Understanding the role of BRD8 in human carcinogenesis
Abstract
The bromodomain is a conserved protein–protein interaction module that functions exclusively to recognize acetylated lysine residues on histones and other proteins. It is noteworthy that bromodomain-containing proteins are involved in transcriptional modulation by recruiting various transcription factors and/or protein complexes such as ATP-dependent chromatin remodelers and acetyltransferases. Bromodomain-containing protein 8 (BRD8), a molecule initially recognized as skeletal muscle abundant protein and thyroid hormone receptor coactivating protein of 120 kDa (TrCP120), was shown to be a subunit of the NuA4/TIP60-histone acetyltransferase complex. BRD8 has been reported to be upregulated in a subset of cancers and implicated in the regulation of cell proliferation as well as in the response to cytotoxic agents. However, little is still known about the underlying molecular mechanisms. In recent years, it has become increasingly clear that the bromodomain of BRD8 recognizes acetylated and/or nonacetylated histones H4 and H2AZ, and that BRD8 is associated with cancer development in both a NuA4/TIP60 complex-dependent and -independent manner. In this review, we will provide an overview of the current knowledge on the molecular function of BRD8, focusing on the biological role of the bromodomain of BRD8 in cancer cells.
1 INTRODUCTION
Dynamic changes in chromatin structure are closely linked to transcriptional regulation. The basic unit of chromatin is the nucleosome,1 which consists of approximately 146 bp of DNA wrapped around a histone octamer composed of histones H2A, H2B, H3, and H4. In the static state, DNA is densely packed into chromatin, while during essential cellular processes such as transcription, replication, recombination, and DNA repair, DNA becomes accessible through changes in chromatin structure. Accessibility to the DNA is controlled by two classes of enzymatic complexes, ATP-dependent chromatin remodelers and histone-modifying enzymes.2 The first class, represented by the SWI/SNF complex, is composed of ATP-dependent chromatin remodeling enzymes that utilize the energy from ATP hydrolysis to reorganize chromatin. The second class includes GNAT- and MYST-related complexes that acetylate a subset of the lysine residues on the tails of core histones.3 In many cases, these chromatin-modifying complexes contain a component with a bromodomain(s) that acts as an acetyl-lysine binding module.
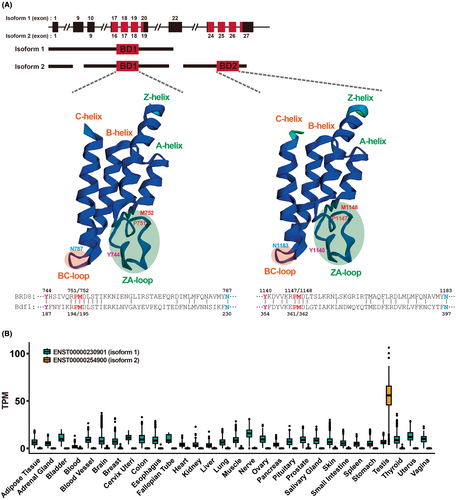
A motif of 77 amino acids (currently recognized as a larger domain of approximately 110 amino acid residues), called the “bromodomain”, was originally identified as a sequence common to the Drosophila brahma and the yeast SWI2/SNF2 proteins, involved in transcriptional activation.4 “Bromo-” was named after brahma by analogy to the methyl-lysine binding motif, the chromodomain, and is not related to the chemical element bromine. Subsequently, the bromodomain was found to interact specifically with acetylated lysine and to cooperate with histone acetyltransferase in the regulation of gene transcription.5 As proteins containing specific domains that recognize the modification status of a histone tail are often referred to as “epigenetic readers”, bromodomain-containing proteins are also classified as epigenetic readers. However, it should be noted that the term “readers” is discussed as an overstated metaphor. As an alternative, the use of “binders” is suggested here.6 The characteristic structure of this domain is a bundle of four α-helices (αZ, αA, αB, and αC) linked by flexible loop regions (ZA- and BC-loops) (Figure 1A). These loops, which form a hydrophobic pocket for acetyl-lysine binding, have large sequence variations, resulting in substrate specificity.5, 7, 8
