SPOP Suppresses Hepatocellular Carcinoma Growth and Metastasis by Ubiquitination and Proteasomal Degradation of TRAF6
Funding: This work was supported by National Natural Science Foundation of China, 82272975. National Key Research and Development Program of China, 2023YFC2306800. The Innovative and Entrepreneurial Team of Chongqing Talents Plan. Chongqing Medical Scientific Research Project (Joint project of Chongqing Health Commission and Science and Technology Bureau), 2023DBXM007. Senior Medical Talents Program of Chongqing for Young and Middle-aged, Kuanren talents and Joint project of Pinnacle Disciplinary Group, the Second Affiliated Hospital of Chongqing Medical University.
Wenyi Chang and Kaiying Feng contributed equally to this work.
Ni Tang is the lead contact.
ABSTRACT
Tumor necrosis factor receptor-associated factor-6 (TRAF6) is a well-established upstream regulator of the IKK complex, essential for the modulation of the NF-κB (nuclear factor kappa B) signaling pathway. Aberrant activation of TRAF6 has been strongly implicated in the pathogenesis of various cancers, including hepatocellular carcinoma (HCC). The speckle type BTB/POZ protein (SPOP), an E3 ubiquitin ligase substrate-binding adapter, constitutes a significant component of the CUL3/SPOP/RBX1 complex, which is closely linked to tumorigenesis. In this study, we demonstrated that the E3 ubiquitin ligase SPOP shielded TRAF6 from proteasomal degradation, leading to the hyperactivation of the NF-κB pathway. Notably, a liver cancer-associated S119N mutation in SPOP resulted in a failure to mediate the ubiquitination and subsequent degradation of TRAF6. Moreover, both gain-of-function and loss-of-function experiments revealed that SPOP inhibits the proliferation and invasion of HCC cells through the TRAF6-NF-κB axis in vitro and in vivo. Taken together, our findings elucidate the underpinning mechanism by which SPOP negatively regulates the stability of the TRAF6 oncoprotein, thus offering a new therapeutic target for HCC intervention.
Abbreviations
-
- CCND1
-
- cyclin D1
-
- HCC
-
- hepatocellular carcinoma
-
- IL-8
-
- interleukin-8
-
- IRAK
-
- interleukin-1 receptor-associated kinase
-
- MAP3K7
-
- mitogen-activated protein kinase kinase kinase 7
-
- MYD88
-
- myeloid differentiation primary response 88
-
- NF-κB
-
- nuclear factor kappa B
-
- SPOP
-
- speckle type BTB/POZ protein
-
- TAB1
-
- TGF-beta-activated kinase 1 binding protein 1
-
- TAB2
-
- TGF-beta-activated kinase 1 binding protein 2
-
- TLR4
-
- toll-like receptor 4
-
- TRAF6
-
- tumor necrosis factor receptor-associated factor-6
-
- VEGFA
-
- vascular endothelial growth factor A
1 Introduction
Liver cancer ranks the sixth most common cancer and stands as the third leading cause of cancer mortality around the world [1]. Hepatits B and C viruses infections, alcohol consumption, metabolic diseases, and aflatoxin B1 intake are the main risk factors for liver cancer development and hepatocellular carcinoma (HCC) is the most common primary liver cancer [2, 3]. There are, however, limited treatment options for advanced HCC [4]. Therefore, it is essential to understand HCC progression and develop novel approaches to early diagnosis, prognosis prediction, and treatment of patients with HCC.
