Immunosuppressive Effects of Multiple Myeloma-Derived Extracellular Vesicles Through T Cell Exhaustion
Funding: This work was in part supported by the National Cancer Center Research and Development Fund (2020-J-3, 2023-J-3).
ABSTRACT
Extracellular vehicles (EVs) are reported to be involved in several processes relating to tumor progression, including angiogenesis, osteolysis, and drug resistance in multiple myeloma (MM). However, the role of EVs in the immune-suppressive milieu of MM is poorly understood. Here, we investigated the effects of MM-derived EVs on T cells, focusing on markers of T cell exhaustion. Using activated peripheral blood mononuclear cells from healthy donors, we observed immunosuppressive effects such as upregulated expression of immune checkpoint markers on CD8+ T cells treated with MM-derived EVs. Proteomic analysis identified several proteins, such as IL-8, SLC1A5, PIN2, and FSP1, associated with regulation of T cell exhaustion and chronic inflammation. Surprisingly, sphingosine kinase 1 (SPHK1) was enriched in MM cell line-derived EVs, implicating SPHK1/S1P signaling in the immunosuppressive effect of MM EVs. Thus, MM-derived EVs may promote T cell exhaustion via upregulating the expression of immune checkpoint markers and thereby contribute to the formation of the immune-suppressive milieu of MM, resulting in impaired T cell activity.
Abbreviations
-
- EVs
-
- extracellular vesicles
-
- HD
-
- healthy donor
-
- MM
-
- multiple myeloma
-
- PBMC
-
- peripheral blood mononuclear cell
-
- S1P
-
- sphingosine-1-phosphate
-
- SPHK1
-
- sphingosine kinase 1
1 Introduction
Multiple myeloma (MM) cells proliferate in the bone marrow and cause several symptoms such as anemia, pathological fractures, hypercalcemia, and renal failure, so-called CRAB symptoms. MM cells also often trigger lymphoid cell immune dysfunction associated with severe infections and suppression of antitumor immunity.
Despite the remarkable development of new therapies, MM is still considered incurable. Recently, the microenvironment surrounding MM cells has been suggested to play an important role in the progression and relapse of the disease [1]. Communicating between tumors and the surrounding microenvironment, it has been hypothesized that extracellular vehicles (EVs) contribute to the formation of an immune-suppressive milieu [2].
EVs consist of unique particles surrounded by a lipid bilayer with no nucleus (cannot replicate) released from cells. EVs include particles described as exosomes, microvesicles, ectosomes, oncosomes, and apoptotic bodies, among other designations. They are distinguished by differences in size and mode of production. EVs play a role in cell–cell communication by carrying lipids, proteins, small RNAs, and possibly DNA [3].
In the microenvironment of several types of tumors, EVs play important roles in tumor progression and immunosuppression [4]. Consistent with other types of tumors, the same is true in the case of myeloma [5, 6]. With regard to the role of EVs in MM, they are involved in angiogenesis, osteolysis, immunosuppression, and resistance to therapeutic drugs. In addition, several studies have reported an emerging role for EVs as novel therapeutic targets for the treatment of MM and proposed their potential use as clinical biomarkers for early diagnosis, disease classification, and therapy monitoring.
There are few reports in the literature regarding the immunosuppressive effects of EVs from MM cells. MM- and MM bone marrow stromal cell-derived EVs induced activation and expansion of myeloid-derived suppressor cells, resulting in inhibition of T cell proliferation [7, 8]. It has also been reported that MM-derived EVs tended to induce apoptosis of CD4+ T cells while increasing the viability of CD8+ T cells when tested on healthy donors, but that the cytotoxicity of the latter was decreased. The viability of regulatory T cells was increased by exposure to MM-derived EVs, resulting in increased immune suppression. Taken together, these reports suggest that MM-derived EVs trigger an altered T cell phenotype and contribute to dampening their activity [9].
