Outcome of implants placed to retain craniofacial prostheses – A retrospective cohort study with a follow-up of up to 30 years
Abstract
Objectives
To retrospectively assess the treatment outcomes of endosseous implants placed to retain craniofacial prostheses.
Material and Methods
Patients with craniofacial defects resulting from congenital disease, trauma, or oncologic treatment had implant retained prostheses placed in the mastoid, orbital, or nasal region and then assessed over a period of up to 30 years. Implant survival rates were calculated with the Kaplan–Meier method. Clinical assessments consisted of scoring skin reactions under the prosthesis and the peri-implant skin reactions. Possible risk factors for implant loss were identified. Patient satisfaction was evaluated using a 10-point VAS-scale.
Results
A total of 525 implants placed in 201 patients were included. The median follow up was 71 months (IQR 28–174 months). Implants placed in the mastoid and nasal region showed the highest overall implant survival rates (10-year implant survival rates of 93.7% and 92.5%, respectively), while the orbital implants had the lowest overall survival rate (84.2%). Radiotherapy was a significant risk factor for implant loss (HR 3.14, p < 0.001). No differences in implant loss were found between pre- and post-operative radiotherapy (p = 0.89). Soft tissue problems were not frequently encountered, and the patients were highly satisfied with their implant-retained prosthesis.
Conclusion
Implants used to retain craniofacial prostheses have high survival and patient satisfaction rates and can thus be considered as a predictable treatment option. Radiation is the most important risk factor for implant loss.
What is known
- Implant placement to retain a craniofacial prothesis in patients with defects in the craniofacial region is a predictable treatment option with high implant survival and patient satisfaction scores.
- Most studies report short-term outcomes and small patient groups.
What this study adds
- This study presents the outcomes of a large patient group with a history of craniofacial congenital disease, trauma, or oncological disease, treated over a period up to 30 years.
- Radiotherapy has a negative effect on implant survival irrespective of whether implants are placed before or after radiotherapy.
1 INTRODUCTION
Implant placement to retain a prothesis in patients with defects in the craniofacial region due to oncologic treatment, congenital disease, or trauma is a predictable treatment option, with high implant survival and patient satisfaction scores.1 Craniofacial prostheses are a durable solution, that mimic the contour of the missing facial region, blend into the surrounding regions, and can be worn and placed with relative ease and comfort.2, 3 When compared with autologous surgical reconstructions, which usually require several extensive procedures, implant-retained prostheses lead to a more acceptable combination of a relatively limited surgical procedure and satisfactory aesthetic results.4, 5
An optimal treatment outcome (from a prosthetic as well as a surgical point of view) crucially requires careful pre-operative implant placement planning.6, 7 Poor bone quality and low bone volume are important risk factors for craniofacial implant loss, with the highest reported implant loss occurring from the orbital regions.8-10 In addition, radiotherapy is negatively associated with implant survival as radiation has a high impact on bone quality.11, 12 However, the timing of craniofacial implant placement in oncology patients, e.g., before or after radiotherapy, is still an issue of debate.13-15 Some studies recommend placing implants before starting radiation therapy (during ablative surgery), while others recommend implant treatment after radiotherapy.16, 17
Although implant survival and patient satisfaction rates are high, most studies in the literature present short-term results on small patient groups. This is in line with the conclusion of the Chrcanovic et al. review on craniofacial implant survival and complications.11 Our aim, therefore, was to assess the treatment outcome of endosseous implants placed to retain craniofacial prostheses in a large group of patients with a craniofacial defect.
2 MATERIAL AND METHODS
2.1 Patients and treatment protocols
All consecutive patients treated with implants in the mastoid, auricular or nasal region to retain a craniofacial prosthesis between May 1988 and December 2018 at the University Medical Center Groningen (UMCG) were included in this retrospective study. All the implants were placed by two experienced oral and maxillofacial surgeons in the native bone under general anesthesia using a 2-stage method; each patient received antibiotic prophylaxis at general anesthesia induction (Augmentin 1200 mg i.v.). The irradiated patients then continued with a broad-spectrum antibiotic for a further 2 weeks. The oncology patients' implants were placed during or after ablative surgery and, when applicable, before or after radiotherapy.