According to the Human Genome Organization (Gene group: Bromodomain containing, https://www.genenames.org/data/genegroup/#!/group/1232), the human proteome contains at least 43 bromodomain-containing proteins, clustered into eight major families (I–VIII).7 Bromodomain-containing 8 (BRD8) belongs to subgroup III along with BAZ1B, BRWD1, BRWD3, CREBBP, EP300, PHIP, and WDR9.7 In 1996, the human BRD8 cDNA was isolated from a Jurcat acute T-cell leukemia cell line and named it SMAP (skeletal muscle abundant protein).9 The SMAP cDNA contained an ORF of 2271 nt encoding a deduced 81.4 kDa protein with a bromodomain, but its function was unknown. Another group isolated a thyroid hormone receptor (TR)-interacting protein of 920 amino acids with a deduced molecular size of 120 kDa and named it p120.10 The amino acid sequence of p120 was similar to that of SMAP, and p120 enhanced TR-mediated transactivation in the presence of T3. SMAP and p120 are now regarded as different forms of the BRD8 protein. BRD8 was later found in a large multiprotein complex of the NuA4/TIP60 (nucleosome acetyltransferase of H4/Tat interacting protein, 60 kDa) histone acetyltransferase. Importantly, BRD8 has been implicated in carcinogenesis and our recent study has identified that there exists both NuA4/TIP60 complex-dependent and -independent mechanisms.11 This review summarizes current knowledge on the molecular features and functions of BRD8 in cancer cells.
1.1 Splicing variants of BRD8 and their expression in human organs
According to the Ensembl database in EMBL-EBI, there are 11 protein-coding transcripts of human BRD8. Two major structural forms, isoform 1 or Q9H0E9-2 (951 amino acids) and isoform 2 or Q9H0E9-1 (1235 amino acids), are encoded by transcript variants ENST00000230901/NM_006696 and ENST00000254900/NM_139199, respectively. BRD8 isoform 2 differs from isoform 1 in the C-terminal region and lacks 73 amino acids in the central region. Importantly, both isoforms have bromodomain 1 (BD1), but isoform 2 has another bromodomain (BD2) at the C-terminus (Figure 1A). Immunoblotting of BRD8 seems to detect bands above the calculated molecular weight: isoforms 1 (102,839 Da) and 2 (135,336 Da) appear between 150 and 250 kDa and close to 250 kDa, respectively.11
RNA sequencing data from the GTEx project (https://gtexportal.org/) revealed the expression patterns of BRD8 variants across human tissues. A variant ENST00000254900, corresponding to isoform 2, is expressed abundantly and exclusively in the testis (Figure 1B). On the other hand, ENST00000230901, corresponding to isoform 1, is broadly expressed in all tissues. This variant (ENST00000230901) is also expressed in colonic mucosa and tumors.11 Thus, understanding the splicing mechanism and the role of the testis-specific variant could help to determine its phenotype(s), including the “preweaning lethality” in Brd8 KO mice shown by the International Mouse Phenotyping Consortium (https://www.mousephenotype.org).
1.2 Bromodomains of BRD8
As described above, BRD8 isoform 2 has two bromodomains, BD1 between codons 705 and 813 and BD2 between codons 1101 and 1209 at the C-terminus. The amino acid sequences of BD1 (109 a.a.) and BD2 (109 a.a.) are 54.1% identical (ClustalW: https://www.genome.jp/tools-bin/clustalw). The BD1 of human BRD8 has been reported to interact at least with histone H411 and H2AZ.12 In addition, the BD1 bound to acetylated K73/76 of Twist family bHLH transcription factor 1 (TWIST1), while BD2 bound to acetylated K5/8 of histone H4.13 Regarding the interaction with histone H2AZ, BRD8 binds to both the unacetylated and acetylated forms. Unexpectedly, acetylated H2AZ has a relatively lower binding affinity for BRD8 than the unacetylated H2AZ, implying a recognition preference of the bromodomain.12