Sphingosine kinase 1 (SPHK1) is an enzyme that converts sphingosine to sphingosine-1-phosphate (S1P). SPHK1/S1P signaling is closely involved in regulating tumor growth, tumor immune evasion, and drug resistance [10, 11]. EVs are identified as the key transporters of SPHK1 from tumor cells, and SPHK1-packaged EVs are known to elevate S1P levels in the tumor microenvironment [12-15]. In ovarian cancer, S1P has been reported to induce T cell exhaustion by the upregulation of PD-L1 expression through E2F1-mediated transcription [14, 16, 17]. Regarding MM, the association of SPHK1/S1P signaling with immunosuppressive effects in the tumor microenvironment is unclear.
Based on previous findings, for further elucidation of the effect of MM-derived EVs on immune cells, here we investigated the immunosuppressive effects of these EVs on CD8+ T cells and the association of SPHK1 with any such effects.
2 Material and Methods
2.1 Preparation of Extracellular Vesicles
Blood plasma samples were collected from HDs (n = 10) or from untreated MM patients newly diagnosed at the Department of Hematology and Oncology, Nagoya City University Hospital (Aichi, Japan) between June 2006 and March 2018 (n = 30) after their written agreement had been obtained using informed consent forms approved by our institutional review board (IRB) (ID: 70-00-0113). For separation of EVs from plasma samples, ExoQuick Plasma Prep and Exosome Precipitation Kits (System Biosciences, Palo Alto, CA, USA) were used according to the manufacturer's instructions. For the preparation of MM cell line-derived EVs from culture medium, ExoQuick-TCTM Tissue Culture Media Exosome Precipitation Solution (System Biosciences) was used.
2.2 Nanoparticle Tracking and Confirmation by Transmission Electron Microscopy
Size and particle concentration of all EV preparations was measured by FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan) using a NanoSight system (Malvern Technologies, Malvern, UK) with a high-sensitivity CMOS camera. Samples were diluted to an appropriate concentration in Milli-Q water and analyzed under constant flow conditions at 25°C with five captures at 60 s/capture. Specimens were imaged by transmission electron microscopy (JEM1400Flash at 100 kV; JEOL Ltd., Tokyo, Japan) with negative staining performed by Hanaichi UltraStructure Research Institute Co. Ltd. (Aichi, Japan).
2.3 Flow Cytometry for EV Markers
PS Capture Exosome Flow Cytometry Kits (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) with Tim 4 beads were used according to the manufacturer's instructions.
2.4 Uptake of EVs Into CD8-Positive T Cells
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood of an HD, and CD8+ T cells were isolated by MACS depletion (Miltenyi Biotec, Bergisch Gladbach, Germany). EVs from HD or MM were labeled with ExoGlow protein and ExoGlow Membrane (System Biosciences) according to the manufacturer's protocol. CD8+ T cells were cultured with these EVs at 37°C in a CO2 incubator for 24 h, then washed, stained with DAPI, and inspected by fluorescence microscopy using a 100× objective and ZEN imager software.
2.5 Immunoblotting
Total proteins from EVs derived from an HD or a patient with MM were isolated and analyzed by SDS-PAGE followed by Western Blotting. The bands were detected using ImageQuant LAS-4000 mini (GE Healthcare, USA).
2.6 Flow Cytometry
PBMCs were stained with antibodies according to the manufacturer's protocol, and events were collected using a BD FACS Canto II or BD FACSymphony A1 (BD Biosciences, Franklin Lakes, New Jersey). Data were analyzed with Kaluza Software 2.1.
2.7 Effects of EVs on Activation Markers in CD8-Positive T Cells
PBMCs from an HD were stimulated with anti-CD3 agonist antibody (3 μg/mL), anti-CD28 agonist antibody (3 μg/mL), and interleukin (IL)-2 (150 U/mL) and cocultured with EVs derived from MM patients or HDs for 24 h. The expression of CD25 and CD69 as activation markers on CD8+ T cells was assessed by flow cytometry.
