Metformin inhibits the proliferation and invasion of ovarian cancer cells by suppressing tripartite motif-containing 37-induced tumor necrosis factor receptor-associated factor 2 ubiquitination
Ya Zheng and Haiyan Zhang contributed equally to this work.
Abstract
Ovarian cancer is the leading cause of death in gynecological malignancies worldwide. Our previous studies have proved that metformin inhibited the proliferation and invasion of ovarian cancer in vitro and in vivo. However, the underlying mechanisms have not been fully elucidated. Immunohistochemistry was carried out to detect the expression of tripartite motif-containing 37 (TRIM37), Ki-67, and MMP-9 in ovarian cancer and normal tissues. The influence of TRIM37 on the proliferation and invasion of ovarian cancer cells was verified by the real-time cellular analysis proliferation test, colony formation test, and Transwell assay. Western blot analysis and immunoprecipitation were used to detect the expression of the nuclear factor-κB (NF-κB) pathway and the interaction between TRIM37 and tumor necrosis factor receptor-associated factor 2 (TRAF2). Ubiquitination detection was carried out to detect the ubiquitination level of TRAF2. The present study revealed that TRIM37 expression was significantly increased in ovarian cancer tissues compared with normal control tissues, and its overexpression was closely associated with proliferation and metastasis. Metformin inhibited the NF-κB signaling pathway by downregulating TRIM37. Metformin also inhibited the ubiquitination of TRAF2 induced by TRIM37 overexpression. Metformin inhibits the proliferation and invasion of ovarian cancer cells by suppressing TRIM37-induced TRAF2 ubiquitination.
Abbreviations
-
- EDD
-
- E3 isolated by differential display
-
- IKB
-
- inhibitor of NF-κB
-
- IKK
-
- IKB kinase
-
- MEF
-
- murine embryonic fibroblast
-
- NF-κB
-
- nuclear factor-κB
-
- PARP
-
- poly(ADP-ribose) polymerase
-
- PDTC
-
- pyrrolidinedithiocarbamate
-
- PRC2
-
- polycomb inhibitory complex 2
-
- RIP
-
- receptor interacting protein
-
- RTCA
-
- real-time cellular analysis
-
- TNF-α
-
- tumor necrosis factor-α
-
- TRAF2
-
- tumor necrosis factor receptor-associated factor 2
-
- TRIM37
-
- tripartite motif-containing 37
-
- UPS
-
- ubiquitin proteasome system
1 INTRODUCTION
Ovarian cancer is the leading cause of death in gynecological malignancies worldwide. Due to the lack of effective screening methods, and the lack of specific symptoms and signs in patients with early ovarian cancer, most patients are diagnosed with advanced stages of ovarian cancer. The 5-year survival rate is only 15%–20% in advanced patients. On the basis of ideal cytoreductive surgery, adjuvant chemotherapy is the standardized treatment for ovarian cancer.1, 2 At present, paclitaxel plus platinum is the first-line chemotherapy for epithelial ovarian cancer. Many clinical studies have proved that they are superior to any other regimen, but eventually more than 75% of patients have drug resistance and disease recurrence.3
Our previous studies have proved that metformin inhibited the proliferation and invasion of ovarian cancer in vitro and in vivo.4 However, the underlying mechanisms have not been fully elucidated. The UPS is a highly selective and specific protein degradation pathway, which can degrade more than 80% of intracellular proteins. The UPS plays an important role in the pathogenesis of a variety of diseases, including malignant tumor,5, 6 neurodegenerative disease,7 immune and inflammatory response,8 cardiovascular disease,9 and virus infection.10 E3 ubiquitin ligase is a key component of UPS, which plays an important role in the occurrence and development of many kinds of tumors. Tripartite motif-containing 37 is a new E3 ubiquitin ligase discovered in recent years.11, 12 It was reported that TRIM37 played a cancer promoting role in breast cancer and lung cancer.13-15 In 2014, an article in Nature reported that TRIM37, as a ubiquitin ligase, can ubiquitinate histone H2A in breast cancer, and then bind to the promoter of tumor suppressor gene, resulting in tumor suppressor gene silencing and cancer promoting effects.15 In addition, when esophageal cancer was stimulated by genotoxic drugs, TRIM37 interacted with TRAF6 and ubiquitinated NF-κB essential modulator (NEMO), activated the NF-κB pathway, and thus played a cancer-promoting role.16 However, the effect of TRIM37 in ovarian cancer has not been clarified.