Tumor necrosis factor receptor-associated factor-6 (TRAF6), a member of the TNF receptor associated factor (TRAF) protein family, has been delineated as a key mediator of inflammation and plays an important role in the nuclear factor kappa B (NF-κB) pathway [5-7]. NF-κB, an essential regulatory protein, is involved in the growth and spread of cancerous cells, especially in HCC [8-10]. Upon toll-like receptor 4 (TLR4) stimulation, myeloid differentiation primary response 88 (MYD88) recruits and activates interleukin-1 receptor-associated kinase (IRAK). After being activated, IRAK then interacts with TRAF6 and induces TRAF6 dimerization, which activates its E3 ubiquitin ligase activity. TRAF6-mediated Lys63 (K63)-linked ubiquitin chains activate a complex comprising mitogen-activated protein kinase kinase kinase 7 (MAP3K7, also known as TAK1), TGF-beta-activated kinase 1 binding protein 1 (TAB1), and TGF-beta-activated kinase 1 binding protein 2 (TAB2) [5, 11]. The complex subsequently phosphorylates the IKK (inhibitor of IκB kinase) complex, thereby inducing the activation of NF-κB, which comprises p65/RelA, p50/NF-κB1, p52/NF-κB2, RelB, and c-Rel. This activation results in the upregulation of multiple transcriptional targets, including cyclin D1 (CCND1), interleukin-8 (IL-8), and vascular endothelial growth factor A (VEGFA), all of which play critical roles in oncogenesis across various tissue types [8, 12-16]. Overexpression of TRAF6 has been documented in a range of tumor types, including colon, gastric, breast and liver carcinomas [17-20]. Even though TRAF6 plays a significant role in tumor progression, little is known about the molecular mechanisms involved in ubiquitination-mediated degradation of TRAF6.
The speckle type BTB/POZ protein (SPOP) binds substrates for Cullin 3-RING E3 ubiquitin ligases [21]. Substrates of SPOP are selectively recruited via the N-terminal MATH domain, while the BTB and BACK domains are involved in oligomerization and binding to CUL3. SPOP has been identified as a tumor suppressor capable of recognizing and degrading various oncoproteins, including bromodomain-containing protein 4 (BRD4), CYCLIN E1 and fatty acid synthase (FASN) [22-24]. Although SPOP mutations are frequently observed in prostate cancer and endometrial cancers, their occurrence in other tumor types is less documented [25-27]. Our previous studies have suggested that down-regulation of SPOP in HCC results in the loss of its ability to ubiquitinate neurite outgrowth inhibitor-B (Nogo-B) and cAMP response element binding 5 (CREB5), thereby facilitating HCC progression [28, 29]. However, the molecular mechanisms and biological roles identified for the SPOP-TRAF6 stability regulation pathway have yet to be further elucidated.
In the current study, we found an increase in TRAF6 protein levels in HCC tissues relative to normal tissues. Additionally, our findings suggest that SPOP mediate the degradation of TRAF6 in HCC cells, resulting in the suppression of NF-κB activation and the progression of HCC.
2 Materials and Methods
2.1 Cell Culture
PLC/PRF/5 and HEK293 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). MHCC-97H, Huh7 and HEK293T cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). DMEM (C11995500BT, Gibco, New York, NY, USA) supplemented with 10% fetal bovine serum (HY-K1006, NTC, Cordoba, Argentina) and 1% penicillin/streptomycin (HY-K1006, MedChem Express, Monmouth Junction, NJ, USA) was used for cell culture. The cells were transfected with Lipofectamine 3000 (L3000150, Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions.
2.2 Antibodies
A list of the antibodies used is provided in Table S1.
2.3 Plasmids
The full-length cDNA of Human TRAF6 and SPOP was amplified using PCR and then ligated into pBudCE4.13-HA vector or pSEB-3Flag vector respectively. TRAF6 and SPOP deletion mutants were generated using two-step PCR amplification. Site-directed mutagenesis was used to generate the SPOP mutants S119N using the wild-type (WT) SPOP-3xFlag plasmid. In addition, the pT7-TRAF6-6 × His (P64553), pCMV-His-UB (P4836), pCMV-UB-K63 × His (P45634) and pCMV-UB-K48 × His (P45628) constructs were purchased from Miaoling Biologica Technology Co. LTD (Wuhan, China). The specific primers are listed in Table S1.
