Concomitant pulmonary vein isolation and percutaneous closure of atrial septal defects: A pilot project
Abstract
Background
Patients with an atrial septal defect (ASD) are at increased risk of developing atrial fibrillation (AF). Currently percutaneous ASD closure is the preferred therapeutic strategy and although pulmonary vein isolation (PVI) for AF is feasible after ASD closure, the transseptal puncture can be technically challenging and probably increases the perioperative risk. A staged approach, with PVI several months before ASD closure, has been recommended for patients already scheduled for closure, but no data are available on combined procedures.
Purpose
This pilot study evaluates the feasibility of a combined procedure of PVI and ASD closure in patients with a hemodynamic important ASD and documented AF.
Methods
In one procedure, PVI was performed prior to placement of the ASD closure device. Transseptal access for PVI was obtained via wire passage through the ASD in all patients. Patients were followed with 5-day-holter monitoring at 3, 6, and 12 months. Recurrence of AF was defined as a documented, symptomatic episode of AF.
Results
The study population consisted of five patients (four females, mean age: 58 (±3) years). Acute PVI was achieved in all patients. Only one patient had a small residual ASD after closure. Besides a small groin hematoma in two patients, no complications occurred. After 12-month follow-up, three patients were free of AF recurrence (60%).
Conclusion
This study shows that a combined PVI with ASD closure is feasible with an acceptable success rate of AF free survival. These preliminary results in a small patient group warrants a larger trial.
1 INTRODUCTION
Evolving treatment options in patients with congenital heart disease (CHD) resulted in an improved survival and a rapidly expanding patient population of grown-ups with CHD. Atrial septal defect (ASD) is one of the most common CHD with a reported birth prevalence of 1–2 per 1000 live births.1-3 As these patients age, atrial tachyarrhythmias are common complications, mainly atrial fibrillation (AF).4-6 The prevalence of AF is high in ASD patients (reported between 20% and 45%), with the first episode of new-onset AF at a younger age as compared to the general population.4, 7-12 Both surgical and percutaneous closure of an ASD have been proven as an effective treatment of a significant ASD with different procedural complication risks and rates of residual shunting.13 Closure of the ASD has been associated with a higher risk of developing AF as well as an effective treatment of AF, but evidence is inconsistent.9, 11, 14 In the general population, various treatment options for AF have extensively been studied, of which pulmonary vein isolation (PVI) has been accepted as a safe and effective procedure.15, 16 Less is known about the preferred treatment strategy for AF in patients with an ASD, especially PVI, timing of PVI, for example before, after, or during ASD closure. Although several studies have shown that a PVI can be safely performed in patients after ASD closure, transseptal puncture can be troublesome and challenging in the presence of a closure device or patch. Successful transseptal punctures at native septal sites or directly through the device or patch have been described in several studies, often requiring additional tools such as intracardiac echo and balloon dilatation through an Amplatzer device.17-21
Pulmonary vein isolation in patients with an uncorrected or ASD is feasible with reasonable success rates ranging between 56% and 75% during a follow-up of 20 months in 18 and 4 patients, respectively. Left atrial access can be obtained through the defect and rarely requires transseptal puncture.22, 23 In case if transseptal puncture is required, it is not associated with additional risks compared to patients without ASD.22, 23 A staged procedure of PVI, several months before percutaneous closure, has been advocated.22 One of the drawbacks of this approach is that these patients will have to undergo two separate procedures, each with the risk of procedural complications. Thus far only one patient with concomitant PVI and ASD closure has been described with transseptal access through the ASD and successful cryoballoon ablation prior to placement of a septal occluder with no residual shunt or atrial arrhythmia at one year follow-up.24 Our aim was to study the feasibility and safety of a combined approach of PVI and ASD closure in a small cohort of ASD patients with symptomatic, documented AF and the one year success rate.
2 METHODS
This pilot study was approved by the local ethics committee in the Radboud University Medical Center Nijmegen (METC number: 2018-4860).
2.1 Inclusion
Patients with an indication for ASD closure, defined according to current clinical guidelines; significant left-to-right shunt with echocardiographic signs of right ventricle volume overload 25 and documented, symptomatic AF.