Auricular prostheses involved placing 2 or 3 implants in the mastoid bone, approximately 18 mm from the external auditory canal. A minimum distance of 11 mm was maintained between the implants. In the nasal region, two implants were placed in the maxillary bone of the nasal floor after trimming the sharp edges from the caudal site of the piriform aperture. Implants in the orbital region were placed in the supraorbital (2 or 3 implants) and infraorbital rim (1 or 2 implants). The Nobel Biocare (Zurich, Switzerland) and Entific Medical Systems Inc (Gothenburg, Sweden) implant systems were used. Second stage surgery in the non-irradiated patients involved retrieving the implants under local anesthesia and thinning the subcutaneous tissue surrounding the implants after 3 months of osseointegration. Regarding the irradiated patients, the second stage surgery took place 3 months after the last radiotherapy session. Gauze dressings with antibiotic ointment (Terra-Cortril, Pfizer Inc., New York, NY) were draped around the abutments to guarantee good skin positioning and to prevent abutment overgrowth. The gauze dressings were changed weekly and removed completely after 3 weeks. Prosthetic rehabilitation was carried out after the second stage surgery by a team of experienced maxillofacial prosthodontists. The patients were seen at the regular yearly follow-ups.
A waiver of exemption regarding the Medical Research Involving Human Subjects Act (WMO) was granted by the Medical Ethics Committee of the University Medical Center Groningen (reference number M19.235062).
2.2 Data collection and treatment outcome assessment
The patients' demographics, implant treatment variables, and data on implant survival and complications were collected retrospectively from the patient records. With respect to the radiotherapy patients, the data on the timing of the implant placement (before or after radiotherapy) and radiation dose on the tumor area were also recorded. In the survival analysis, implant loss was defined as the loss of an implant for any reason during the follow-up period. Implant survival was defined as the time from implant placement until the date of implant loss (event) or the last known follow-up. Death was censored and did not count as an event. Implants which were never retrieved after being placed were excluded from the survival analysis. The subgroup analysis was based on implant location, implant indication, the presence of radiotherapy, and the timing of the implant placement.
2.3 Clinical assessment
The patients who were still attending the follow-ups were clinically assessed by an oral and maxillofacial surgeon (JA) and maxillofacial prosthodontist (IE) who had not been involved in their treatment. A minimal follow-up period of 1 year after the prosthesis placement was required. The clinical assessment included scoring the skin reactions around the implants and underneath the prosthesis. The peri-implant tissues were scored according to the Tolman and Taylor criteria18: 0, no irritation; 1, slight redness; 2, red and moist tissue; 3, granulation, red and moist tissue; 4, active infection. Skin reactions under the prosthesis were scored as being present or not present. The patients were also asked to score their overall satisfaction with the prosthesis using a 10-point VAS-scale (1, absolutely not satisfied; 10, very satisfied). The clinical outcomes of the irradiated and non-irradiated patients were compared.
2.4 Statistical analysis
The categorical data from the calculated descriptive statistics were presented as number and percentages. In case of normality, the groups were compared using one-way ANOVA. The Mann–Whitney U-test was used to compare the groups with a categorical variable. Implant survival rates were determined with the Kaplan–Meier method and reported as a percentage of survival. The survival curves were compared with the log-rank test. In order to identify possible risk factors for implant loss, a multivariate analysis using a Cox-proportional-hazards model was performed. The following covariates were added to the analysis: age at implant placement, gender, implant location (mastoid, nasal, orbit), and radiotherapy (yes or no). A p-value <0.05 was regarded as statistically significant. Graphpad Prism 8 for Windows was used for the survival analyses and curve comparison. All the remaining statistical analyses were carried out with IBM SPSS statistics 23 (SPSS, Chicago, IL, USA).