2.4 Immunohistochemistry Analysis
Liver tissue samples were fixed in formalin and processed for immunohistochemical analysis of PCNA and TRAF6. Imaging was conducted using Caseviewer software (3DHISTECH, Budapest, Hungary). The histoscore were evaluated as previously described [30].
2.5 Production of Adenoviruses
The recombinant adenoviruses AdSPOP and AdGFP were generated as previously described [28].
2.6 Clinical Specimens and Mouse Xenograft Assay
During surgical procedures conducted at the First Affiliated Hospital of Chongqing Medical University, tumor tissues and corresponding non-tumorous tissue samples were collected from a cohort of 30 patients. All participants provided informed consent and had not undergone chemotherapy or radiation therapy prior to the surgical intervention.
At 4 weeks of age, BALB/c male nude mice (n = 5 per group) were randomly received 2 × 106 MHCC-97H cells subcutaneously, and tumor size was measured every 3 days thereafter, starting on the sixth day. Tumors from nude mice were extracted, fixed, and stained with PCNA after 30 days.
2.7 Statistics
The differences between two groups were analyzed using the Student's t-test, while differences among multiple groups were evaluated through one-way or two-way ANOVA. Fisher's exact test were performed to analyze the relationship between TRAF6/SPOP expression and clinicopathological features. The data were presented as mean ± SD, and a p-value < 0.05 was defined statistically significant. GraphPad Prism 8 or SPSS v 13 was used for statistical analysis. *p < 0.05, **p < 0.01, ***p < 0.001.
2.8 Others
Other materials and methods are described in the Data S1.
3 Results
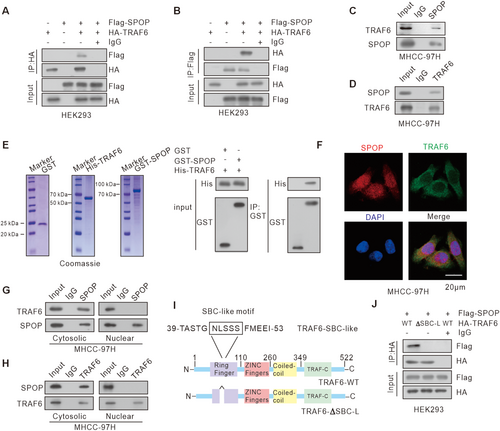
3.1 TRAF6 Is a Novel SPOP-Interacting Protein
Considering that TRAF6 plays a role in NF-κB pathway, we sought to determine whether SPOP exerts tumor-suppressive functions by interacting with TRAF6. We firstly verified the binding between TRAF6 and SPOP using co-immunoprecipitation (Co-IP) assays, which was observed upon ectopic overexpression (Figure 1A,B). This interaction was further substantiated in MHCC-97H cells, where endogenous SPOP and TRAF6 were found to engage in a complex (Figure 1C,D). In addition, the GST pull-down assay confirmed the direct interaction between SPOP and TRAF6 (Figure 1E). Immunofluorescence data further indicated the colocalization of SPOP and TRAF6 in MHCC-97H cells (Figure 1F). To elucidate the subcellular localization of the SPOP and TRAF6 interactions, we conducted Co-IP experiments using extracted cytoplasmic and nuclear proteins. The results demonstrated that the interaction occurs in the cytoplasm (Figure 1G,H). The SPOP-binding consensus (SBC) motif is usually responsible for SPOP binding to its substrates [3]. Therefore, we sought to determine the similar motif in TRAF6 that is required for SPOP-TRAF6 interaction. Despite the absence of a canonical SBC motif, our bioinformatics analysis identified a SBC-like motif (44-NLSSS-48 aa) within TRAF6 sequence (Figure 1I). This motif differs from the classic SBC motif in which the first and second residues were replaced by a polar amino acid, asparagine, and a non-polar amino acid, leucine. We then generated a TRAF6 ΔSBC-like mutant in which the putative motif sequence was deleted. The Co-IP assay showed that TRAF6-ΔSBC-like mutant totally abolished the SPOP-TRAF6 interaction (Figure 1J). Collectively, our findings indicate that SPOP interacts with TRAF6 via the SBC-like motif in TRAF6.