2.8 Effects of EVs on Immune Checkpoint Markers on CD8-Positive T Cells
PBMCs from an HD were activated with anti-CD3 agonist antibody (1 μg/mL) and IL-2 (100 U/mL) for 24 h, and then cocultured with EVs (100 μg/mL) isolated from patients with MM or HD or myeloma cell lines for 72 h. Altered expression of checkpoint receptors (PD-1, TIGIT, and LAG3) on CD8+ T cells was then assessed by flow cytometry. For cytokine production, PBMCs were cocultured with phorbol 12-myristate 13-acetate (PMA) (20 ng/mL), ionomycin (2 μg/mL), and BD GolgiPlug (BD Biosciences) (1 μL/mL) containing brefeldin A for 4 h. Intracellular staining for interferon (IFN)-γ was performed using the Intracellular Fixation and Permeabilization Buffer Set (88-8824-00; Thermo Fisher Scientific) according to the manufacturer's instructions.
2.9 Proteomic Analysis of Peripheral Blood Mononuclear Cells Treated With EVs From MM Cell Lines
Activated PBMCs treated with MM cell line-derived EVs were subjected to proteomic analysis according to the protocol of Proteobiologics Co. Ltd. (Osaka, Japan). Activated PBMCs were divided into two groups according to the origin of EVs to be tested. One group was treated with EVs that promoted the expression of immune checkpoint markers (Group A; AMO1, XG7, and OPM2) and the other with EVs that did not (Group B; KMS12BM, KMS11, and RPMI8226). Peptide samples were analyzed by nano-LC–MS/MS (UltiMate 3000 RSLCnanoHPLC; Thermo Fisher Scientific) coupled to a Q Exactive Orbitrap mass spectrometer (Thermo Fisher Scientific). The obtained Raw file was subjected to protein identification and quantitative analysis using DIA-NN 1.8. Carbamidomethyl and N-terminal protein acetylation were included as variable modifications. A maximum of two missed cleavages was allowed. The mass spectrometry data were searched against all proteins in the UniProtKB human database. Protein–protein association networks and functional enrichment analysis provided by String (https://string-db.org/) were performed [18]. The data used for the analysis were the differences (log2 value) between the two groups in the quantities of the proteins identified. For proteins for which no quantification values could be obtained in either group, the analysis was performed using the minimum quantification value.
2.10 Effects of Sphingosine Kinase 1 Inhibitor on CD8-Positive T Cells
PBMCs were stimulated as described above, and then cocultured with the EVs (20 μg/mL) from myeloma cell lines together with the SPHK1 inhibitors PF506 (100 nM) or FTY720 (100 nM) (Cayman Chemical, Ann Arbor, MI) for 72 h. The expression of checkpoint receptors (PD-1 and TIGIT) on CD8+ T cells was then assessed by flow cytometry.
2.11 CRISPR/Cas9-Mediated Knockout of SPHK1
Clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9)-mediated gene knockout was performed using the Alt-R CRISPR/Cas9 system from Integrated DNA Technologies (IDT, Coralville, IA). To generate a ribonucleoprotein (RNP) complex, chemically synthesized crRNA (predesigned, IDT) and tracrRNA (IDT) were mixed and annealed at 95°C for 5 min, and then gradually cooled to room temperature. The annealed guide RNA (300 pmol) was then incubated with Alt-R Cas9 Nuclease V3 (20 μg, IDT) at 37°C for 15 min. Electroporation of the RNP complex was performed using the ELEPO21 electroporator (Nepa Gene, Ichikawa, Japan). The Alt-R Cas9 Electroporation Enhancer (IDT) was added just before electroporation at a final concentration of 2 μM. The following parameters were used for electroporation: poring pulse voltage, 275 V; pulse length, 1.0 ms; pulse interval, 50 ms; number of pulses, 2; polarity, +; and transfer pulse: voltage, 20 V; pulse length, 50 ms; pulse interval, 50 ms; number of pulses, 5; polarity, +/−.
Genomic DNA was extracted 48–72 h after electroporation using NucleoSpin DNA RapidLyse kits (Macherey Nagel, Düren, Germany). Genomic regions surrounding the target sites were amplified by PCR and submitted to Sanger sequencing. The knockout efficiency was assessed using Inference of CRISPR Edits (ICE) analysis. We knocked out SPHK1 in AMO1 (Table S4) and used EVs from this cell line to perform the immunosuppression experiments described above.
2.12 Antibodies and Reagents
A variety of antibodies were used for flow cytometry and immunoblotting. Detailed information can be found in Appendix S1.