2.2 Periprocedural management
Prior to the PVI, a computed tomography scan was obtained to examine the three-dimensional anatomy of the pulmonary veins (PVs). All patients used oral anticoagulation therapy prior to the ablation procedure. All patients were on a novel oral anticoagulant (NOAC), for which we used an interrupted regimen consisting of a NOAC-free interval of 12–24 hours prior to the procedure.
2.3 Ablation procedure
All procedures were performed under general anesthesia or conscious sedation, with transesophageal echocardiography (TEE) to exclude thrombi in the left atrium.
After gaining right femoral vein access and sheath placement, a steerable decapolar diagnostic catheter was positioned in the coronary sinus. Access to the left atrium and PVs was obtained using direct wire passage through the septal defect. In case if this could not be achieved, transseptal puncture (single or double) under fluoroscopic and TEE guidance would have been performed. During the procedure, the patient heparin was administered targeting an activated clotting time >300 seconds. PVI using cryo energy was performed using the second generation cryoballoon (Medtronic, Minneapolis, MN, USA) in combination with the 12-French steerable sheath (Medtronic, Minneapolis, MN, USA). All the PVs were mapped with an inner lumen mapping catheter. Every vein was treated with at least two applications. The freeze cycles were 180 seconds. Balloon size of the cryoballoon was 28 mm in all cases. Phrenic nerve palsy was monitored during ablation of the right-sided PVs. PVI using radiofrequency (RF) energy was performed, guided by a 3-D electroanatomical mapping system (Ensite Precision, Abbott), and a 3.5 mm irrigated open-tip catheter was used. Ablative therapy was performed at 25–30 W, 43°C and an irrigation rate of 17 mL/min. A point-by-point series of interconnecting focal ablation lesions was used to encircle the left- and right-sided PV pairs. PVI was confirmed using a steerable multi-electrode lasso catheter. No additional lines or focal lesions were made. After confirming isolation of the veins an over the wire change to an Amplatzer Torque 45° delivery system was performed for the ASD closure. After TEE sizing, the appropriate closure device was delivered, using fluoroscopy guidance, to close the defect. Using contrast injection, residual defects were ruled out and after confirmation of a good and stable position, the implantation tool was released and retracted. The day after the procedure, a transthoracic echocardiogram (TTE) was performed to exclude pericardial effusion and confirm a good position of the closure device. The patient was discharged hereafter.
2.4 Follow–up
Data during follow-up were systematically collected and included a resting ECG and physical examination during regular outpatient visits, as well as 6-day holter monitoring at 3, 6, and 12 months after ablation, but also symptom driven consultation. As recommended in current guidelines, a blanking period of three months after PVI was applied.15 Oral anticoagulation was continued for at least three months after the procedure, after which life-long treatment was based on the CHA₂DS₂-VASc score. Anti-arrhythmic drugs were stopped or continued at the discretion of the treating physician.
Recurrence of all documented AF episodes, all procedure related complications, procedure time, and duration of hospital admission were collected.
3 RESULTS