3.2 SPOP Promotes the Ubiquitination and Degradation of TRAF6
Given the established role of SPOP as an E3 ubiquitin ligase in mediating ubiquitination and degradation of its substrates, we first investigated whether SPOP affected TRAF6 protein expression levels. We ectopically expressed HA-tagged TRAF6 in HEK293 cells and observed a significant reduction in TRAF6 protein levels upon co-expression with Flag-SPOP (Figure 2A). The treatment with proteasome inhibitor MG132 effectively rescued TRAF6 levels, implicating the proteasomal pathway in SPOP-mediated TRAF6 degradation. Consistent with these findings, overexpression of SPOP decreased both exogenous and endogenous TRAF6 in a dose-dependent manner, without a concomitant alteration in TRAF6 mRNA level (Figures 2B–D and S1A). Furthermore, we also observed an increased TRAF6 protein levels in SPOP knockout (SPOP-KO) HCC cells (Figure 2E). Upon cycloheximide (CHX) treatment to assess the half-life of TRAF6 protein, we found that SPOP significantly accelerated the degradation of endogenous TRAF6 in MHCC-97H cells (Figure 2F,G). In alignment with these findings, overexpression of SPOP was observed to enhance the ubiquitination of TRAF6 in HCC cells (Figure 2H). It is well-established that K48-linked ubiquitination is generally associated with proteasomal degradation, whereas K63-linked ubiquitination is implicated in non-degradative functions such as cell signaling, inflammatory responses, and cell cycle regulation [31]. Specifically, overexpression of SPOP facilitated the K48-linked polyubiquitination of TRAF6, a modification typically linked to proteasomal degradation (Figure 2I). Conversely, substitution of the lysine at position 48 of ubiquitin with arginine significantly reduced SPOP-mediated ubiquitination of TRAF6, whereas substitution of the lysine at position 63 had no such effect (Figure 2J,K). Collectively, these results suggest that SPOP facilitates the K48-linked polyubiquitination and subsequent degradation of TRAF6 in HCC.
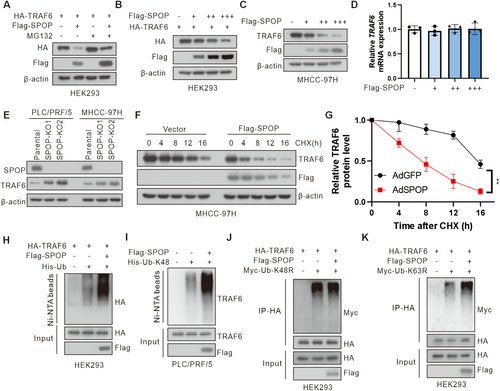
Furthermore, given that the SBC-like motif of TRAF6 is crucial for its interaction with SPOP, we next investigated whether SPOP could promote the ubiquitination and degradation of the TRAF6 ΔSBC-like mutant. The results revealed that the TRAF6 ΔSBC-like mutant abrogated SPOP-mediated degradation and ubiquitination of TRAF6, thereby extending the half-life of TRAF6 protein (Figure S1B–E). These findings collectively demonstrate that SPOP-mediated degradation of TRAF6 is dependent on its SBC-like motif.