3 RESULTS
A total of 220 patients with 575 craniofacial implants were initially evaluated. Fifty implants in 19 oncology patients (11.2%) were never retrieved before the second stage surgery due to tumor recurrence (11 patients) or death (8 patients) and so were excluded. The remaining 525 implants placed in 201 patients were included in this retrospective study. The patient characteristics are presented in Table 1, and the clinical aspects from baseline to long-term follow-up are shown in Figures 1-4. More males than females were involved. The traumatology patients and those with congenital deformities were significantly younger at the time of implant placement compared to the oncology patients (p < 0.001).
| Implant placement indication | |||
|---|---|---|---|
| Oncology | Traumatology | Congenital | |
| Number of patients per group (%) | 150 (74.6) | 18 (9.0) | 33 (16.4) |
| Mean age at implant placement | |||
| Years (SD) | 67.0 (14.5) | 42.1 (14.8) | 31.4 (17.9) |
| Gender | |||
| Male (%) | 101 (67.3) | 11 (61.1) | 20 (60.6) |
| Female (%) | 49 (32.7) | 7 (38.9) | 13 (39.4) |
| Number of patients per implant location | |||
| Mastoid | 50 | 14 | 33 |
| Nasal aperture | 44 | 1 | 0 |
| Orbit | 56 | 3 | 0 |
| Number of implants per patient | |||
| Mastoid | 2 or 3 | ||
| Nasal aperture | 2 | ||
| Orbit | 3 or 4 | ||
| Timing of implant placement | |||
| During ablative surgery, no radiotherapy (%) | 52 (34.7) | ||
| During ablative surgery, before radiotherapy (%) | 40 (26.7) | ||
| During ablative surgery, after radiotherapy (%) | 32 (21.3) | ||
| After ablative surgery, no radiotherapy (%) | 19 (12.7) | ||
| After ablative surgery, before radiotherapy (%) | 1 (0.7) | ||
| After ablative surgery, after radiotherapy (%) | 6 (4.0) | ||
| Radiotherapy dose on tumor area (gray) | |||
| Median (min-max) | 64 (30–70) | ||
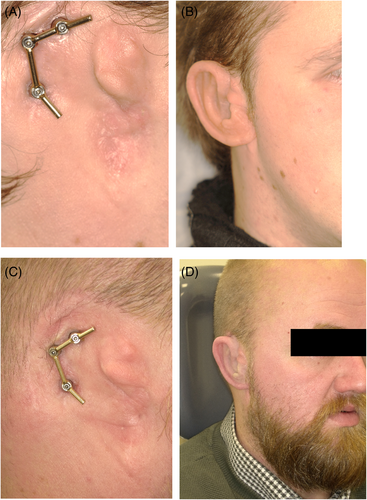
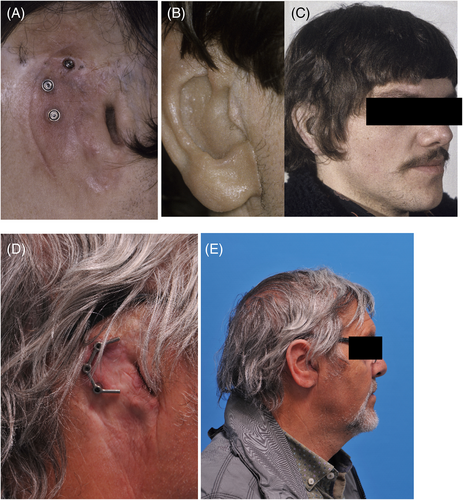
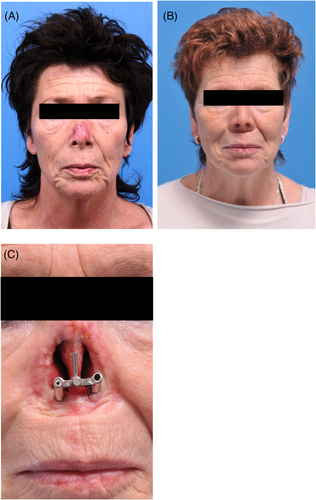
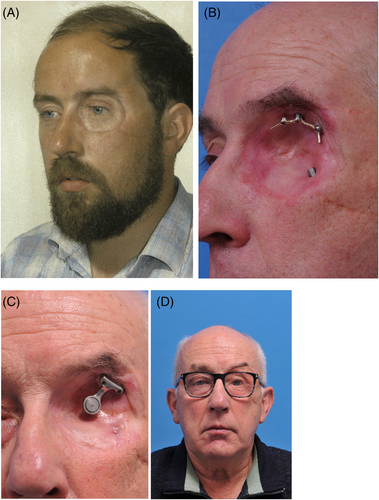
The mean time between implant placement and second stage surgery was 6 months (IQR 4–8) for the irradiated patients and 4 months (IQR 3–6) for the non-irradiated patients. The median time between implant placement and radiotherapy commencement was 6 weeks (IQR 4–8.75). Regarding the patients treated after radiotherapy (secondary implant placement), the median time between the end of radiotherapy and implant placement was 108.5 weeks (IQR 34–232.5).
