of released titanium ions in the obtained supernatants of the bacterially colonized surfaces and the surfaces placed in pure medium were determined by atomic absorption spectrometry (AAS) with electrothermal sputtering (ZEEnit 650, Analytik Jena AG, Jena, Germany). To quantify the titanium ions in the supernatants, the measured intensity was compared with that of a standard titanium reference (1000 mg/L; Merck KGaA, Darmstadt, Germany). For this purpose, a calibration curve was first constructed based on the expected concentration (40 μg/L) using a stock solution. To stabilize the released titanium ions, 1% nitric acid was added as a diluent. The droplets from the nebulizing process of the different sample liquids (20 μl) were evaporated in a graphite tube in a multistage drying process (90°C for 20 s, 105°C for 20 s, 110°C for 10 s). In the subsequent pyrolysis phase (1900°C for 10 s), all organic components were eliminated, and solid particles were burned. A rapid heat increase (1000°C/s) to 2625°C for 10 s is required for solid particle evaporation and atomization. Because the initiated transition of electrons to a higher energy level within the atoms and the subsequent return to the ground state releases a quantum of light, elemental analysis was performed using a hollow cathode lamp with a titanium cathode radiating at 365.4 nm.
For electron microscopic analysis, half of the samples were placed in Sekusept (Ecolab, Reinach, Switzerland) overnight and then cleaned for 10 min in an Extran cleaning solution (Merck KGaA, Darmstadt, Germany) in an ultrasonic bath (BactoSonic 35 kHz, Bandelin Electronic GmbH & Co. KG Berlin, Germany). Afterwards, cleaned samples and biofilm-covered samples were washed with PBS and fixed in 2.5% glutaraldehyde solution in 0.1-M sodium phosphate buffer (Merck KGaA, Darmstadt, Germany) for at least 24 h at 4°C. After the samples were washed again with 0.1-M sodium phosphate buffer (Merck KGaA, Darmstadt, Germany), they were dehydrated through a series of graded ethanol solutions (30, 50, 70, 90, 2 × 100%). After they were dried with an EMITECH K850 critical point dryer (Emitech, Lohmar, Germany) according to the manufacturer's instructions, the samples were gold-coated (BalTec SCD 004; BAL-TEC AG, Principality of Liechtenstein). The images were obtained with a field emission SEM MERLIN VP compact (Carl Zeiss AG, Oberkochen, Germany) up to an enlargement of 25 000×.
2.4 Influence of titanium ions on an existing biofilm
To evaluate the influence of titanium ions on an existing biofilm, S. sanguinis and P. gingivalis were cultured in 96-well microtiter plates in BHI/saliva medium, as explained previously, for 72 h and then incubated with graded concentrations of a titanium stock solution (200–1000 mg/L) and in pure medium. After the biofilm was washed twice with PBS, its mass was photometrically determined using crystal violet staining, as described above. To determine the live bacterial count, the medium was removed from the wells of the microtiter plates, and the biofilm structures were washed once with PBS. The remaining biofilms were scraped from the surface and resuspended in 1 ml PBS. After preparing a dilution series in PBS, 100 μl of each dilution stage was plated on Columbia blood agar using an Eddy Jet Spiral Plater (IUL Instruments GmbH, Königswinter, Germany). After 48 h of incubation under aerobic or anaerobic conditions, the bacterial count (CFU/ml) was determined using a counting template (IUL Instruments GmbH, Königswinter, Germany).
2.5 Bacterial recolonization
The pretreated samples from the initial biofilm formation assay were placed in Sekusept (Ecolab, Reinach, Switzerland), cleaned in Extran cleaning solution (Merck KGaA, Darmstadt, Germany) for 10 min in an ultrasonic bath, and sterilized by autoclaving. Subsequently, bacterial recolonization was performed analogous to the initial biofilm formation for 7 and 21 days. The blank cp-Ti and Ti6AlV samples were used as negative references. Biofilm formation was quantitatively evaluated using crystal violet staining, as described above.
