Fate of the Fontan connection: Mechanisms of stenosis and management
Abstract
Background
Stenosis of the venous connections and conduits is a well-known late complication of the Fontan procedure. Currently, data on the outcomes of percutaneous intervention for the treatment of extra- or intracardiac conduits and lateral tunnel baffles obstruction are limited. In an attempt to better define the nature and severity of the stenosis and the results of catheter interventional management, we reviewed Fontan patients with obstructed extra- or intracardiac conduits and lateral tunnel baffles.
Methods
Retrospective review of all Fontan patients who had cardiac catheterization from January 2002 to October 2018 was performed. Hemodynamic and angiographic data that assessed extra- or intracardiac conduit, or lateral tunnel baffle obstruction/stenosis were evaluated.
Results
Twenty patients underwent catheter intervention because of conduit stenosis, including calcified homografts, stenotic Gore-Tex conduits and obstructed lateral tunnels. Six other patients had Fontan obstruction but were referred for surgical revision. After stenting, there was a significant reduction in the connection gradient [2.0 mm Hg (IQR 2; 3) vs 0 mm Hg (IQR 0; 1), P < .0001]. Fontan conduit/connection diameter increased [10.5 mm (IQR 9; 12) vs 18 mm (IQR 14.9; 18); P < .0001] and New York Heart Association class [III (IQR II; III) vs I (IQR II; III); P = .03) with stent placement.
Conclusions
We demonstrated the hemodynamics and angiographic subtypes of conduit stenosis in patients after Fontan, We showed that calcified homografts, stenotic Gore-Tex conduits and lateral tunnels pathways can be safely and effectively stented to eliminate obstruction. Percutaneous stenting is associated with a decrease in connection gradients and improvement in functional capacity.
1 INTRODUCTION
Since Fontan and Baudet originally described the Fontan palliation of tricuspid atresia in 1971,1 there have been many modifications to the operation. With the concept of conservation of energy, de Leval et al2 confirmed the best model to be of the total cavopulmonary connection, utilizing either the lateral tunnel or eventually the intra- or extracardiac conduit (ECC).
The lateral tunnel is not commonly performed because of increased incidence of arrhythmia, and one report3 noted a significant incidence of lateral tunnel stenosis. However, the fate of ECC has not been clearly defined in long-term studies. Most postoperative studies have focused primarily on mortality or the need for early reoperation. Several studies evaluated modification of the orientation and placement of the ECC to maximize energy conservation and provide equal bilateral pulmonary artery flow distribution.4 They reported an optimal size for the ECC of 18-20 mm diameter based on flow dynamics during catheterization. There are concerns that in patients younger than 3 years old, the use a large bore (18 mm) conduit will not fit small patient vasculature.5, 6 Often a 14, 16, or 18 mm conduit has been used for repair. One study reported a high incidence of Dacron conduit stenosis for ECC in Fontan patients.7 Another report described stenting of Dacron ECC noting the morphology of different stenoses.8 They reported thick neo-intima in Dacron grafts. Because of the significant incidence of neointimal peel, Dacron grafts have been abandoned. Improved results have been reported with the routine use of Gore-Tex tubes (W. L. Gore and Associate Inc., Flagstaff, Arizona) usually of 18 or 20 mm diameter.8
Recently long-term Fontan follow-up reports have noted chronic liver failure and cirrhosis as progressive events in Fontan survivors.9 While pulmonary artery (PA) hypoplasia and stenosis have been recognized and treated in postoperative Fontan patients, the long-term fate of the inferior vena cava (IVC) conduit itself is often overlooked. Several studies have noted the occurrence of conduit stenosis requiring surgical conversion,10 but the mechanism of the conduit failure has not been accurately investigated.
Late results in 1138 Fontan patients at the Mayo Clinic were recently reviewed.11 We wished to focus on Fontan patients sent to the cardiac catheterization laboratory to define postoperative hemodynamics and to define potential etiologies for Fontan failure. The aim of this report is to define the hemodynamics and mechanisms of IVC conduit obstruction and describe successful treatment of Fontan conduit obstruction with intravascular stent placement.