Results from our previous studies showed that metformin inhibited the proliferation, migration, and invasion of epithelial ovarian cancer cells.4 We proposed that metformin could inhibit epithelial ovarian cancer by influencing epigenetic enzymes. In the present study, the effect of TRIM37 in ovarian cancer was explored and we investigated whether metformin could inhibit epithelial ovarian cancer by interfering with E3 ubiquitin ligase TRIM37.
2 MATERIALS AND METHODS
2.1 Cell lines and reagents
Human immortal ovarian epithelial cell line HOSEpiC and human ovarian cancer cell lines including A2780, SKOV3, OVCAR3, OVCAR5, and OVCAR433 were purchased from ATCC and cultured in RPMI-1640 medium supplemented with 10% FBS (Gibco, Life Technologies), 1% penicillin, 1% streptomycin, and 1% amphotericin B at 37°C with 5% CO2. Metformin were purchased from Sigma-Aldrich and dissolved in PBS. Both PDTC (50 μM) and TNF-α were purchased from MedChemExpress Corporation. Lipopolysaccharide (100 ng/ml) was purchased from Beyotime Biotechnology.
2.2 Patients and samples
A total of 30 control samples of normal ovarian epithelial tissues and 60 cases of epithelial ovarian cancer tissues used in the study were applied from the tissue bank of the Obstetrics and Gynecology Hospital of Fudan University and were approved by the ethics committee of our hospital. All patients signed informed consent. Patients in the ovarian cancer group were pathologically diagnosed as epithelial ovarian cancer and treated, and were without other malignant tumors. For the control group, the tissues were taken from the patients who underwent adnexectomy for hysteromyoma, and other ovarian lesions were excluded.
2.3 Plasmids and lentivirus transfection
The interference and overexpression lentiviral vectors of TRIM37 were constructed by Shanghai Genechem Chemical Technology Co., Ltd. The TRAF2 plasmid (pCDH-CMV-Human TRAF2 [NM_021138]-C-Myc-EF1-Puro) was constructed by Lnc Biotechnology Co., Ltd. According to the TRIM37 sequence, the company designed four pairs of interference sequences. The target information is shown in Table 1, and the sequence information is shown in Table 2. 293T cells were seeded into 6-well plates, and the density was approximately 70% before transfection the next day. Transfection system A, including 125 μl (Opti-MEM, Thermo Fisher scitific, USA) 2.5 μg DNA, and 5 μl (p3000 reagent, Thermo Fisher scitific, USA) (2 μl/μg DNA) was prepared and vortexed for 3 s. Transfection B system including 125 μl Opti-MEM and 7.5 μl (Lipofectamine 3000, Thermo Fisher scitific, USA) was prepared and vortexed for 3 s. Solution A was mixed with solution B and incubated at room temperature for 10 min, added to a 6-well plate, and supplemented with 2 ml RPMI-1640 medium. After 8 h, the RPMI-1640 medium was replaced with fresh medium containing 10% FBS. Western blot analysis was used to verify the transfection efficiency of four pairs of sequences, and the most effective pair was selected for virus packaging. Puromycin was used to select the stable cell lines.