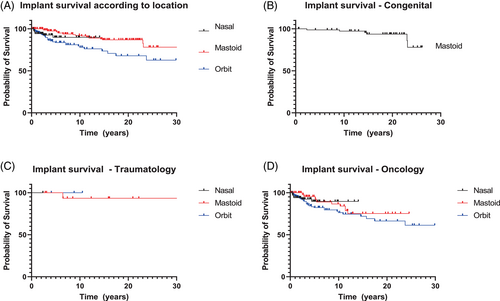
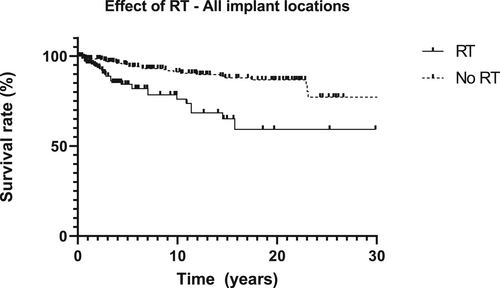
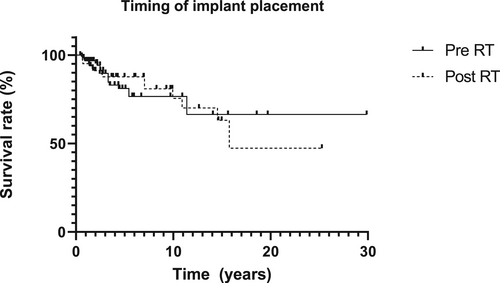
The Kaplan–Meier survival curves per patient group (oncology, traumatology, and congenital disease) and location (mastoid, orbit, nasal aperture) are presented in Figure 5A–D. The implant survival rates differed per patient group and implant location. The percentage of implants lost per location did not vary greatly: the mastoid region 21/231 implants (10-year implant survival rate 93.7%); the nasal region 7/90 implants (10-year implant survival rate 92.5%); and the orbital region 33/204 implants (10-year implant survival rate 84.2%) (Tables 2–4 show the implant loss per subgroup). Implant survival in the various implant locations differed significantly (p < 0.001). Radiotherapy had a negative effect on implant survival (p < 0.001) (Figure 6). A comparison of the survival curves in Figure 7 did not result in a statistically significant implant survival difference between the implants placed before or after radiotherapy (p = 0.89).
| Implant region | ||||
|---|---|---|---|---|
| Mastoid | Nasal | Orbit | Total | |
| Number of placed implants (number of lost implants) | 108 (13) | 88 (7) | 194 (33) | 390 (53) |
| During ablative surgery, no RT | 47 (3) | 26 (3) | 49 (4) | 122 (10) |
| During ablative surgery, pre RT | 26 (6) | 16 (0) | 78 (11) | 120 (17) |
| During ablative surgery, post RT | 5 (0) | 40 (4) | 28 (6) | 73 (10) |
| After ablative surgery, no RT | 23 (4) | 4 (0) | 23 (6) | 50 (10) |
| After ablative surgery, pre RT | 0 (0) | 0 (0) | 5 (1) | 5 (1) |
| After ablative surgery, post RT | 7 (0) | 2 (0) | 11 (5) | 20 (5) |
| Median follow-up in months (IQR) | 51.5 (23–124) |
|||
| Implant region | ||||
|---|---|---|---|---|
| Mastoid | Nasal | Orbit | Total | |
| Number of placed implants (number of lost implants) | 41 (2) | 2 (0) | 10 (0) | 53 (2) |
| Median follow-up in months (IQR) | 102 (43–193) | |||
| Implant region | ||||
|---|---|---|---|---|
| Mastoid | Nasal | Orbit | Total | |
| Number of placed implants (number of lost implants) | 82 (6) | 0 (not applicable) | 0 (not applicable) | 82 (6) |
| Median follow-up in months (IQR) | 234 (153.8–264.3) | |||
Multiple variables (age at implant placement, gender, implant location and the presence of radiotherapy) were included in the Cox proportional-hazard model. Within the study population, two variables (gender and the presence of radiotherapy) remained statistically significant in the multivariate analysis (Table 5).