2 METHODS
We reviewed 233 patients after Fontan who had diagnostic cardiac catheterization at Mayo Clinic, Rochester, Minnesota between January 2002 and October 2018. Patients who had superior vena cava (SVC) stenosis, pulmonary artery balloon dilation/stenting, or atriopulmonary/right ventricle connection stenosis were excluded. One patient who had had Fontan procedure and previously undergone catheterization at our institution but underwent stenting by one of authors (D.J.H.) elsewhere was also included in the analyses. We identified 28 patients with obstruction of the Fontan IVC conduit. Two patients who had not provided research authorization were excluded. The final cohort included 26 patients, 6 of these patients were referred to surgery for conduit revision because of other surgical indications. The purpose of this report was to review the hemodynamic findings and interventional treatment of 20 patients with intracardiac or extracardiac conduit or lateral tunnel stenosis.
Clinical, hemodynamic, and angiographic data were reviewed. Patients were indexed by the first catheterization in which a stent was implanted into the stenotic Fontan connection or conduit. Angiographic and hemodynamic assessments were made prior to the decision for catheter intervention. Angiographic descriptions and measurements were obtained from the operator’s observations during the procedure and/or from prior radiologic computerized tomography (CT) or magnet resonance imaging (MRI) data. When possible, measurements were obtained from the anterior-posterior and lateral angiographic projections. The minimum stenotic dimensions were recorded and the type and location and apparent mechanism of the stenosis defined. One or more stents were deployed using standard techniques. Palmaz XL, Palmaz Genesis XD stents (Cordis Endovascular), Intrastent Max LD stents (Medtronic, Minneapolis, Minnesota) or Covered CP stents (Numed, Hopkinton, New York) were used depending upon the operator’s preference. The length of the stenosis determined the length of the stent and the number of stents deployed. In some cases, multiple dilations were performed or high pressure (Atlas Bard Peripheral Vascular, Inc., Tempe, Arizona) dilation balloons were employed to satisfactorily dilate resistant stenoses. The minimum final stent diameters were then recorded after final stent redilation.
Demographic, clinical, and surgical data, as well as follow-up information were obtained from the medical records. The type of Fontan connection and the material utilized for the surgical repair were identified. Adverse events during or after the catheterization procedure were recorded. New York Heart Association (NYHA) classification was determined before and at follow-up after stent implantation.
Continuous variables are presented as median and interquartile ranges (25th, 75th percentile) unless stated otherwise; nominal variables are presented as counts (%). Groups were compared using the Fisher’s exact test. Pre- and poststenting variables were compared by paired analysis using the Wilcoxon signed-rank test or the McNemar’s test for continuous and nominal variables, respectively. Analyses were performed with JMP software (version 13.0; SAS Institute Inc, Cary, North Carolina) and a P value < .05 considered statistically significant. The study was approved by the Mayo Clinic Institutional Review Board.
3 RESULTS
Twenty patients with Fontan conduit obstruction had percutaneous intervention and stenting. The intervention occurred after a median of 13 years (7.6; 19) after Fontan palliation. Thirteen (65%) patients had undergone Fontan operation at Mayo Clinic; seven patients had had their Fontan completion performed elsewhere. Two patients had surgical intervention for conduit replacement after the catheter stent intervention. The demographic and clinical data of the 20 patients are included in Table 1.