2.13 Statistical Analysis
Statistical analyses were performed with GraphPad Prism software. Student's t-test and Mann–Whitney U test were used to evaluate between-group differences, and one-way ANOVA followed by Dunnett's test was used to evaluate within-group differences. Statistical significance was set at a p value of < 0.05. For proteomic analysis, Welch's T-test and fold-change analysis were used to evaluate the two groups. The fold-change threshold was set at 2.
3 Results
3.1 Confirmation of the Presence of Extracellular Vesicles Derived From MM Patients
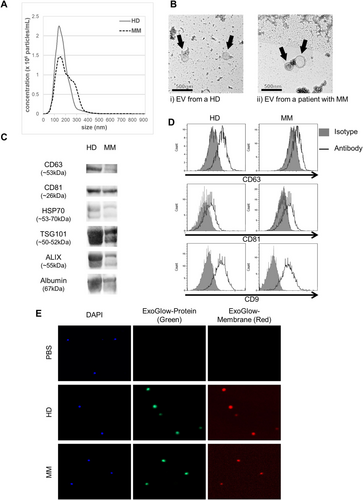
Characterization of EVs from patients with MM or from HDs was performed using nanoparticle tracking analysis (NTA), transmission electron microscopy (TEM), and immunoblotting. Representative EVs isolated from MM patients and HDs were similar in morphology and size (Figure 1A,B). EVs from both sources were positive for the typical EV markers CD63, CD81, HSP70, TSG101, and Alix (Figure 1C). CD9, CD63, and CD81 expression on the surface of these EVs was also confirmed by flow cytometry (Figure 1D). When cocultured with EVs that were simultaneously stained with two dyes that specifically fluoresce intra-EV or EV membrane proteins, CD8+ T cells were found to capture these EVs (Figure 1E). These results suggest that EVs from MM patients and HDs were similarly taken up by CD8+ T cells, with no significant difference between the two.
3.2 Effect of EVs Derived From MM Patients on Activated T Cells
Initially, PBMCs from HDs were stimulated with a mixture of anti-CD3 and anti-CD28 antibodies in the presence of IL-2, and cocultured with EVs derived from either HDs or MM patients. Expression of immune-related factors was then quantified. In some cases, exposure to MM patient-derived EVs resulted in a reduction of activated CD25- or CD69-positive T cells, but there were no significant differences depending on the source of EVs (median 83.1% vs. 83.3% for CD25, p = 0.52, 91.0% vs. 90.0% for CD69, p = 0.21 for HD and MM, respectively) (Figure 2A).
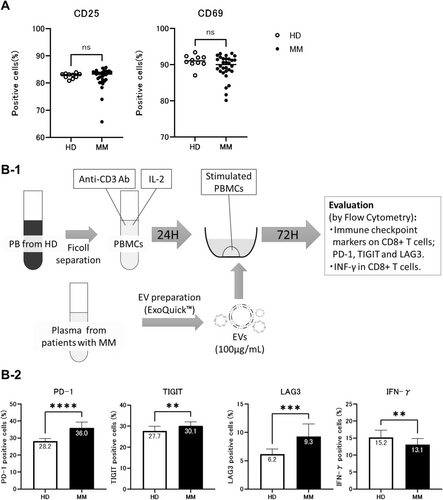
Next, the expression of immunosuppressive markers on activated CD8+ T cells was assessed after coculture with EVs (Figure 2B-1). Coculture with MM patient-derived EVs resulted in a significantly higher proportion of PD-1+ CD8+ T cells than treatment with HD-EVs (36.0% vs. 28.2%, p < 0.0001) (Figure 2B-2). Unlike HD-EVs, coculture with MM patient-derived EVs resulted in sustained increases of PD-1+ T cell expression even after the initial stimulation. Similarly, a significant increase in TIGIT positivity was observed in CD8+ T cells treated with MM patient-derived EVs relative to EVs from HD (30.1% vs. 27.7%, p = 0.0024). A significant difference was also observed for LAG-3 in MM patient-derived EV cocultures (9.3% vs. 6.2%, p = 0.0001). Finally, a significantly decreased proportion of IFN-γ-producing T cells was observed after treatment with MM patient-derived EVs compared to EVs from HD (13.1% vs. 15.2%, p = 0.0042).