2.6 Statistics
The results were statistically analyzed and graphs were generated by using SPSS (IBM Deutschland GmbH, Ehningen, Germany). Normal distribution of the data was demonstrated using the Kolmogorov–Smirnov test. Data homogeneity was tested using Levene's test. Analysis of variance (ANOVA) and Scheffé post hoc tests were used to detect significant differences between data. In the case of atomic absorption spectroscopy, analysis of covariance (ANCOVA) and Bonferroni-corrected post hoc analysis were performed. Here, the normal distribution of the error terms was checked using the quantile-quantile plot and histogram of the residuals. Since this showed a violation of normal distribution, bootstrapping was performed with 95% Bias-corrected and accelerated (BCa) confidence intervals and 1000 samples. Statistical significance was set at p ≤ 0.05. All data are presented as the mean ± SD. At least three biological replicates and three technical replicates were included in the statistical analyses of all experiments.
3 RESULTS
3.1 Stable biofilm structures over 7 and 21 days
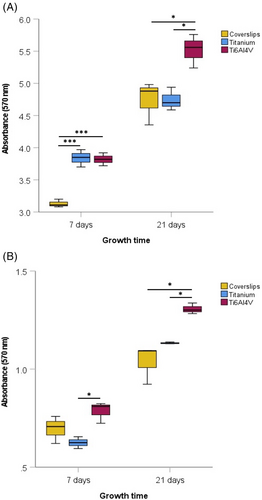
Comparison of the microscopic images after live/dead staining (Figure 1) and the measured absorbance (Figure 2) showed that both S. sanguinis and P. gingivalis were able to form stable biofilm structures on titanium and Ti6Al4V samples over 7 and 21 days. After 7 days, there was a significant difference between the S. sanguinis biofilm mass on the plastic cover slips and on the titanium (p < 0.001) and Ti6Al4V samples (p < 0.001). After 21 days, the highest S. sanguinis biofilm mass was detected in the Ti6Al4V samples. In comparison, significantly smaller values were measured in the control group (p < 0.05) and the titanium samples (p < 0.05). ARegarding the biofilm mass of P. gingivalis, a significantly higher absorption was measured after 7 and 21 days on the Ti6Al4V samples than on the titanium samples (p < 0.05). In addition after 21 days, a larger biofilm mass was detected on the Ti6Al4V samples compared to that of the negative control (p < 0.05).
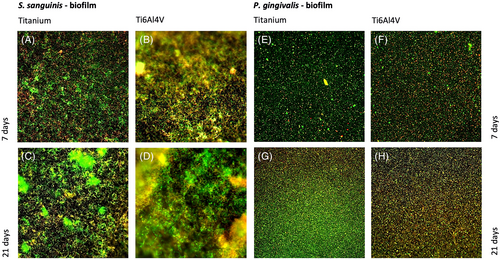
3.2 Biocorrosion
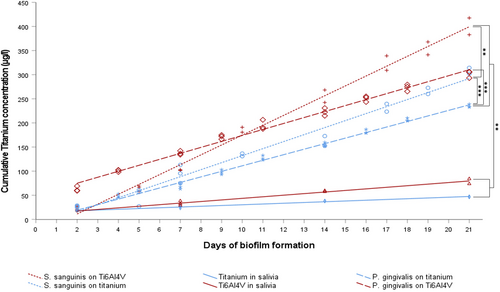
The measured increase in titanium ions in the supernatant by the AAS demonstrated bacteria-induced corrosion. Figure 3 shows the cumulative Titanium concentration. In order to evaluate the increases as well as the statistically significant differences between the experimental groups, a linear regression of the measured data was calculated in each case. Table 1 shows the coefficients of the linear regression as well as the coefficient of determination R2. Subsequently, analysis of covariance (ANCOVA) and Bonferroni-corrected post hoc analysis with BCa-bootstrap were used to detect significant differences between experimental groups (Table 2).