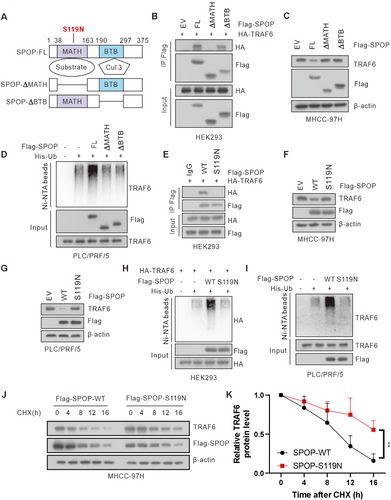
3.3 Cancer-Derived SPOP Mutant Lost the Ability to Interact With and Degrade TRAF6
The MATH domain of SPOP is responsible for its substrates recruitment in SPOP (Figure 3A) [21]. We found that deletion of the MATH domain, but not the BTB domain, abolished the interaction between SPOP and TRAF6, and both the MATH and the BTB domain was required for SPOP-mediated TRAF6 ubiquitination and degradation (Figure 3B–D). Subsequently, we investigated the impact of a liver cancer-associated SPOP mutation, S119N, located in MATH domain, on TRAF6 stability [28, 29]. As a result, the S119N mutant was unable to interact with TRAF6 and thereby failed to facilitate the degradation and ubiquitination of TRAF6 proteins in HCC cells (Figure 3E–I). In addition, ectopic expression of wild-type SPOP (SPOP-WT), but not SPOP-S119N mutant, significantly shortened the half-life of TRAF6 (Figure 3J,K). In conclusion, these results indicate that cancer-derived SPOP mutant (S119N in MATH domain) lost the ability to interact with and degrade TRAF6.
3.4 SPOP Negatively Regulates NF-κB Activation Through TRAF6
TRAF6, an upstream regulator of the IKK complex, plays a key role in the NF-κB signaling pathway. We firstly evaluated whether SPOP suppresses the NF-κB pathway. Our findings demonstrated that overexpression of SPOP, in contrast to its knockdown, resulted in a decrease or increase in the levels of phosphorylated TAK1 and p65 in HCC cells, respectively (Figure 4A–D). We next evaluated the impact of SPOP on TRAF6-meditated NF-κB activation and observed that co-expression of SPOP and TRAF6 decreased the levels of phosphorylated TAK1 and p65 when compared to TRAF6 overexpression alone (Figure S2A). Furthermore, we identified an elevation in phosphorylated TAK1 and p65 levels in SPOP-KO cells treated with TRAF6 short hairpin RNA (shRNA) relative to TRAF6 knockdown (Figure S2B). TRAF6 mediated polyubiquitylation of TAK1 has been shown to be required for TAK1 activation [11, 32]. We thus determined whether SPOP could suppress TRAF6-meditated TAK1 ubiquitylation. Results showed that TRAF6 promoted TAK1 ubiquitination and co-transfection of SPOP suppressed TRAF6-meditated TAK1 ubiquitylation in HCC cells (Figure 4E,F). Furthermore, SPOP also suppressed the TRAF6-mediated activation of NF-κB reporter in HCC cells (Figure 4G,H).
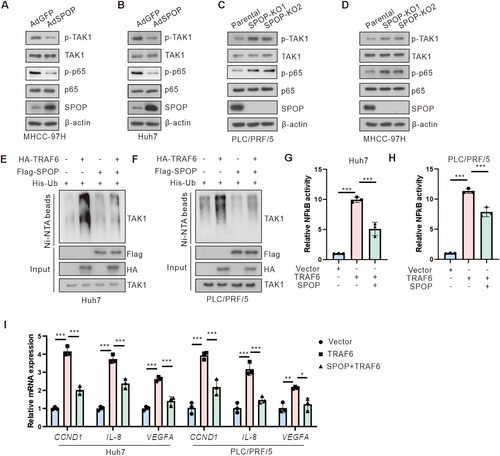
NF-κB has been reported to regulate the expression of numerous genes associated with tumor cell survival, proliferation, and invasion [8]. Consequently, we evaluated the expression of NF-κB-dependent oncogenes. Our findings revealed that overexpression of TRAF6 enhanced the expression of NF-κB target genes, including CCND1, IL-8, and VEGFA, in HCC cells. Conversely, this effect is abrogated by co-transfection of SPOP (Figure 4I). In summary, these results suggest that SPOP acts as a negative regulator of TRAF6-mediated NF-κB activation in HCC cells.