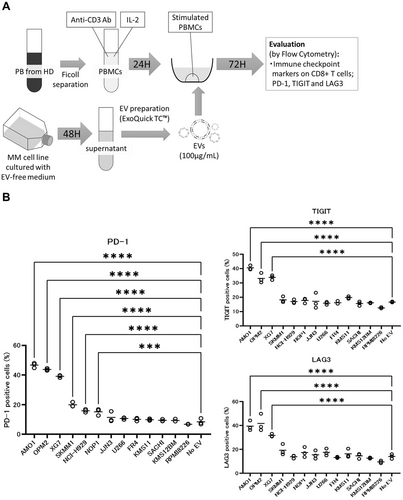
3.3 Effect of EVs Derived From MM Cell Lines on Activated T Cells
Although increased proportions of immune checkpoint marker-positive CD8+ T cells were observed on coculture of PBMCs with EVs derived from peripheral blood of MM patients, it was unclear whether EVs derived from the MM cells themselves directly caused this effect on T cells. Therefore, we asked whether the same result would be obtained with EVs derived from MM cell lines. To test this possibility, a similar experiment was performed with EVs derived from each of several different MM cell lines (Figure 3A). Treatment with EVs from 6 of 13 MM cell lines (AMO1, OPM2, XG7, SKMM1, NCI-H929, and NOP1) significantly sustained the elevated proportions of PD-1-positive cells in activated PBMCs, relative to PBS alone (“No-EVs” in Figure 3B). Similar significant effects were also seen for TIGIT and LAG3 with EVs from 3 of these 6 lines (AMO1, OPM2, XG7) (all p < 0.01).
3.4 Proteomic Analysis of Peripheral Blood Mononuclear Cells Treated With EVs From Different Myeloma Cell Lines
To identify specific proteins related to potentially immune-suppressive effects of MM cell line-derived EVs on activated T cells, we performed comprehensive proteomic analysis on PBMCs cocultured either with EVs from MM cell lines enhancing or not enhancing the proportion of PD-1+ T cells. Specifically, PBMCs were divided into two groups: group A consisted of PBMCs treated with EVs that promoted the increased proportions of immune checkpoint marker-positive cells (n = 3; AMO1, XG7, and OPM2), group B consisted of PBMCs treated with EVs that did not do so (n = 3; KMS12BM, KMS11, and RPMI8226). The overlapping presence or absence of proteins between the two groups is shown in a Venn diagram (Figure 4A). Proteins expressed only in group A but not in group B were FSP1, TNFA, AGRIN, NOTCH3, and others. Proteins expressed only in group B were ACTG, AN13D, MED25, and NALP2 (Figure 4A, Table S1).
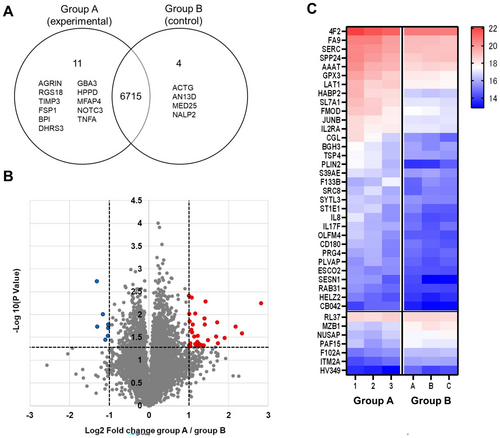
A volcano plot and heatmap are shown in Figure 4B,C. The selection criteria were set at an absolute log2 change ≥ 1 and p < 0.05. Proteins meeting these criteria are listed in Table S2. Compared to group B, there were 32 proteins (e.g., IL-8, SLC1A5 also known as “AAAT” or “neutral amino acid transporter B(o),” PLIN2) with higher expression and seven proteins with lower expression in group A.
Pathways with an enrichment score > 1 were selected by enrichment analysis (Table S3). Retinoic acid metabolism-related pathways and inflammation- or immune-related pathways were identified in this manner.