| Variable | Univariate model hazard ratio (95% CI) | p-value | Multivariate model hazard ratio (95% CI) | p-value |
|---|---|---|---|---|
| Age at implant placement | 1.02 (1.00–1.03) | 0.011 | 1.01 (0.99–1.03) | 0.227 |
| Gender (female) | 0.51 (0.28–0.92) | 0.018 | 0.47 (0.26–0.86) | 0.015 |
| Implant location | ||||
| Nasal | Reference | 0.135 | ||
| Mastoid | 0.51 (0.21–1.23) | 0.402 | ||
| Orbit | 1.42 (0.62–3.25) | |||
| Radiotherapy | 3.4 (2.03–5.76) | <0.001 | 3.14 (1.80–5.47) | <0.001 |
Forty-five out of the 61 implants which were lost (73.8%) were not replaced because the patients could still wear a functioning prosthesis on the remaining implants. The 24 implants lost by 10 patients were replaced to increase prosthesis retention but three were lost again. At follow-up, 96.9% of the auricular prostheses, 93.4% of the nasal prostheses, and 89.8% of the orbital implant-retained prostheses were still functional. A new craniofacial prosthesis was made every 2 to 2.5 years.
During the follow-up, seven patients (two patients with nasal implants and five patients with orbital implants) developed osteoradionecrosis. In the majority of these patients (n = 5), the exposed bone developed outside the implanted region and the implants were not affected. Two patients experienced loss of an orbital implant as a result of progressive osteoradionecrosis. Osteoradionecrosis was usually treated with a combination of hyperbaric oxygen, antibiotics and debridement therapy.
3.1 Clinical assessment
Of the initial 220 patients, 126 patients died during the follow-up period. Unfortunately, 28 out of the remaining 94 patients were lost to further follow-up (due to moving to another part of the country or multiple no-shows). Sixty-two patients (20 irradiated and 42 non-irradiated patients) were available for the clinical assessment (Tables 6–8). The mean follow-up period in this group was 164.4 months (SD: 100.8). There was no statistically significant difference in skin reactions under the prosthesis between the irradiated and non-irradiated patients (p = 0.76). Peri-implant soft tissue reactions were more frequent in the irradiated patients (p = 0.02). The presence of soft tissue problems did not vary between the various implant locations (p = 0.34 for the presence or absence of skin reactions; p = 0.06 for peri-implant reactions).
| N (%) | |
|---|---|
| Clinical assessment | |
| Skin reaction under the prosthesis | |
| Present | 17 (27.4) |
| Not present | 45 (72.6) |
| Reaction around the abutments | |
| No irritation | 39 (62.9) |
| Slight redness | 11 (17.7) |
| Red and moist tissue | 7 (11.3) |
| Granulation, red and moist tissue | 3 (4.8) |
| Active infection | 2 (3.2) |
| Patient-reported outcomes | |
| Overall satisfaction with implant retained prosthesis | |
| Mean score (SD) | 8.4 (1.7) |
| No irritation | Slight redness | Red and moist tissue | Granulation, red and moist tissue | Active infection | Total | |
|---|---|---|---|---|---|---|
| Implant location | ||||||
| Mastoid | 26 | 5 | 4 | 0 | 0 | 35 |
| Nasal | 8 | 2 | 2 | 3 | 0 | 15 |
| Orbit | 5 | 4 | 1 | 0 | 2 | 12 |
| Total | 39 | 11 | 7 | 3 | 2 | 62 |
| No irritation | Slight redness | Red and moist tissue | Granulation, red and moist tissue | Active infection | Total | |
|---|---|---|---|---|---|---|
| Indication for implant placement | ||||||
| Oncology | 21 | 9 | 3 | 3 | 2 | 38 |
| Traumatology | 8 | 0 | 2 | 0 | 0 | 8 |
| Congenital | 12 | 2 | 2 | 0 | 0 | 16 |
| Total | 39 | 11 | 7 | 3 | 2 | 62 |
4 DISCUSSION
This study presents the treatment outcomes for implants used to retain craniofacial prostheses in maxillofacial defects. The overall survival rates of the endosseous implants placed in patients with craniofacial defects due to trauma (96.2%) and congenital diseases (92.7%) were higher compared to the implants placed in patients with oncological defects (86.4%). The lower survival rates in the latter might be because the oncology group consisted of more patients with orbital implants and patients who had to undergo radiotherapy. These findings are in accordance with earlier studies on craniofacial implant placement.8, 10, 12, 19, 20
When treating patients with orbital defects, various challenges need to be addressed, for example the poor bone quality in the orbit region and potential issues with cleaning the peri-implant skin due to the local anatomy and the visual handicaps that patients with monocular vision encounter.21 Although older age (frailty) and a decline in visual capacities during aging can also be mentioned as factors affecting peri implant hygiene, age was not identified from the current study's multivariate regression analysis data as a significant risk factor for implant survival. Adding radiotherapy, however, to areas comprised of bone and soft tissues increased the susceptibility of implant failure, such as in the orbital region, even more, resulting in lower implant survival rates.