Between September 2016 and August 2017, five patients (four female, mean age 58 ± 3 years) were eligible for the combined procedure of PVI and ASD closure; baseline and procedural characteristics are listed in Table 1. All patients had symptomatic paroxysmal AF with a median time from onset of symptoms to the procedure of 26 months, ranging from 2 to 54 months. The maximal CHA₂DS₂-VASc score was 2 and all patients used a NOAC. Transseptal access for PVI was obtained via wire passage through the ASD in all patients and no additional transseptal puncture was needed.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Age (years) | 58 | 49 | 59 | 66 | 58 |
| Sex | F | F | F | F | M |
| Comorbidity | M. Graves | None | Hypertension | Hypercholesteromia | Hypertension |
| BMI | 25 | 24 | 24 | 29 | 26 |
| ASD size (mm) | 16 | 15 | 19 | 18 | 4 and 1 |
| RVEDD (inlet,mm) | 47 | 43 | 40 | 43 | 52 |
| Left ventricle systolic function | Normal | Normal | Normal | Normal | Normal |
| LVEDD (mm) | 46 | 43 | 38 | 35 | 50 |
| Valve disease | No | No | No | No | No |
| left atrial size (m1/m2) | 29 | 43 | 32 | 25 | 48 |
| duration of AF (months)a | 26 | 2 | 42 | 54 | 25 |
| CHADSVASC score | 2 | 1 | 2 | 2 | 1 |
| Anticoagulation | Apixaban bid | Edoxaban od | Dabigatran bid | Apixaban bid | Apixaban bid |
| Antiarrythmic drug | propranolol | metoprolol | nebivolol | sotalol | flecainide |
| PVI technique | Cryoballoon | Cryoballoon | Cryoballoon | Cryoballoon | RF point-by-point |
| Balloon size (mm) | 28 | 28 | 28 | 28 | n.a. |
| Acute isolation pulmonary veins | Yes | Yes | Yes | Yes | yes |
| ASD closure device | Amplatzer | Amplatzer | Amplatzer | Amplatzer | Gore Cardioform Septal Occluder |
| Size closure device (mm) | 28 | 18 | 24 | 20 | 30 |
| Total procedure time (min) | 153 | 183 | 236 | 153 | 218 |
| Fluoroscopy time (min) | 24 | Unknown | 23 | 24 | 27 |
| Hospital admission (days) | 1 | 1 | 1 | 1 | 2 |
| Complication | Groin hematoma | No | No | Groin hematoma | No |
| AF recurrence at 3months | No | Yes | No | No | No |
| AF recurrence at 6 months | No | Yes | No | No | No |
| AF recurrence at 12 months | No | No | No | No | Yes |
- Abbreviations: LVEDD, left ventricle end diastolic dimension; PVI, pulmonary vein isolation; RVEDD, right ventricle end diastolic dimension.
- a Duration defined as time in months of diagnosed atrial fibrillation before the procedure.
Pulmonary vein isolation was achieved in all patients using cryothermal (N = 4) or RF (N = 1) energy. Either an Amplatzer closure device (N = 4) or a Gore Cardioform Septal Occluder (N = 1) was implanted. Median procedure and fluoroscopy time were 183 (153–236) and 24 (23–27) minutes, respectively. Figures 1 and 2 show the fluoroscopy and TEE images of one of the procedures.
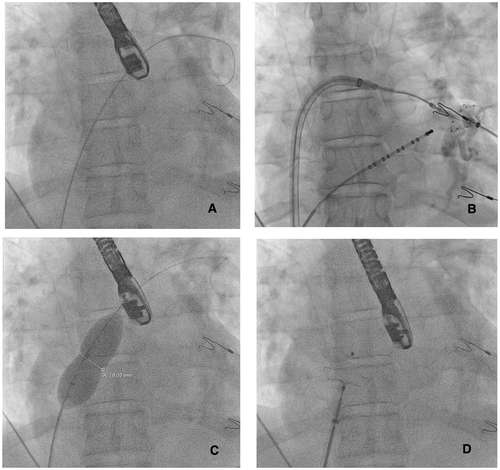
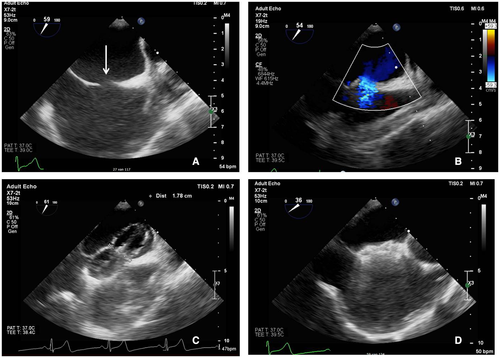
Immediately after closure, TEE showed no residual ASD. TTE performed on the first postprocedural day confirmed stable position of the closure device and absence of pericardial effusion in all patients. Four patients were discharged on the first postprocedural day and one on the second. Every patient continued to use oral anticoagulants. At the end of the 12-month follow-up period, three patients were free from AF recurrence. One patient had a documented recurrence of AF at the three and six month follow-up period. Both episodes converted spontaneously to sinus rhythm and additional therapy was not given. Another patient had a recurrence of AF at 12-month follow-up, which was treated with an electrical cardioversion.
3.1 Complications
Besides two small groin hematoma at the vascular access sites, controlled by means of manual compression, no major periprocedural complications occurred.