3.5 SPHK1 Carried by EVs From Myeloma Cells Can Cause T Cell Exhaustion
Based on other studies reporting the association of SPHK1/S1P signaling with immunosuppressive effects, EVs from the same two groups (group A: AMO1, XG7, OPM2; group B: KMS12BM, KMS11, RPMI8226) were tested for enrichment of SPHK1 protein by immunoblotting. As shown in Figure 5A, only EVs from group A had enriched SPHK1 protein, which was not observed for group B.
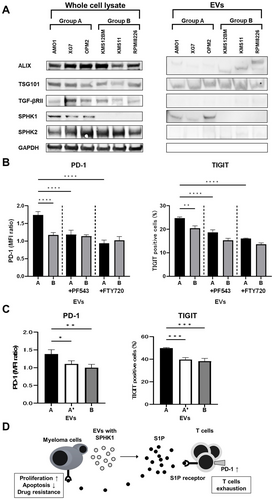
Next, to investigate whether SPHK1 might be involved in any immunosuppression induced by EVs, we tested whether inhibition of SPHK1/S1P signaling by PF543 or FTY720 prevented the potentially immunosuppressive effects. PF543 inhibits the activation of SPHK1, whereas FTY720 acts as a potent agonist at the S1P receptor type 1 (S1P1), internalizes S1P1 on lymphocytes, and consequently inhibits S1P activity. These two drugs were added to cocultures of EVs and activated PBMCs, and exhaustion markers on T cells were then examined. As shown in Figure 5B, both drugs blocked EV-induced upregulated expression of PD-1 on CD8+ T cells and the increased proportions of TIGIT+ cells. Similarly, EVs derived from the SPHK1-expressing AMO1 cells showed increased expression of PD-1 and TIGIT on CD8+ T cells, but these effects were abolished when SPHK1 was knocked out (Figure 5C, Figure S1).
4 Discussion
Here, we focused on the potential role of EVs in immunosuppression in MM and identified possible immune-suppressive effects on T cells of MM-derived EVs from both MM cell lines and samples from MM patients. Plasma-derived EVs from melanoma patients are reported to suppress CD8+ T cell activation, as shown by the downregulation of activation markers on these cells [19]. However, in our study, no alterations of the activation markers CD69 and CD25 were observed on CD8+ T cells cocultured with MM patient-derived EVs, except for a few samples showing a weak suppression, and no clear significant suppression was observed overall. This result prompted us to investigate immunosuppressive markers on T cells after coculture with MM patient-derived EVs. PD-1, TIGIT, and LAG3, known as immune checkpoint markers of immune cells, are also considered major markers of T cell exhaustion. During prolonged antigen stimulation, T cell exhaustion, represented as a loss of proliferation and effector function, is known to occur at a late phase of T cell activation. Such exhaustion is often observed in the tumor microenvironment of several cancers and in situations of chronic inflammation such as immune-mediated disease. In addition, not only a continuous stimulation by antigen presenting cells but also interaction with supportive cells modulating the immune microenvironment promotes the exhaustion of activated T cells in a direct or indirect manner. In our study, MM-derived EVs could induce the exhaustion of T cells through increasing the proportion of checkpoint-positive cells and by the elevation of exhaustion markers on T cells.
We identified several specific proteins by proteomic analysis in relation to the activation or exhaustion of T cells in PBMCs treated with EVs promoting or not promoting the increase of immune checkpoint marker-positive cells (Group A). Eleven of the proteins detected in Group A have been reported to be associated with immune exhaustion and inflammatory disease [13, 20, 21]. Of these, IL-8, a chemotactic factor contributing to inflammatory responses, is reported to upregulate PD-1 expression on CD8+ T cells and to induce an immunosuppressive milieu in the tumor microenvironment [22]. Furthermore, IL-8 is intimately involved in gastric cancer metastasis to the lymph node through upregulation of PD-1 on CD8+ T cells. Therefore, MM-derived EVs may upregulate PD-1 expression on CD8+ T cells, trigger the exhaustion of these cells, and contribute to the progression of MM through the immune escape from cytotoxic T cells.