It is noteworthy that the double bromodomain-containing yeast Bdf1 (BD1, 149–256 a.a.; BD2, 314–423 a.a.), a part of the SWR chromatin remodeling complex, is thought to be homologous to human BRD8 isoform 2.14-16 The amino acid sequences of BD1 and BD2 between human BRD8 isoform 2 and yeast Bdf1 are 25.0% and 23.9% identical, respectively (ClustalW). Bdf1 was first identified as a protein to interact with histone H4 by yeast two-hybrid screening.17 Subsequently, Matangkasombut and Buratowski showed that point mutations in the bromodomains of Bdf1 disrupted histone binding in vitro, which leads to defects in its transcription.18 Bdf1 contains an additional extra terminal domain, and thus may belong to the BET family (https://www.yeastgenome.org/). Of note, mutant Bdf1 proteins with a substitution in the ZA-loop of BD1 (Y187A) or BD2 (Y354A) showed reduced histone H4 binding. In addition, its binding to the core histones, H2A, H2B, H3, and H4 was impaired by substitutions of P194/P361 or M195/M362 in the ZA loop, or N230/N397 in the BC loop (of BD1 and BD2). The Y187/Y354, P194/P361, M195/M362, and N230/N397 correspond to Y744/Y1140, P751/P1147, M752/M1148, and N787/N1183 of BD1 and BD2 in human BRD8 (Q9H0E9-1, NP_631938), respectively (Figure 1A).19, 20
The bromodomain also acts as a binding module for the nonhistone proteins.11, 13, 21 BRD8 has been known to bind MRG-binding protein (MRGBP)11, 21 and TWIST113 through BD1. Although it is likely that BRD8 interacts directly with EP400 and EP400 N-terminal like (EP400NL), it remains unclear whether the bromodomain(s) mediates interactions with these proteins.22
1.3 BRD8 is a coactivator for nuclear hormone receptors
Enhancement of the transcriptional activity of the TR was the first reported function of BRD8.10 In addition, BRD8 acts as a co-activator for other nuclear receptors including androgen receptor (AR),10, 23, 24 progesterone receptor,24 estrogen receptor,24 glucocorticoid receptor,23 and retinoid X receptor (RXR)/peroxisome proliferator-activated receptor γ (PPARγ).25, 26 The BRD8 protein contains multiple short sequence motifs “LXXLL” (amino acids 271–275 and 340–344 in BRD8 isoform 1) that is necessary for binding nuclear receptors.27 Consistent with these data, a variant of BRD8 lacking the N-terminal LXXLL motif (p120β) lost ligand-inducible enhancement by TR, PPARγ, and RXR, while the variant retained the ability of AR transcriptional activity.23 This may imply that the mechanism by which BRD8 functions as an AR coactivator may differ from other nuclear hormone receptors. BRD8 has also been reported to play an important role in the regulation of PPARγ target genes during adipogenesis through the recruitment of chromatin-modifying complex including EP400 and TIP60.25
1.4 BRD8 is part of the multiprotein complex of NuA4/Tip60
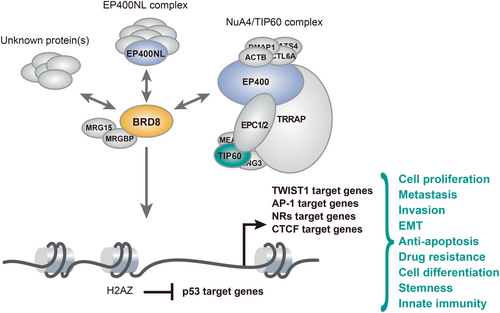
Previous proteomic analysis revealed that BRD8 is a member of the human NuA4/TIP60 histone acetyltransferase complex, suggesting that BRD8 is involved in transcriptional regulation in concert with this complex (Figure 2).28 NuA4/TIP60 is an evolutionarily conserved and multifunctional histone acetyltransferase complex that to date consists of at least 20 subunits (Table 1).15, 16, 22, 28-35 The catalytic subunit TIP60 (KAT5: lysine acetyltransferase 5) acetylates nonhistone proteins as well as histones H4, H2A, and H2A variants.36-39 EP400, originally identified as an interacting partner of the adenovirus E1A protein,40 is a functional subunit with ATP-dependent chromatin remodeling activity, allowing the replacement of conventional histone H2A with variant histone H2AZ.34 This complex is well-known to be recruited to DNA damage sites and protect the cells against damage.