4 DISCUSSION
This pilot study shows that a combined procedure of PVI and ASD closure in symptomatic AF patients and an indication for ASD closure is feasible and safe. During the 12-month follow-up period three of five patients were free from AF recurrence (60%). Interestingly, both patients with AF recurrences had an enlarged left atrium, known as a predictor of AF in general, and of PVI failure.26, 27
Our recurrence rate is comparable to the rate described in patients undergoing PVI with an uncorrected ASD (44%).23 Another study showed a comparable recurrence rate after PVI in patients with a previously closed ASD (44%).20
Lakkireddy et al compared outcomes of PVI during a mean follow-up of 15 months between patients with a corrected ASD (51% percutaneous closure device and 49% surgical repaired ASD) and a matched AF control group and found no significant in recurrence rate recurrence rate (18% vs 24%, P = 0.6) 18 Our recurrence rates are similar to those reported for PVI in the general population, which ranged between 45% and 89%.15 Also, complications did not occur.
The median procedure time of 183 minutes in the present study seems reasonable, particularly when taking into account that this is a combined procedure. Our procedure time is only slightly longer than the mean PVI procedure time of 124 (cryoballoon) and 141 minutes (RF) in the FIRE and ICE study and the 162 (cryoballoon) and 166 minutes (RF) in a recent meta-analysis on ablative therapy in PAF.28, 29
The consideration to perform PVI several months before closure seems reasonable but has a clear downside of performing two procedures and subsequent exposure to twice the procedural complication risks. The disadvantage of performing a PVI and ASD closure in one single procedure is that, in case of an AF recurrence, a redo PVI may be more challenging. Nevertheless, previous studies showed the feasibility of transseptal access, although requiring the need of extra image modalities or other tools. These studies even reported no residual defects, independently of the transseptal access sites including the native septum or the closure device itself.17-21
Taking a recurrence rates of 40% after PVI into account, every 4 out of 10 patients will be a potential candidate for a redo PVI. The actual decision on performing the redo procedure will depend on severity of symptoms and will be a joint decision of the patient and physician. Nevertheless, if all patients with AF recurrences would opt for a redo procedure, four patients will be subjected to the complication risks of a redo procedures.
However, in the stepwise approach, every patient will at least have two procedures. It might be questioned whether a redo PVI in these patients should be performed before the closure procedure or concomitant. With a presumed recurrence rate of 40% and all patients with AF recurrences undergoing a redo procedure, at least 6 out of 10 patients will have had two procedures and four patients will even have undergone three procedures, when redo PVI and ASD closure done separately. Another issue to be resolved in this approach is the advised waiting times between procedures.
Although the available data are positive on feasibility of performing PVI in patients with a closure device, we do recognize that a redo PVI does logically introduce a slightly higher complication risk. This is, to our opinion, insufficient reason to expose all patients to at least two procedures and thereby doubling the complication risk in all patients. Moreover, our limited data show AF recurrences in patients with a dilated left atrium in which it could even be reasonable to consider a surgical (eg, epicardial or hybrid) approach in case of a redo procedure. This will overcome the additional challenge of puncturing through or passed a closure device and its inherent additional complication, such as device dislodgement and septal laceration. With limited experience and possibly a publication bias, this might even be the preferred option in these patients. On the contrary, left atrial dilatation is probably a marker of disease progression and it would be interesting to be able to treat patients earlier, possibly through screening tools, yet to be developed.
In conclusion, the preferred strategy for symptomatic patients with AF accepted for ASD closure could be a concomitant procedure of a PVI followed by an ASD closure with a low complication risk. However, larger studies are needed to provide further evidence that this is indeed the best strategy and better define patient characteristics and risk factors associated with outcome.
Conflict of interest
None.
AUTHOR CONTRIBUTION
The final version of the article was approved by all authors.
Study design: Reinder Evertz, Charlotte A. Houck, Tim ten Cate, Anthonie L. Duijnhouwer, Rypko Beukema, Sjoerd Westra, Kevin Vernooy, Natasja M.S. de Groot
Data analysis: Reinder Evertz, Charlotte A. Houck, Tim ten Cate, Anthonie L. Duijnhouwer, Rypko Beukema, Sjoerd Westra, Kevin Vernooy, Natasja M.S. de Groot
Paper review and final approval: Dr. K. Vernooy and Prof. N de Groot