3.5 SPOP Negatively Regulates TRAF6-Mediated Cell Proliferation and Invasion
In order to ascertain the involvement of SPOP in TRAF6-mediated HCC cell proliferation and migration, SPOP-KO cells were subjected to TRAF6 shRNA treatment. The results from 5-ethynyl-2′-deoxyuridine (EdU) incorporation assays, clonogenic assays, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays indicated that knockdown of TRAF6 led to a reduction in cellular proliferation. Conversely, depletion of SPOP resulted in increased cell proliferation and colony-forming capacity in PLC/PRF/5 and MHCC-97H cells (Figures 5A,B, S3A and S4A–C). Moreover, cells with simultaneous depletion of endogenous TRAF6 and SPOP exhibited an enhanced proliferation rate comparable to those with TRAF6 depletion alone (Figures 5A,B, S3A and S4A–C). In keeping with these findings, the wound healing and transwell assays demonstrated that the knockdown of TRAF6 resulted in a decrease in the migration and invasion capabilities of PLC/PRF/5 and MHCC-97H cells, whereas simultaneously knockout of SPOP rewired the effect (Figures 5C, S3B and S4D,E). In addition, simultaneously overexpression of TRAF6 and SPOP reduced the proliferation and invasion capabilities of Huh7 cells when compared to TRAF6 alone (Figure S5A–E). Previous studies have demonstrated that TRAF6 could also reduce apoptosis in cancer cells including HCC cells [20, 33]. Therefore, we further investigated the role of SPOP in TRAF6-mediated reduction of apoptosis in HCC cells. The results revealed that cell apoptosis was increased following treatment with TRAF6 siRNA whereas concurrent knockout of SPOP mitigated this effect in HCC cells (Figures 5D and S4F). Additionally, the simultaneous overexpression of TRAF6 and SPOP led to a higher apoptosis rate in Huh7 cells compared to TRAF6 overexpression alone (Figure S5F).
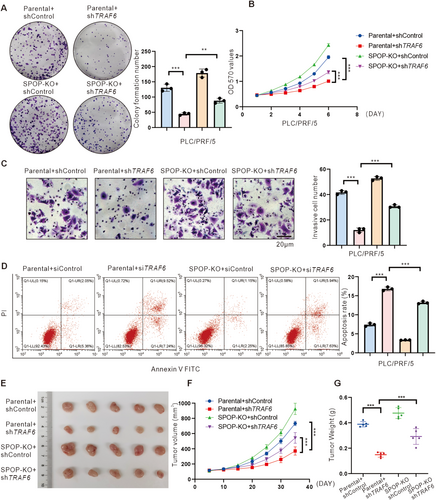
To extend our in vitro findings, a tumorigenicity model in nude mice injected with MHCC-97H cells was employed to further probe the tumor-suppressive effect of SPOP-mediated ubiquitylation on TRAF6 in vivo. As expected, knockdown of TRAF6 suppressed tumor growth, while knockout of SPOP rescued tumor growth in vivo (Figures 5E–G and S6A). We further validated the expression of downstream molecules of NF-κB, specifically Cyclin D, IL-8, and VEGFA, in xenograft tumors. The results indicated that TRAF6 knockdown reduced the expression levels of Cyclin D, IL-8, and VEGFA, whereas SPOP knockout counteracted this effect (Figure S6B). Altogether, these findings suggest that SPOP can inhibit the proliferation and invasion of HCC cells in vivo and in vitro by targeting TRAF6.