| Yeast | Human | Approved name (human) | |||
|---|---|---|---|---|---|
| NuA4 | SWR1 | TIP60 | SRCAP | EP400NL-associated | |
| *Eaf5 | Bdf1 | BRD8 | BRD8 | BRD8 | Bromodomain containing 8 |
| Esa1 | KAT5 (TIP60) | Lysine acetyltransferase 5 | |||
| Eaf1 | Swr1 | EP400 | SRCAP | EP400P1 (EP400NL)/SMARCA4 (BRG1) | E1A binding protein p400 |
| Snf2 related CREBBP activator protein | |||||
| EP400 pseudogene 1 | |||||
| SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 4 | |||||
| Tra1 | TRRAP | Transformation/transcription domain associated protein | |||
| Epl1 | EPC1 | Enhancer of polycomb homolog 1 | |||
| EPC2 | Enhancer of polycomb homolog 2 | ||||
| Eaf2 | Eaf2 | DMAP1 | DMAP1 | DMAP1 | DNA methyltransferase 1 associated protein 1 |
| Rvb1 | Rvb1 | RUVBL1 | RUVBL1 | RUVBL1 | RuvB like AAA ATPase 1 |
| Rvb2 | Rvb2 | RUVBL2 | RUVBL2 | RUVBL2 | RuvB like AAA ATPase 2 |
| Arp4 | Arp4 | ACTL6A | ACTL6A | ACTL6A | Actin-like 6A |
| Yng2 | ING3 | Inhibitor of growth family member 3 | |||
| Eaf7 | MRGBP | MRGBP | MRG domain binding protein | ||
| Eaf3 | MORF4L1 (MRG15) | MORF4L1 (MRG15) | Mortality factor 4 like 1 | ||
| MORF4L2 (MRGX) | MORF4L2 (MRGX) | Mortality factor 4 like 2 | |||
| Act1 | Act1 | ACTB | β-Actin | ||
| Yaf9 | Yaf9 | YEATS4 | YEATS4 | YEATS4 | YEATS domain containing 4 |
| Eaf6 | MEAF6 | MYST/Esa1 associated factor 6 | |||
| MBTD1 | MBT domain containing 1 | ||||
| ACTR6 | Actin related protein 6 | ||||
| Vps71 | ZNHIT1 | Zinc finger HIT-type containing 1 | |||
| Vps72 | VSP72 | VSP72 | Vacuolar protein sorting 72 homolog | ||
| JAZF1 | JAZF zinc finger 1 | ||||
- * BRD8 may be a human functional homolog of yeast Eaf5.22
Unexpectedly, yeast NuA4 likely lacks Bdf1, the yeast counterpart of human BRD8.16 However, deletion of yeast Bdf1 was lethal in combination with a mutant allele of Esa1, the yeast counterpart of human TIP60, suggesting that the presence of human BRD8 in the NuA4/TIP60 complex may reflect the functional interaction between Bdf1 and Esa1 in yeast.18 Consistent with their observation in yeast, BRD8 facilitated acetylation of histone H4K16 where it is catalyzed by TIP60 at DNA damage sites.41, 42 In addition, BRD8 mediated the incorporation of H2AZ by EP400 and the subsequent induction of PPARγ target genes during adipogenesis.25 Taken together, BRD8 may play an important role in transcription and DNA repair as a subunit of the NuA4/TIP60 complex.
1.5 BRD8 interacts with EP400NL to form a nuclear complex distinct from the NuA4/TIP60 complex
We recently reported that BRD8 regulates the expression of multiple subunits of the prereplicative complex, such as minichromosome maintenance genes (MCMs) and origin recognition complex genes (ORCs), independently of TIP60.11 This can be explained by the formation of an independent functional complex. Indeed, a recent interactome analysis indicated that BRD8 interacts directly with EP400NL to form a tetrameric protein complex named TINTIN (Trimer independent of NuA4 for transcription interactions with nucleosomes) (Figure 2, Table 1).22 The EP400NL gene is located adjacent to EP400 and encodes a 51.8 kDa protein that shares a similar N-terminal sequence with the large EP400 protein (343.5 kDa). Another group also showed that EP400NL induced PD-L1 expression in the presence of serum- and γ-interferon-stimulation, suggesting a possible link between this complex and tumor immune evasion.35 Interestingly, BRG1, a core ATPase subunit of the human SWI/SNF complex, was found in the EP400NL-associated complex instead of EP400, suggesting that this complex also has chromatin remodeling activity.35 Although the components of the EP400NL-associated complex are not fully understood, this complex can control gene expression in the absence of histone acetyltransferase activity.35 Collectively, BRD8 may govern gene expression in concert with different complexes.