| Linear regression model y = β0 + β1⋯x | |||
|---|---|---|---|
| β0 | β1 | R2 | |
| Streptococcus sanguinis on Ti6Al4V | −29.630 | 20.433 | 0.991 |
| Streptococcus sanguinis on titanium | −13.230 | 14.541 | 0.973 |
| Porphyromonas gingivalis on titanium | −3.824 | 11.494 | 0.994 |
| Porphyromonas gingivalis on Ti6Al4V | 50.233 | 12.386 | 0.988 |
| Titanium in salivia | 14.713 | 1.564 | 0.973 |
| Ti6Al4V in salivia | 10.844 | 3.278 | 0.959 |
| ANCOVA | |||||
|---|---|---|---|---|---|
| Dependent variable: | |||||
| Source | Type III Sum of Squares | df | Mean Square | F | Sig. |
| Corrected model | 936108,809a | 6 | 156018,135 | 140,701 | 7,07E-46 |
| Intercept | 22323,194 | 1 | 22323,194 | 20,132 | 1,97E-05 |
| Days of biofilm formation | 703928,742 | 1 | 703928,742 | 634,819 | 1,32E-44 |
| Experimental groups | 327623,467 | 5 | 65524,693 | 59,092 | 4,63E-28 |
| Error | 108668,877 | 98 | 1108,866 | ||
| Total | 3325424,823 | 105 | |||
| Corrected total | 1044777,686 | 104 | |||
- a R Squared = 0.896 (Adjusted R Squared = 0.890).
The concentration of titanium released was significantly increased in the titanium samples with bacterial colonization when compared to the negative control (p < 0.01). Titanium release was significantly higher in Ti6Al4V samples than in titanium samples incubated with S. sanguinis (p < 0.01) or P. gingivalis (p ≤ 0.001; Figure 3). On comparing titanium release between the samples colonized with S. sanguinis and P. gingivalis, significantly higher titanium ion concentrations were measured with titanium (p ≤ 0.001) incubated with S. sanguinis. Electron micrographs (Figure 4) showed biofilm structures without any noticeable changes to the specimen surfaces, such as pitting attack or surface erosion.
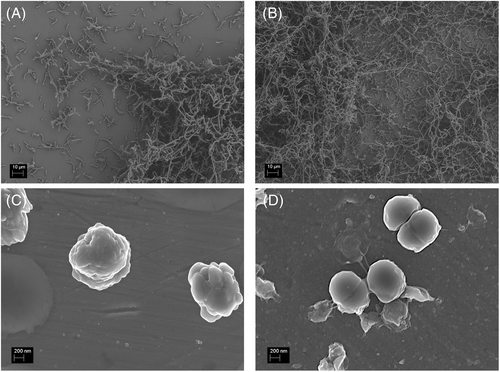
3.3 Incubation with different titanium concentrations during the early phase of biofilm formation
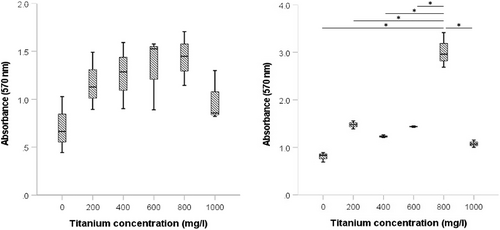
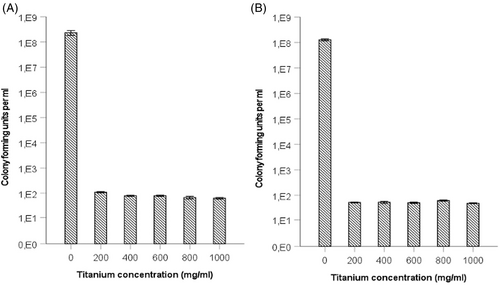
As shown in Figure 5, incubation with increasing titanium concentrations in the early phase of biofilm formation indicated different responses between S. sanguinis and P. gingivalis regarding the quantification of biofilm mass by crystal violet staining. Incubation of a P. gingivalis biofilm with titanium ions resulted in significantly (p < 0.05) increased absorbance after crystal violet staining for all groups, but no statistical significance (p = 0.139) was observed for S. sanguinis biofilms. Subsequent determination of the live cell count showed that the addition of titanium ions into the medium had a significant negative (p < 0.001) effect on the number of living cells in the biofilm. A significant decrease in the bacterial count for both S. sanguinis and P. gingivalis occurred due to the presence of titanium ions in the culture medium (Figure 6).
3.4 Bacterial recolonization experiments
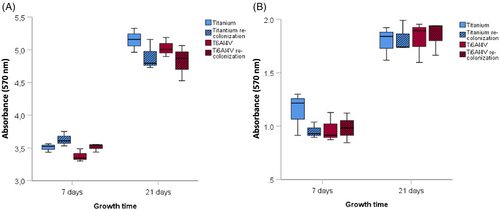
Stable biofilm formation by S. sanguinis and P. gingivalis was detected after 7 and 21 days in the previously treated and sterilized implant samples, as well as in the newly manufactured titanium and Ti6Al4V samples. There were no significant differences in biofilm mass formation between any of the test groups (Figure 7).