SPHK1 is an enzyme that converts sphingosine to S1P, which has been reported to play a critical role in the formation of the immunosuppressive tumor microenvironment in several solid tumors [11]. In MM, SPHK1/S1P signaling has been reported to act as a tumor growth and antiapoptotic factor in the tumor cells, to promote IL-6-dependent cell growth, and to facilitate the emergence of resistance to proteasome inhibitors [23-25]. Also, it was reported that the serum concentration of S1P was higher in MM patients than in healthy age-matched donors [26].
One report on ovarian cancer showed that (1) SPHK1 was highly expressed in ovarian cancer cell lines, and EVs derived from these cell lines also contained large amounts of SPHK1, (2) these EVs produced S1P independent of cells in the tumor microenvironment, and (3) S1P promoted an increase in PD-1 in CD8+ T cells [14]. Unlike the case with solid tumors, little is known about the role of this signaling in the MM tumor microenvironment. In particular, the involvement of SPHK1 in the action of MM-derived EVs, specifically in the context of immune escape, is under investigated. However, as with ovarian cancer, in our current study we found enrichment of SPHK1 protein in EVs from specific groups of MM cell lines and demonstrated an association of the SPHK1/S1P pathway with EV-induced potential immunosuppressive effects on T cells by using specific pharmacological inhibitors. Data from experiments with EVs from an SPHK1-knockout cell line also support this mechanism. Thus, the SPHK1/S1P pathway could support cell growth, apoptosis- and drug-resistance via direct effects on MM cells, as well as contributing to immune escape by causing T cell exhaustion due to elevating S1P levels via packaged EVs (Figure 5D). Taken together, these data suggest that the SPHK1/S1P pathway could represent a potential novel target for future MM treatment strategies.
This study has some limitations, and although it proposes an association of SPHK1-packaged EVs with immunosuppressive effects in MM, detailed mechanisms related to the following points have not been fully addressed: SPHK1 production by MM cells, S1P elevation in the tumor milieu, and elevation of immune checkpoint markers on T cells when treated with MM-derived EVs.
In conclusion, our results suggest that MM-derived EVs could induce T cell exhaustion via the elevation of immune checkpoint markers, subsequently suppress T cell function, and support immune escape of MM from cytotoxic T cells. Moreover, we propose that this immune suppression is highly associated with the SPHK1/S1P pathway through SPHK-1 in packaged EVs and the subsequent elevation of S1P near immune cells. This study offers improved understanding of the mechanism of action of the immune-suppressive milieu of MM, which impairs T cell function and thus contributes to the exploration of new strategies for overcoming tumor immune escape and to the development of effective immune therapies targeting the EVs from MM.
Author Contributions
Shinya Hagiwara: conceptualization, data curation, formal analysis, investigation, validation, visualization, writing – original draft. Masaki Ri: methodology, project administration, writing – review and editing. Toru Ebina: writing – review and editing. Yoshiaki Marumo: writing – review and editing. Tomoyuki Nakamura: writing – review and editing. Kentaro Hirade: writing – review and editing. Takahiro Nakashima: writing – review and editing. Arisa Asano: writing – review and editing. Shiori Kinoshita: writing – review and editing. Tomotaka Suzuki: writing – review and editing. Tomoko Narita: writing – review and editing. Ayako Masaki: writing – review and editing. Hirokazu Komatsu: writing – review and editing. Shinsuke Iida: supervision, writing – review and editing.
Acknowledgments
We would like to thank Ms. Chiori Fukuyama (Nagoya City University Graduate School of Medical Sciences, Nagoya) for storing the samples for this study. We acknowledge the assistance of the Research Equipment Sharing Center at Nagoya City University. We thank FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan) for nanoparticle tracking analysis, Hanaichi UltraStructure Research Institute (Okazaki, Aichi, Japan) for TEM analyses, and Proteobiologics Co. Ltd. (Osaka, Japan) for proteomic analysis.
Ethics Statement
Approval of the research protocol by an Institutional Review Board: This study was approved by the Ethical Committee of Nagoya City University Graduate School of Medical Sciences (ID: 70-000113).
Informed Consent: All informed consent was obtained from the subjects.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.
Conflicts of Interest
Dr. Shinsuke Iida is an Editorial Board member of Cancer Science. Other authors do not have any conflicts of interest to declare.