| n | ||
|---|---|---|
| Age at catheterization, years | 20 | 17 (11.5; 26) |
| Age at Fontan palliation, years | 20 | 4 (2; 6.7) |
| Sex, male | 20 | 17 (85%) |
| Weight at catheterization, kg | 20 | 59 (42.5; 72) |
| Height at catheterization, cm | 20 | 168.5 (149.8; 176.5) |
| Body surface area, m2 | 20 | 1.6 (1.3; 1.9) |
| Fontan connection | 20 | |
| Extracardiac homograft | 10 (50%) | |
| Extracardiac Gore-Tex | 6 (30%) | |
| Intraatrial conduit Gore-Tex | 1 (5%) | |
| Lateral tunnel | 3 (15%) | |
| Clinical status | ||
| Protein-losing enteropathy | 20 | 3 (15%) |
| Cyanosis | 20 | 8 (40%) |
| Edema | 20 | 4 (20%) |
| Fatigue | 20 | 13 (65%) |
| Cirrhosis | 20 | 11 (55%) |
| NYHA | 20 | 3 (2; 3) |
| Baseline catheterization data | ||
| Fontan pressure, mm Hg | 19 | 14 (12; 17) |
| Gradient, mm Hg | 19 | 2 (2; 3) |
| Pulmonary artery wedge pressure, mm Hg | 17 | 9 (6.5; 11) |
| Ventricular end-diastolic pressure, mm Hg | 14 | 10 (7; 11.3) |
| Cardiac index, L/min/m2 | 18 | 3 (2.5; 3.8) |
| Arterial O2 saturation, % | 19 | 93 (89; 94) |
| Poststenting catheterization data | ||
| Fontan pressure, mm Hg | 19 | 15 (13; 17) |
| Gradient, mm Hg | 19 | 0 (0; 1) |
| Follow-up NYHA | 19 | 2 (2; 3) |
3.1 Hemodynamics
The catheterization hemodynamics varied based on age after Fontan completion, severity of stenosis and presenting symptoms. Overall hemodynamic data are presented in Table 1 and the details of hemodynamics and stenting in each patient are outlined in Table 2. Mean Fontan conduit pressure >15 mm Hg was present in 44% adults (age ≥18) and 30% of the pediatric patients. Moreover, older patients with significant associated symptoms of edema and protein-losing enteropathy (PLE) uniformly had elevated Fontan pressures. There was a significant reduction in the connection gradient from a prestent median gradient of 2 mm Hg (2; 3) to a poststent median gradient of 0 mm Hg (0; 1), P < .0001.
| # | Age | Age at Fontan | Fontan type | Stenosis | Symptoms/comorbidities | IVC pressure | VEDP | Gradient pre | Gradient post | Conduit sizea | Size pre | Size post | Post NYHA improvementb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 3 | ECC homograft | PA | Cyanosis, fatigue | 12 | 7 | 0 | 1 | 20 | NA | 15 | Yes |
| 2 | 6 | 5.9 | ECC Gore-Tex | PA | Edema, cirrhosis | 26 | 16 | 4 | 1 | 24 | 12 | 20 | No |
| 3 | 32 | 7 | Lateral tunnel | IVC | Cyanosis, fatigue, cirrhosis | 16 | 8 | 2 | 0 | NA | 14 | 18 | Yes |
| 4 | 26 | 4 | Lateral tunnel | Central | Cirrhosis | NA | NA | NA | NA | NA | 14 | 18 | No |
| 5 | 9.5 | 2 | ECC homograft | PA | Fatigue | 13 | 10 | 1 | 0 | 20 | 8 | 16 | Yes |
| 6 | 24 | 4 | ECC homograft | Diffuse | Cirrhosis | 12 | 7 | 2 | 1 | 20 | 13 | 18 | No |
| 7 | 18 | 2 | ECC Gore-Tex | Diffuse | Cyanosis, fatigue, edema, PLE, cirrhosis | 28 | 24 | 3 | 0 | 14 | 11 | 17 | No |
| 8 | 13 | 4 | ECC homograft | PA | Cyanosis, fatigue, PLE, cirrhosis | 13 | 7 | 2 | 0 | 20 | 9 | 14 | No |
| 9 | 4 | 2 | ECC homograft | PA | Fatigue | 13 | 7 | 2 | 0 | 17 | 8 | 14 | Yes |
| 10 | 31 | 9 | Intraatrial conduit | Diffuse | Cirrhosis | 16 | 12 | 2 | 0 | NA | 10.5 | 13.5 | No |
| 11 | 15 | 3 | Lateral tunnel | Central | Cyanosis | 17 | 2 | 2 | NA | 10 | 18 | No | |
| 12 | 21 | 12 | ECC Gore-Tex | Diffuse | Fatigue, edema, PLE, cirrhosis | 26 | 10 | 6 | 1 | 20 | 3 | 14 | Yes |
| 13 | 15 | 5 | Intraatrial Gore-Tex | Central | Cyanosis, fatigue, hepatic congestion | 12 | 9 | 1 | 0 | 16 | 10 | 14.8 | No |
| 14 | 23 | 15 | ECC Gore-Tex | Diffuse | Fatigue, hepatic congestion | 14 | 14 | 5 | 2 | 18 | 12 | 19 | No |
| 15 | 27 | 11 | ECC Gore-Tex | Multiple | Fatigue | 14 | NA | 3 | 0 | 20 | 12 | 19 | No |
| 16 | 18 | 2 | ECC homograft | IVC | Cyanosis, fatigue | 7 | NA | 7 | 1 | 19 | 15 | 18 | Yes |
| 17 | 26 | 5 | ECC homograft | PA | Fatigue, cirrhosis | 10 | NA | 2 | 0 | 22 | 7 | 20 | No |
| 18 | 16 | 2 | ECC homograft | IVC | Fatigue, cirrhosis | 19 | 11 | 2 | 0 | 18 | 9.5 | 15 | No |
| 19 | 11 | 4 | ECC Gore-Tex | Central | Fatigue | 14 | NA | 2 | 1 | 18 | 12 | 18 | Yes |
| 20 | 16 | 2 | ECC homograft | Cyanosis, fatigue | 12 | 11 | 2 | 0 | 18 | 9 | 18 | No |
Note:
- Pressure recordings measured in mm Hg, whereas conduit size represents measurements in mm.