The effect of ionizing radiation on peri-implant bone was confirmed by animal studies and it seems that radiation therapy negatively influences the microarchitecture and biomechanical properties of bone tissue, especially near the surface of the implant.22, 23 Earlier research concluded, though, that the survival of nasal implants is not influenced by radiotherapy.5, 12 Our study confirms this observation as an equal proportion of nasal implants were lost by irradiated and non-irradiated patients.
The radiation dosages on the tumor varied between 30 and 70 Gray but, because radiation techniques have evolved greatly, it cannot be concluded that the radiation dose on the tumor was equal to the radiation dose on the implant area. Thus, firm conclusions on the effect of implant-specific radiation dosages on implant survival cannot be drawn. Literature on site-specific radiation dosages, and the effect of ionizing radiation on basic bone biology in the craniofacial region, is not available yet.
The results of the multivariate analysis show that female gender has a positive effect on implant survival (hazard ratio 0.47, p = 0.015). Even though Toso et al. reported a similar finding,20 ours could also have been a result of the skewed gender ratio in the studied population. The male predominance, which was also mentioned in a recent systematic review, can be due to the higher incidence of congenital aural atresia and craniofacial tumors in males.12, 24
Besides the timing of implant placement in relation to radiation therapy, placing implants during or after ablative surgery is also a common issue of debate. The few available studies that treated patients during and after ablative surgery infer that placing endosseous implants during ablative surgery does not lead to worse or better function than implants placed in a secondary setting.15, 16, 25 We could confirm this observation. It is sometimes argued that secondary placement offers better implant positioning but, with the current advances in digital planning techniques and early involvement of a maxillofacial prosthodontist, we believe that optimal implant positioning can also be achieved during ablative surgery.5, 26
An additional phenomenon when treating head and neck cancer patients during ablative surgery is the issue of some implants possibly not being used due to various disease- or patient-related factors. In our study, 50 implants in 19 oncology patients (11.2%) had to be excluded from the analysis because it was not possible to perform second-stage surgery due to the earlier mentioned reasons. This seems to be an inevitable risk when treating oncology patients during ablative surgery. However, we still advise implant placement during ablative surgery because of the clear functional benefits in the majority of patients (earlier prosthetic rehabilitation, implant placement before radiotherapy, and no need for an additional operation [patients are often tired and do not feel up to the treatment at a later stage, even though they can really benefit from it]).
Performing regular clinical examination on implants placed in craniofacial regions is important in the aftercare period. Contrary to implants placed in the oral cavity, radiographic evaluation of the peri-implant bone level is not common is extraoral regions for a number of reasons: 1) Because of local anatomy, perpendicular placement of the x-ray tube to the sensor is not possible in extraoral regions. 2) 3-dimensional imaging modalities such as (conebeam) computed tomography ([CB]CT) tend to show a lot of scattering (especially when extraoral implants with a wider flange are use) resulting in unreliable measurements. Also, taking multiple, repeated radiographs and exposing patients to these levels of radiation when applying a (CB)CT does not adhere to the ALARA (‘as low as reasonably possible’) concept of radiation. 3) Implants used for extraoral application are generally short (maximum length of 7 mm, often shorter). When peri-implant bone loss occurs, the implants will presumably already show mobility due to the shortness of the implants.