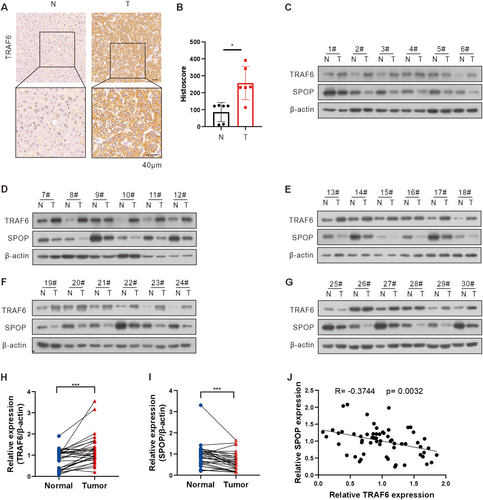
3.6 SPOP Is Correlated With TRAF6 Expression in HCC Tissues
To examine the effect of TRAF6 in HCC patient specimens, we performed the immunohistochemistry (IHC) analysis on six pairs of fixed HCC tissue samples and corresponding non-tumor liver tissues. The results showed that TRAF6 exhibited strong or intermediate staining in HCC tissues compared with non-tumor liver tissues (Figure 6A,B). Subsequently, in order to elucidate the relationship between SPOP and TRAF6 at the protein level in HCC patient specimens, proteins were extracted from 30 pairs of HCC tissue samples and their corresponding non-tumor liver tissues. Western blot analysis revealed a significant upregulation of TRAF6 and a concomitant downregulation of SPOP protein levels in HCC tissues compared to non-tumor liver tissues (Figure 6C–I). Additionally, an inverse correlation was identified between the expression levels of SPOP and TRAF6 (Figure 6J). Furthermore, TRAF6/SPOP expression ratio was positively correlated with the differentiation status and pathological stage of HCC, suggesting potential insights into HCC progression (Table 1). These findings collectively underscore a robust association between the SPOP-TRAF6 axis and human HCC.
| Characteristic | Low TRAF6/SPOP expression ratio | High TRAF6/SPOP expression ratio | p |
|---|---|---|---|
| n | 15 | 15 | |
| Sex | |||
| Male | 12 | 11 | ns |
| Female | 3 | 4 | |
| Age (years) | |||
| < 60 | 9 | 8 | ns |
| ≥ 60 | 6 | 7 | |
| HBsAg | |||
| Positive | 14 | 14 | ns |
| Negative | 1 | 1 | |
| AFP | |||
| ≥ 20 | 8 | 14 | 0.035 |
| < 20 | 7 | 1 | |
| Tumor size (cm) | |||
| ≥ 5 | 2 | 9 | 0.021 |
| < 5 | 13 | 6 | |
| Tumor number | |||
| Single | 12 | 8 | ns |
| Multiple | 3 | 7 | |
| Differentation | |||
| I–II | 15 | 10 | 0.042 |
| III | 0 | 5 | |
| TNM | |||
| I | 12 | 3 | 0.003 |
| II–III | 3 | 12 | |
4 Discussion
Abnormal activation of the NF-κB signaling pathway is a pervasive characteristic across multiple cancer types, including HCC15. In the context of hepatocarcinogenesis, the activation of NF-κB is a common and early occurrence, often linked to the acquisition of a transformed phenotype [10]. However, the precise tumorigenic mechanisms underlying this process remain inadequately understood. TRAF6, a critical adaptor protein, is integral to the regulation of the NF-κB signaling pathway. Numerous studies have demonstrated that TRAF6 is aberrantly overexpressed in various malignant tumors, including lung cancer, colorectal cancer, leukemia, and breast cancer, where it significantly contributes to the proliferation, migration, and invasion of tumor cells [19, 34-36]. In this study, we assessed the expression of TRAF6 protein in human HCC tissues and explored its potential role in HCC pathogenesis. Our analysis of clinical samples corroborates these findings, revealing elevated TRAF6 expression in HCC tissues compared to adjacent non-cancerous tissues. This upregulated expression pattern is consistent with prior research and suggests a role for TRAF6 in the pathogenesis of HCC [20, 37].
The activation of certain signaling pathways, including NF-κB and AKT, has been implicated in TRAF6-mediated oncogenesis and is associated with various inflammatory, apoptotic, and gene regulatory pathways [38, 39]. Functional investigations have revealed that TRAF6 enhances the viability, migratory potential, and invasive properties of HCC cells while simultaneously reducing their susceptibility to apoptosis. Furthermore, previous studies, along with our findings, indicate that SPOP, an E3 ubiquitin ligase, is downregulated in HCC [28, 29, 38, 40]. In this study, we identified an interaction between SPOP and TRAF6, which results in impaired proteasomal degradation and subsequent accumulation of TRAF6.