- Abbreviations: ECC, extracardiac conduit; IVC, inferior vena cava; NA, not available; PA, pulmonary artery; PLE, protein-losing enteropathy.
- a Original conduit size at time of Fontan procedure.
- b Decrease in New York Heart Association (NYHA) class at follow-up.
3.2 Stenting
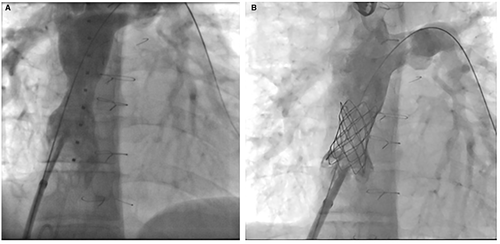
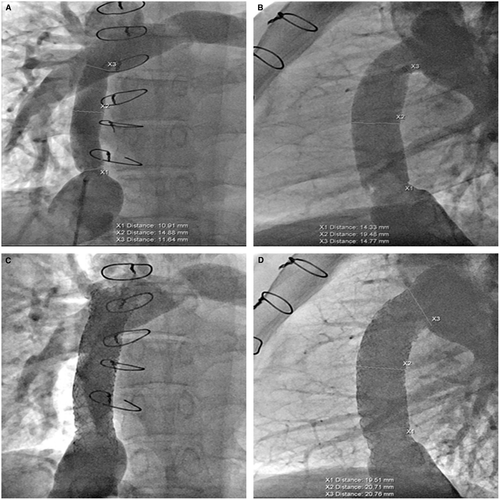
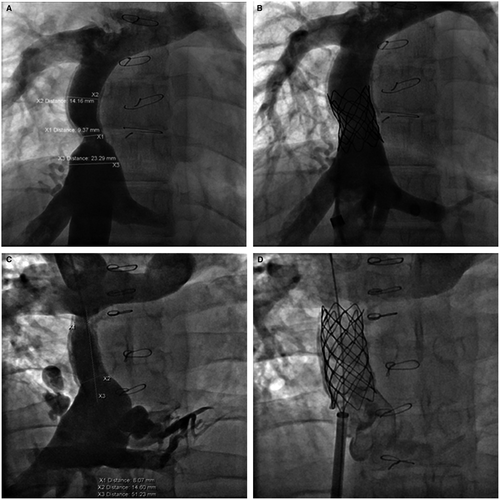
Various bare metal stents were utilized for enlargement of the stenosis in 15 of the cases with a final diameter of 14-20 mm. covered Cheatham Platinum stent was placed in six patients. In one patient with a lateral tunnel Fontan (Figure 1), a covered stent was used to avoid risk for development of right to left shunt and in the remaining patients it was used to protect against disruption of a calcified, stenotic homograft. In six patients, multiple stents were implanted at the same intervention (Figures 2, 4, and 5). Two patients had implantation of a second stent at a later catheterization, and three patients had subsequent redilation of a previous stent.