Further studies on the influence of aftercare and soft tissue reactions on implant survival are needed. These issues should, preferably, be studied prospectively in larger groups. This is challenging because each treatment centre carries its own treatment protocol according to the specialties available. No statistically significant differences were found in the presence of skin reactions under the prosthesis between the irradiated and non-irradiated patients. An earlier study on the aftercare of craniofacial prostheses, however, reported that skin reactions were significantly milder in irradiated patients than in non-irradiated patients.27 The authors hypothesized that irradiated skin is thinner and drier than healthy skin and thus less susceptible to peri-implant problems. We could not confirm this finding but concluded from our study that although severe peri-implant skin reactions are not common, some redness is present around the abutments in 17.7% of patients. There is a tendency for healthier peri-implant skin in the mastoid region but to what extent this is related to anatomic factors such as thinner skin in the mastoid region and a less moist environment, self-care, or other patient-related factors such as frailty, could be a subject for further research. Some researchers17 stated that the main reasons for implant loss are soft tissue problems while other authors claimed the opposite: implant loss is not related to adverse skin reactions but to loss of integration.28 We could not draw any conclusions on a potential causal relationship between peri-implant skin reactions and implant loss.
Few studies on craniofacial implant placement mentioned the development of osteoradionecrosis and, when reported, the incidence was low.5, 13, 15, 28-30 This could imply that osteoradionecrosis is not a significant issue in craniofacial implant therapy. In all the current study's patients with osteoradionecrosis, the exposed bone did not originate from the region with the implants and the implants were not affected in most of the patients (5 out of 7). It can be stated that all the patients with osteoradionecrosis had extensive mid-face defects due to large tumors (a T4 adenoid cystic carcinoma of the maxillary sinus, and a large basal cell carcinoma). This indicates that the development of osteoradionecrosis probably depends more on the extent of the surgical reconstruction than on the presence or placement of implants.
The finding that the majority of the lost implants were not replaced indicates that the loss of a craniofacial implant does not necessarily lead to the loss of the prosthesis in the long term. Even one implant can, in some patients, be enough for prosthesis retention, resulting in a high percentage of functional prostheses despite implant loss. This is in accordance with the Subramaniam et al. findings.17 The patients also seemed to be highly satisfied with their prosthesis. Nevertheless, more insight can be gained from the patient's specific wishes.
4.1 Strengths and limitations
The current study presents implant survival data with a follow-up of up to 30 years in a large group of patients. The Kaplan–Meier survival method was used to indicate the probability of implant loss or implant survival after a particular point in time. The patients who did not experience implant loss were censored and could have been either lost to follow-up, continued in the follow-up without experiencing implant loss, or died after implant treatment. With the Kaplan–Meier method, an assumption is made that when patients are censored, they are still at risk of experiencing implant loss after the censoring date. This is not a viable assumption for patients who have died. As our study had a large oncology group, death is a competing risk in our analysis and thus a limitation of our study as it can lead to an overestimation of experiencing the event (implant loss) in the long term.31 Another issue in our survival analysis is that each implant was considered an independent sample instead of counting multiple measurements in each patient. Also, because of the long follow-up period, few patients remained (especially in the oncology group), which makes interpretation of the survival rates after 20 years difficult. This is also reflected in the number of patients remaining for the clinical assessment; an unfortunate and inevitable consequence of providing long-term patient care.
4.2 Conclusion
Within the limitations of this study, we can conclude that the outcome of craniofacial implants to retain craniofacial prosthesis is favorable. Congenitally deformed patients and traumatology patients have higher implant survival rates than oncology patients. Orbital implants score worse than nasal and mastoid implants. Radiotherapy has a negative effect on implant survival irrespective of whether the implants are placed before or after radiotherapy.
AUTHOR CONTRIBUTIONS
Jamie Alberga: Concept/Design, Data analysis/interpretation, Drafting of the article, Critical article revision, Statistics, Data collection. Iris Eggels: Critical article revision, Data collection. Anita Visser: Drafting of the article, Critical article revision. Baucke van Minnen: Drafting of the article, Critical article revision. Anke Korfage: Drafting of the article, Critical article revision. Arjan Vissink: Concept/Design, Drafting of the article, Critical article revision, Article approval. Gerry Raghoebar: Concept/Design, Drafting of the article, Critical article revision, Article approval.
ACKNOWLEDGMENTS
The authors would like to thank Jadzia Siemienski for correcting the English grammar in the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