The ubiquitin/proteasomal protein degradation systems play a crucial role in modulating the NF-κB signaling pathway by dynamically regulating protein levels [41, 42]. As an E3 ubiquitin ligase, SPOP is implicated in multiple biological processes [43]. Recent research has garnered significant interest in the interplay between SPOP and the NF-κB signaling pathway, with a focus on SPOP's impact on the molecular function of MYD88 [44-47]. SPOP plays a crucial role in regulating the inflammatory activation of hematopoietic stem cells (HSCs) by targeting MYD88 for ubiquitination and degradation [44], underscoring its regulatory function in emergency hematopoiesis and inflammation sensing within the bone marrow. Furthermore, SPOP may also suppress inflammatory responses by interfering with MyD88 self-association [47]. Also, it was determined that SPOP hinders the proliferation of diffuse large B-cell lymphoma by non-degradatively ubiquitinating MyD88, recognizing an atypical SPOP-binding motif of MyD88, and impeding myddosome assembly [46]. Our current study elucidates a novel mechanism by which SPOP inhibits the NF-κB signaling pathway through the ubiquitination and subsequent degradation of TRAF6. However, the potential involvement of SPOP in modulating this pathway via MYD88 cannot be excluded. The collective findings from prior studies, along with our own data, highlight the association between SPOP and NF-κB, emphasizing the inhibitory role of SPOP in NF-κB signaling pathways.
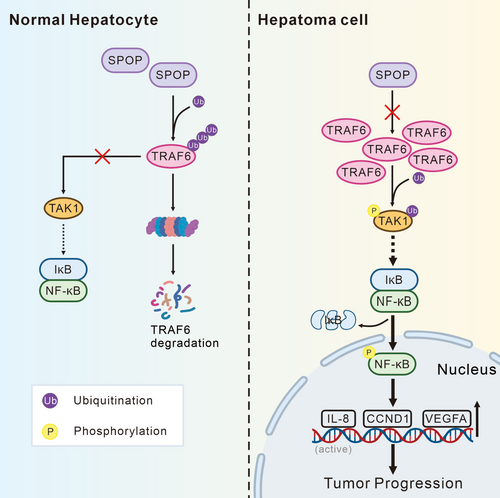
Collectively, our findings provide novel insights into the aberrant accumulation of TRAF6 in HCC, demonstrating that reduced SPOP expression results in impaired degradation of TRAF6, thereby facilitating the activation of the NF-κB pathway and tumor progression (Figure 7). Our study elucidates the expression dynamics of TRAF6/SPOP in HCC, revealing a positive correlation with disease progression, which underscores its pivotal role in disease advancement and identifies it as a potential therapeutic target for HCC. These insights contribute to a deeper understanding of the pathological progression of HCC and inform the development of future therapeutic strategies for its treatment.
Author Contributions
Wenyi Chang: conceptualization, investigation, methodology. Kaiying Feng: data curation, investigation, methodology. Peng Zhou: data curation, investigation. Deao Gong: methodology, validation. Ke Wang: data curation, validation. Ailong Huang: project administration, supervision, writing – original draft. Kai Wang: conceptualization, project administration, supervision. Ni Tang: conceptualization, project administration, supervision.
Acknowledgments
The authors are grateful to Dr. T-C He (University of Chicago, USA) for providing the plasmids pAdEasy system and Prof. Bing Sun (Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, China) for supplying pLL3.7 vectors.
Ethics Statement
Approval of the research protocol by an Institutional Reviewer Board: the use of clinical samples was approved by the Ethics Committee of the Chongqing Medical University (2020007).
Consent
Informed consent was signed before collecting samples.
Conflicts of Interest
The authors declare no conflicts of interest.
Animal Studies
Animal experiments were approved by Chongqing Medical University's Medical Ethics Committee (2020007).