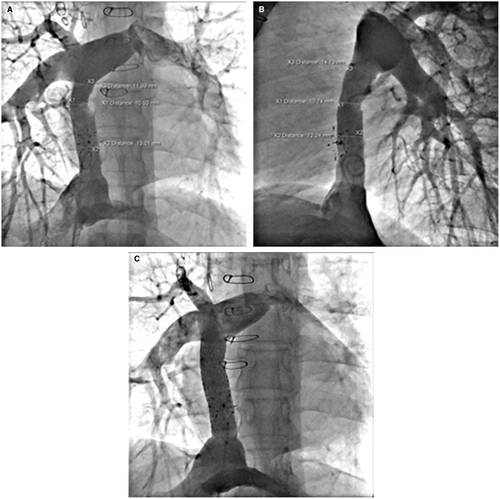
Homograft conduits were found to have diffuse calcification in addition to severe stenosis at the IVC or pulmonary artery ends of the conduit. Figure 3 illustrates diffuse calcific (13 mm) stenosis of a 20-mm homograft conduit placed 20 years earlier. Two 4010 Palmaz stents dilated the conduit successfully to 18 mm. Figure 4 illustrates diffuse central stenosis of a 14-mm Gore-Tex ECC originally placed at age 2 years. Sixteen years later the conduit is stretched causing diffuse narrowed to 12 mm. After placement of two 4010 Palmaz stents, with high pressure balloon dilation to 18 mm, the final conduit diameter averaged 17 mm. One other patient had successful dilation of a Gore-Tex conduit from 3 to 14 mm with improvement of PLE symptoms.
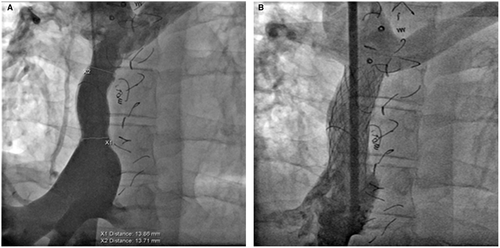
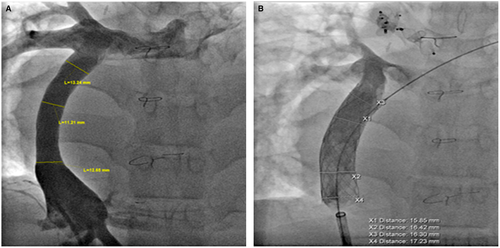
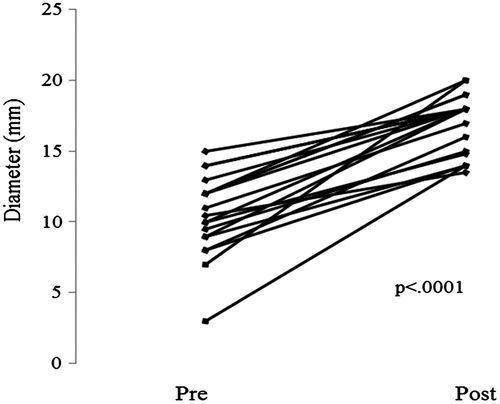
Figure 5 illustrates findings in a 23-year-old patient with diffuse, proximal, and distal stenosis of an 18-mm Gore-Tex ECC with stenosis of 10-12 mm. The entire conduit was stented with three 36-mm long bare metal stents placed with an 18 mm BIB balloon. The stents were then further dilated to 19-mm with a 20-mm Atlas balloon at 14 ATM. High pressure balloon dilation was necessary in both calcified homografts and the Gore-Tex conduits. Figure 6 illustrates two examples of focal stenosis of calcified homograft conduits which appear to be secondary to patient growth and stretch at the end of the conduit. The stenosis was effectively relieved with placement of covered CP stents. Figure 7 illustrates the statistically significant increase in Fontan conduit or connection diameters with percutaneous stent placement [10.5 mm (IQR 9; 12) prestenting vs 18 mm (IQR 14.9; 18) poststenting; P < .0001].
3.3 Adverse events and follow-up in patients undergoing percutaneous intervention
There were no serious adverse events noted in any of the patients who underwent catheter intervention. There were two patients who had a small extravasation of contrast after stent placement in a calcified homograft which fracture upon dilation. The small area of extravasation and fracture was covered with an addition covered CP stent. As observed in Figure 6, the final stented homograft was widely patent and free of residual extravasation of contrast. There was no evidence of compression of other nearby cardiac or extra-cardiac structures.
Follow-up >30 days postprocedure was available for 19 patients. During a median follow-up of 1099 days [220; 3093], median NYHA decreased from III (II; III) to II (II; III), P = .03. NYHA class improved in 6 patients and was unchanged in the remaining patients; postprocedurally, the prevalence of NYHA class ≥3 decreased from 70% to 47% (P = .025).