4 DISCUSSION
In this study, we hypothesized that microbiologically induced corrosion and associated titanium ion release influence bacterial recolonization and thus play an important role in peri-implant inflammation. We developed an in vitro model to investigate the effect of bacterial biofilms on dental implant surfaces. The results suggested that stable biofilms could be cultured on implant surfaces for 21 days under both aerobic and anaerobic conditions to demonstrate the continuous release of titanium ions. There were no changes in surface morphology. This may partly explain why we could not detect any effect on biofilm mass formation upon bacterial recolonization. However, the significant decrease in bacterial count during incubation with titanium-supplemented media shows that strongly elevated titanium concentrations may influence bacterial growth during the early phase of biofilm formation.
Inside the oral cavity, bacteria primarily adhere to desquamation-free surfaces, such as tooth or implant surfaces, and form dental plaques to survive in the long term, fulfilling all the characteristics of a bacterial biofilm.32, 41 S. sanguinis is a gram-positive facultative anaerobic initial colonizer that supports the attachment of subsequent organisms and plays a key role in oral biofilm development.42-44
P. gingivalis, on the other hand, is a gram-negative anaerobe that plays a crucial role in the pathogenesis and progression of periodontal disease.45 Since both surface and bacterial characteristics are crucial for adhesion, the significantly higher adherent-proven biofilm mass of S. sanguinis compared to P. gingivalis can be reconciled with previous findings in the literature. In a study by Mabboux et al.,46 hydrophobic S. sanguinis adhered better to saliva-coated titanium and Ti6A4V alloy than hydrophilic S. constellatus. Previous studies have suggested that this stable adhesion of S. sanguinis ATCC 10556 occurred due to hydrophobic bonds between the bacteria and absorbed salivary proteins.46, 47 Therefore, it can be hypothesized that hydrophilic germs, such as P. gingivalis W83, have poorer adhesion due to the inhibition of chemical bonds by a persistent non-displaceable water film.46 Another argument in favor of a low biofilm mass is that the highly virulent P. gingivalis strain W83 used in this study has a thick capsule, which reduces autoagglutination and shows lower densities than non-encapsulated P. gingivalis strains.48
The literature contains several clinical studies in which metal particles have been detected in hard and soft peri-implant tissues in the presence of inflammation.49-51 Regarding the role of titanium ion release in mucositis and peri-implantitis, Wachi et al.52 investigated cellular responses to titanium ions and/or P. gingivalis in gingival and bone tissues using a rat model. Using plasma mass spectrometry, the authors determined the concentration of titanium released after embedding an implant in a sodium fluoride solution. Published data suggest that monocyte infiltration and osteoclast differentiation are promoted by increased sensitivity of gingival epithelial cells to microorganisms in the oral cavity. Other studies also suggest a cytotoxic inflammatory response to the smallest titanium particles (∼10 μm).53
Nevertheless, few in vitro studies have investigated biocorrosion by periodontal pathogens with the release of titanium ions. In a study by Sridhar et al.,54 dental implants were placed in a Streptococcus mutans liquid culture for 60 days, and the release of titanium ions into the surrounding medium was detected by impedance measurements (start, 10 and 22 days), similar to the measurement time points in our study. Quantification of titanium release was not carried out; hence, a detailed comparison with the present study is not possible. After a longer study period of 60 days, corrosive changes in the surface morphology were also observed.54 A study by Rodrigues et al.55 from the same research group confirmed these visible changes in the implant surfaces and additionally performed an incubation of implants with P. gingivalis for 1 month, in which no corrosive changes in surface morphology were observed. Moreover, the results of Souza et al.,56 who conducted comparative electrochemical tests between bacterially colonized (Streptococcus mutans) and blank samples in pure saliva, confirmed these findings. In their study, free corrosion potential measurements after 48 h indicated a negative impact of biofilm formation on the corrosion resistance of the titanium alloy. Similar results were found in a study by Fukushima et al.,57 in which plasma mass spectroscopy was used to investigate titanium release in the surrounding medium. However, over a shorter study period of 90 min, no increase in titanium release was detected upon incubation with Streptococcus mutans. In addition, Li et al.58 observed electron microscopically visible changes, such as grooves and pits, and an increased corrosion potential in Streptococcus sanguinis biofilm colonization over 14 days.