1.6 Alterations of BRD8 in cancer tissues
It has been reported that a mutation in BRD8 was identified in one of four microphthalmia-associated transcription factor (MITF) family translocation renal cell cancers by whole exome sequencing, and the patient showed a partial response to ipilimumab, an immune checkpoint inhibitor.43 However, BRD8 mutations are not frequently observed in urinary tract cancer (45 of 805 patients in the COSMIC database) or bladder urothelial cancer (13 of 411 patients in The Cancer Genome Atlas [TCGA] PanCancer Atlas dataset).
A rearrangement of BRD8 with the PHD finger protein 1 (PHF1) gene has been reported in low-grade endometrial stromal sarcoma (ESS).44 This rearrangement is expected to lead to an in-frame fusion between exon 16 of BRD8 (NM_006696) and exon 2 of PHF1 (NM_002636), whose transcript preserves the entire coding sequence of PHF1 but lacks the bromodomain from BRD8 (Figure 3A). PHF1 encodes a polycomb-like protein that mediates recruitment of polycomb repressive complex 2 (PRC2) to chromatin, resulting in transcriptional repression of key developmental genes through H3K27 methylation. It is of note that rearrangements of PHF1 with members of the NuA4/TIP60 complex have also been identified in soft tissue sarcomas. Partners of the fusion genes include JAZF1,45 EPC1,45 MEAF6,46 EPC2,47 MBTD1,48 ING3,49 and EP400 (Figure 3B).50 A recent study has shown that the EPC1-PHF1 fusion protein forms a megacomplex in which the NuA4/TIP60 and PRC2 complexes are bound.51 This aberrant complex leads to mislocalized acetylation by TIP60, resulting in the dysregulation of gene expression, particularly in the increased expression of PRC2 target genes.
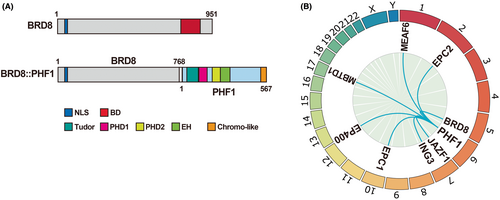
In addition to the BRD8-PHF1 fusion, its reciprocal fusion form (exon 1 of PHF1 and exon 9 of BRD8) was also found in ossifying low-grade ESS.52 Notably, the predicted protein retains the C-terminal region of BRD8 containing the bromodomain. In addition, the expression of this C-terminal BRD8 was probably governed by the PHF1 promoter and was increased in tumor cells compared to the control endometrial stromal cells. The significant oncogenic function of these fusion proteins is still unknown, but given the characteristics of its partners, it might impair DNA damage response, cell cycle, and DNA replication through epigenetic dysregulation.11, 12, 41
Although genetic alterations in BRD8 are rare, increased expression of BRD8 has been found in several tumors including colorectal cancer and glioblastoma.11, 12 According to TCGA data, BRD8 transcripts are highly expressed in cholangiocarcinoma (CHOL), thymoma (THYM), and pheochromocytoma and paraganglioma (PCPG) compared to their matched normal tissues (GEPIA, http://gepia.cancer-pku.cn/). Posttranscriptional modification is also involved in the regulation of BRD8 expression. As the expression of BRD8 was enhanced by the treatment with MG132, a proteasome inhibitor, BRD8 protein is thought to undergo proteasomal degradation through its ubiquitination. Indeed, a total of 31 ubiquitination sites were identified inside and outside of the bromodomain.11 In colorectal cancer cells, BRD8 accumulates through the inhibition of ubiquitin-dependent protein degradation by the interaction with MRGBP. In addition, the isoform-specific regulation by microRNA has been reported. BRD8 isoform 2 is specifically upregulated through the loss of microRNA-185 in prostate cancer, which leads to the activation of AR-mediated transcription.23, 53
1.7 Dysregulation of BRD8 is associated with tumorigenesis