Eleven patients experienced sustained improvement in functional capacity poststenting and did not require additional cardiac surgeries or transplantation during follow-up. One patient had minor redilation of a covered CP stent 6 years after initial implantation. It is noteworthy that Improvement in NYHA was seen in 63% of patients with baseline ventricular end-diastolic pressure ≤10 mm Hg compared to none of those with baseline ventricular end-diastolic pressure >10 mm Hg (P = .03), suggesting that patients with significant elevation of VEDP were more likely to experience continued Fontan failure.
Among those without symptomatic improvement, two patients had surgical revision of the Fontan with an extracardiac Gore-Tex conduit because of persistent nonspecific symptoms. Two patients died during follow-up. One had severe left ventricular dysfunction while on left ventricular assist device support and the other died after heart and liver transplantation of metastatic hepatocellular carcinoma in the setting of hepatitis C and PLE. Four other patients had clinically documented PLE, in two of these patients PLE improved after conduit stenting, and they have had no further cardiac intervention. Two other patients had persistent PLE and underwent cardiac transplantation.
4 DISCUSSION
Compared to previous reports concerning Fontan connection or conduit stenosis, this single-center report has focused on a select group of patients with conduit or lateral tunnel obstruction. As noted in previous reports, internal obstruction in fabric ECC would not be amenable to improvement with stent placement except in the instance of a dislodged obstructive internal peel. With the recognition of conduit stenosis secondary to progressive calcification of homograft conduits and internal obstruction of fabric (Dacron) tubes, the current mode of Fontan completion utilizes Gore-Tex conduits. However, this study does illustrate that calcified homograft conduit obstruction can be effectively stented and dilated to resolve IVC Fontan conduit obstruction. To the best of our knowledge, this is the first study to systematically assess and demonstrate that Gore-Tex ECC conduits can be effectively dilated and stented.
In addition, a unique aspect of our review compared to previous studies focusing on stenting of Fontan conduit obstruction is that most of the patients were considerably older and were found to have significant obstruction many years after the initial Fontan procedure (median 12 years).
Several of the older patients in this study were sent to catheterization for evaluation of hepatic cirrhosis and had not been previously recognized to have conduit obstruction. While these patients experienced effective dilation and stenting of their obstructed conduit, the long-term benefit for reduction in hepatic congestion and cirrhosis warrant further investigation. Logically one would anticipate that earlier and the most complete elimination of obstruction and reduction of IVC pressure, and therefore, hepatic congestion would be the most beneficial for reducing the progression of hepatic fibrosis and dysfunction. Indeed based on these findings and previous studies, replacement of such conduits in these patients with larger Gore-Tex conduits preferable 20 mm or more would seem an attractive option in an attempt to reduce the risk of progressive hepatic fibrosis.
In view of the recognized risk for conduit obstruction, it therefore seems imperative that all Fontan patients have early and serial assessment for possible conduit obstruction with CT, MRI imaging or cardiac angiography. Despite the more widespread use of CT and MRI to assess severity of hepatic fibrosis, CT and MRI had not been effectively utilized to improve the recognition of associated Fontan conduit stenosis, Only 14 patients in our cohort had CT or MR which with careful review defined some conduit obstruction. In addition, standard transthoracic echocardiography had failed to adequate define conduit obstruction. Six of the patients were sent for cardiac catheterization without previous CT or MRI evaluation. Five of the six were catheterized for assessment of hepatic cirrhosis with no previous recognition of possible stenosis of their Fontan connection. Based on this observation we suggest that earlier and more frequent assessment of Fontan patients should be obtained either by careful cross-sectional imaging or cardiac catheterization and angiography in order to assess atrial and pulmonary artery pressures and to define possible stenotic conduits. Our data suggest that particularly those patients being evaluated for hepatic congestion and fibrosis/cirrhosis should have hemodynamic and angiographic assessment to rule out conduit obstruction.
At a minimum, we would suggest follow up catheterization and angiography every 10 years to better recognize and define the progressive stenosis of the calcified homografts. We noted progressive hepatic cirrhosis, ascites, PLE described in older patients who did not have earlier conduit assessment. As observed in Figure 3, some patients with calcified homografts developed diffuse stenosis throughout the homograft and required multiple stents and dilation to effectively relieve the obstruction. In our opinion, a minimum conduit diameter of 18-20 mm should be the goal of dilation to effectively relieve IVC/hepatic obstruction.