In the present study, the absorption measurements after crystal violet staining showed no significant difference in biofilm mass formation on the already corroded surfaces compared to the blank titanium and Ti6Al4V samples, which is in contrast to the results of Barão et al.,59 which revealed a significantly increased P. gingivalis biofilm mass after an incubation period of 6 h following electrochemical corrosion of titanium and Ti6Al4V samples. A conceivable explanation is provided by the results of a previous clinical study that suggested a so-called threshold roughness (<0.2 μm) below which no further effects on bacterial adhesion and/or colonization could be detected after 1 and 3 months.60 Buergers et al.61 also found no correlation between the smallest differences in surface roughness (0.04–0.08 μm) of provisional fixed prosthetic materials and Streptococcus mutans adhesion. In addition, surface characteristics, such as hydrophobicity and zeta potential (electrostatic potential) significantly influence the extent of bacterial adhesion. Thus, an effect with clinical relevance would have been conceivable due to titanium ion release.62
Metals, such as silver, gold, and zinc, have been used for centuries as bactericidal and bacteriostatic agents in a variety of applications.63-65 The electrostatic attraction between positively charged metal ions and the negatively charged bacterial membrane may be crucial for antimicrobial activity.66 In particular, the effect of nanoparticulate silver has been extensively compared to that of other metal nanoparticles, such as copper or zinc.67, 68 Kawahara et al.69 investigated the antibacterial activity of silver zeolite against obligate and facultative anaerobic bacteria in the oral cavity. Gram-negative species, such as P. gingivalis, proved to be more sensitive than gram-positive species, such as S. sanguinis, which might have occurred due to the different amounts of peptidoglycans in the membrane.70 The fact that the antimicrobial effects of many agents, including metal nanoparticles, against planktonic bacteria do not correspond to the effects against a bacterial biofilm is well known and has been demonstrated in various studies, like that by Chaw et al.71 These authors showed that silver ions were effective at minute concentrations against planktonic cells of Staphylococcus epidermidis but only at the periphery of biofilms. In addition, concentration and exposure time play important roles.70 Roy et al.72 suggested that TiO2 nanoparticles may enhance the antimicrobial effect of various antibiotics against methicillin-resistant Staphylococcus aureus strains.
Nevertheless, there are hardly any comparable studies that have evaluated the possible effects of titanium particles. The results of the present study close this gap and show that titanium concentrations (> 200 mg/ml) have a negative effect on the reproductive ability of bacteria. A similar result was obtained by Besinis et al.,73 who showed growth inhibition and “some mortality” in an S. mutans liquid culture from a titanium dioxide concentration ≥ 100 mg/L.
5 SUMMARY
This study confirmed the hypothesis that bacteria associated with peri-implantitis can induce titanium implant surface corrosion–characterized by the release of titanium ions – under in vitro conditions. The biofilm mass, and therefore the thickness of the biofilm, seems to have a significant influence on the release of titanium into the surrounding medium. Obvious surface changes, such as pitting attacks or surface erosion, could not be shown, unlike in other studies. Because of the expected in vivo titanium concentrations, an influence on the proliferation of planktonic bacteria and on early biofilm formation did not occur. Early bacterially induced corrosion was shown to have no influence on bacterial recolonization after the implant surfaces were sterilized. Future investigations may provide further insights into the biological effects of biocorrosion on peri-implant tissue.
AUTHOR CONTRIBUTION
Julia Weller: Experimental procedure, data collection/analysis, article drafting.
Praveen Vasudevan: Study concept/design, critical revision of article.
Bernd Kreikemeyer: Study concept/design, critical revision of article.
Katharina Ekat: Study concept/design, critical revision of article.
Hermann Lang: Study concept/design, critical revision of article.
Mario Jackszis: Experimental experimentation, data analysis.
Armin Springer: Electron microscopic images, data analysis.
Kyriaki Chatzivasileiou: Study design concept/design, critical revision of article.
All authors have approved the final and submitted version of this manuscript.
ACKNOWLEDGMENTS
The authors thank the company DOT GmbH (Rostock, Germany) for providing the titanium implant surfaces.
Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.