The first report linking BRD8 to carcinogenesis identified that accumulation of BRD8 was associated with resistance to spindle poisons in metastatic colon cancer cell lines and advanced rat colon carcinomas.54 Although the growth-promoting effect of BRD8 isoform 2 in prostate cancer may be mediated through activation of AR-mediated transcription,23, 53 the molecular mechanisms by which BRD8 is associated with cancer progression were largely unknown. Recently, integrated analyses of genome-wide mapping of BRD8-binding sites and BRD8-regulated genes revealed direct BRD8 target genes in human lung55 and colorectal cancer cells.11 In lung epithelial cells, BRD8 affected genes essential for the cell cycle, antimicrobial proteins, and chemokines, suggesting an involvement of BRD8 in cell proliferation and immune responses in the mucosal barrier in the airway epithelium.55 In colorectal cancer cells, genes regulated by BRD8 were significantly associated with DNA replication and the cell cycle.11 In glioblastoma, BRD8 blocked the binding of WT p53 to chromatin, thereby inhibiting p53-mediated transactivation.12 In line with the data, knockdown of BRD8 increased the expression of p53 target genes such as p21, Puma, and TIGAR in colorectal cancer, which induced apoptosis and cell cycle arrest.41 However, the involvement of BRD8 in the regulation of p53 expression remains controversial.11 Furthermore, BRD8 mediated the interaction between TWIST1 and the TIP60 complex, and recruited the TIP60 complex to the TWIST1 target loci, thereby promoting epithelial–mesenchymal transition, proliferation, and metastasis of breast cancer.13 Given that the bromodomain of BRD8 is responsible for the recruitment, interaction, and gene expression, it may be a promising therapeutic target for patients with glioblastoma and colorectal, prostate, and breast cancer.
2 CONCLUSIONS AND PERSPECTIVES
Our understanding of the biological roles of BRD8, particularly in cancer, is growing rapidly. There are two major splice forms of BRD8 that give rise to the distinct expression pattern. Isoform 2 is specifically and highly expressed in the testis, suggesting tissue-specific targets and roles for this isoform. It would also be interesting to elucidate whether BD2 has any additional function or merely synergizes the binding affinity of BD1. Therefore, the identification of physiological roles of BRD8 isoform 2 and the function of BD2 are interesting issues to investigate.
Another major challenge that needs to be addressed is the application of BRD8 in the treatment of cancer. As BRD8 is involved in anticancer drug-resistance, cell cycle, and/or proliferation of cancer cells, suppression of its activity and/or expression could be an attractive therapeutic strategy for cancer. Recently, BRD8 probes, DN01 and DN02, that are derivatives of a BRD9 inhibitor BI-9564, have been developed.56 As the association of BRD8 with its target molecules is mediated by the bromodomains, it is interesting to test whether DN01/DN02 alters TIP60 activity, p53 chromatin accessibility, and inhibits the expression of TIP60-independent target genes and/or TWIST1-target genes in cancer cells. In addition, these probes may serve as proteolysis-targeting chimeras (PROTACs).57 As BET protein PROTACs selectively and strongly induce degradation of BET proteins in cancer cells,58, 59 molecular glues that mediate the interaction between BRD8 and cellular enzymes such as E3 ligases may efficiently drive its protein degradation. Alternatively, inhibitors for the BRD8–MRGBP interaction should be another option to decrease the accumulated BRD8 protein because BRD8 is stabilized by the association with MRGBP in cancer cells. These approaches are interesting to be applied for the treatment of tumors with elevated BRD8 expression.
We hope that this review provides a deeper insight into the biology of the bromodomain, which has the potential to offer further novel treatment options for cancer patients.
AUTHOR CONTRIBUTIONS
Kiyoshi Yamaguchi: Visualization; writing – original draft; writing – review and editing. Saya Nakagawa: Writing – review and editing. Yoichi Furukawa: Writing – review and editing.
ACKNOWLEDGMENTS
We thank Yuya Okawara, Meihui Zuo, and Momoa Konno (The University of Tokyo) for helpful discussion.
FUNDING INFORMATION
This work was supported by JSPS KAKENHI Grant Nos. 17K07214 and 24K02318 to K.Y.
CONFLICT OF INTEREST STATEMENT
Yoichi Furukawa is an editorial board member of Cancer Science. The other authors have no conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an institutional review board: N/A.
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.