Figure 6 illustrates a more focal stenosis at the IVC connection of a uniformly calcified homograft. This type of obstruction seems to be a stretch phenomenon producing stenosis at the end of the fixed conduit as the patient grows. This was effectively relieved by placing a 34 mm long covered CP stent at the IVC stenosis. This relief persisted for 6 years when it was redilated from 16 to 17 mm. Figure 6 also illustrated a similar severe focal stenosis at the pulmonary artery connection of a diffusely narrowed calcified homograft which was originally 22 mm. This patient was not evaluated until 21 years later and had clinical evidence of cirrhosis. This stenosis was relieved by placing a 55 mm covered CP stent dilated to 20 mm. Although some calcified homografts were stented with bare metal stents we would recommend the used of the covered CP stent to avoid potential conduit disruption with high pressure dilations.
We noted that there was a higher prevalence of male subjects who had conduit obstruction requiring stent placement. Although, this may partially related to male preponderance in our Fontan population, this number is higher than previously report in the Mayo Clinic cohort (61% males). Male predominance was also present in the Boston study describe the outcomes of stenting in lateral tunnels.3 Whether male patients are more prone to developing Fontan obstruction deserves further investigation.
This study also illustrates the significant occurrence of progressive obstruction of the Gore-Tex ECC by a mechanism that becomes obvious when Fontan patients are evaluated 10-15 years after ECC placement. Patients at 2 years of age have a relatively short 18-20 mm conduit implanted at Fontan completion in order to avoid compression of other structures. Fifteen years later that same conduit has become significantly stretched with diffuse narrowing (Figure 4) or as observed in the homograft the development of stenosis at the proximal or distal anastomosis (Figure 5). Thus, it is not surprising to find that the 18 mm Gore-Tex ECC later is only 12 or 14 mm in diameter. We did not observe evidence of thrombosis of the Gore-Tex conduit. W. L. Gore laboratories now report the availability of new Gore-Tex12 Stretch conduits 14-24 mm in diameter and up to 40 cm in length which will stretch while retaining its original internal diameter. This would appear to offer a significant advantage in the future for Fontan ECCs. However, we feel strongly that even Gore-Tex conduits should be reassessed for conduit obstruction at least 10 years after implantation and periodically thereafter.
In summary, in this study we have observed that severely narrowed and stretched Gore-Tex tubes can be effectively stented to dilate them back to their original 18-20 mm dimensions. While multiple, heavy duty stents and high pressure dilation may be necessary to achieve these results, they seem safe and effective as observed in our patients.
4.1 Limitations
This study is limited by the small cohort of patients who had stenting of their Fontan IVC conduits. Multicenter studies and reports may help to corroborate our observations. The sample size limited our ability to perform additional statistical analyses and regression analyses to predict stenosis. It is particularly noted that many Fontan patients have been lost to effective follow up or have not had an assessment of their IVC conduit.
5 CONCLUSIONS
We present our findings in a group of Fontan patients who had obstruction of their Fontan IVC to PA pathway. Our results demonstrate that stenting of stenotic Fontan conduits can be safely performed, eliminating conduit obstruction of both calcified homograft and stenotic Gore-Tex ECC. While not all stents were fully dilated to 18-20 mm, we would expect that 18-20 mm conduits should be the goal of such procedures in most cases. In some cases, use of a covered stent may be advantageous to avoid potential risk for conduit disruption particularly with the calcified homograft conduit. Earlier recognition of high atrial pressures or minor degrees of ECC conduit obstruction may halt progression of hepatic fibrosis and cirrhosis. Lastly, by demonstrating that conduit obstruction can occur with time and growth in any Fontan conduit, we hope to emphasize earlier and repeated assessment of conduit adequacy and patency.
AUTHOR CONTRIBUTIONS
First author and primary editor of the manuscript: Hagler
Provided statistical support and editor for the manuscript: Miranda
Generated early manuscript data and prepare an abstract: Haggerty
Helped generate early data and provided editorial support: Anderson
Provided early data for manuscript and provided editorial support: Johnson
Repeated reviewed manuscript to provided editorial review and support also provided insight for previous Fontan research: Cetta
Provided editorial support and cardiovascular surgical support for materials and documentation: Said
Intimately involved in catheterization procedures to provide documentation and provided editorial review: Taggart