| ID | Target sequence | Start position | GC (%) |
|---|---|---|---|
| TRIM37-RNAi(34786) | AGTGTAGCAGGTAGTCTAT | 2342 | 42.11 |
| TRIM37-RNAi(34787) | GTTTGGACTTACTCGCAAA | 1182 | 42.11 |
| TRIM37-RNAi(34788) | ACCAGACTTTACCAGTGAA | 838 | 42.11 |
| TRIM37-RNAi(34789) | TAATGCTAAAGGAGGTCAT | 2623 | 36.84 |
| ID | 5′-end | Stem | Loop | Stem | 3′-end |
|---|---|---|---|---|---|
| TRIM37-RNAi(34786)-a | Ccgg | ccAGTGTAGCAGGTAGTCTAT | CTCGAG | ATAGACTACCTGCTACACTGG | TTTTTg |
| TRIM37-RNAi(34786)-b | aattcaaaaa | ccAGTGTAGCAGGTAGTCTAT | CTCGAG | ATAGACTACCTGCTACACTGG | |
| TRIM37-RNAi(34787)-a | Ccgg | ccGTTTGGACTTACTCGCAAA | CTCGAG | TTTGCGAGTAAGTCCAAACGG | TTTTTg |
| TRIM37-RNAi(34787)-b | aattcaaaaa | ccGTTTGGACTTACTCGCAAA | CTCGAG | TTTGCGAGTAAGTCCAAACGG | |
| TRIM37-RNAi(34788)-a | Ccgg | ccACCAGACTTTACCAGTGAA | CTCGAG | TTCACTGGTAAAGTCTGGTGG | TTTTTg |
| TRIM37-RNAi(34788)-b | aattcaaaaa | ccACCAGACTTTACCAGTGAA | CTCGAG | TTCACTGGTAAAGTCTGGTGG | |
| TRIM37-RNAi(34789)-a | Ccgg | gcTAATGCTAAAGGAGGTCAT | CTCGAG | ATGACCTCCTTTAGCATTAGC | TTTTTg |
| TRIM37-RNAi(34789)-b | aattcaaaaa | gcTAATGCTAAAGGAGGTCAT | CTCGAG | ATGACCTCCTTTAGCATTAGC |
2.4 Colony formation test
Cells in logarithmic growth phase were resuspended into single cell suspension with RPMI-1640 medium containing 10% FBS, and counted with a cell counting plate. Each 6-well plate was inoculated with 400 cells, gently shaken and mixed, and then put into a cell incubator for culture. After 2 weeks, the colonies could be observed by the naked eye. The culture medium was discarded. The plates were washed twice with PBS, fixed with the 4% polyformaldehyde for 15 min, and dyed with crystal violet for 10 min. After the plates were dried, photographs were taken and analyzed.
2.5 Real-time cellular analysis proliferation test
The transfected cells were digested and resuspended into single cell suspension with RPMI-1640 containing 10% FBS. The cells were counted by a cell counting plate and the cell concentration was adjusted to 5 × 104 cells/ml. The cell suspension was gently mixed then 5000 cells were implanted into each hole of an E-16 plate. After standing at room temperature for 30 min, the E-16 plate was placed into the RTCA instrument. When the proliferation curve reached the platform period, the test was terminated and data could be derived.
2.6 Immunohistochemistry
The fixed tissue was embedded in paraffin and sliced into 5 μm-thick sections. After deparaffinization and dehydration, the slices underwent antigen retrieval at 95°C for 30 min and were blocked with goat serum for 1 h. The slices were then incubated with primary Abs at 4°C overnight and with HRP-conjugated secondary Abs at 37°C for 45 min. 3,3′-Diaminobenzidine (Jiehao Biotechnology) was used for color development. The slides were examined under the microscope, and representative images were captured. For each slide, five visual fields were randomly selected at 400×. The anti-TRIM37, anti-Ki-67, and anti-MMP-9 Abs were purchased from Abcam. Image-Pro Plus 6.0 (Media Cybernetics) was used to analyze the captured images. Average staining score of five fields was calculated by the software.
2.7 Western blot analysis
Cells were harvested and lysed with RIPA (Beyotime) supplemented with PMSF (Beyotime) and phosphatase inhibitors. The protein concentration was quantified with a BCA kit (Beyotime), and 30 μg protein was loaded onto SDS-PAGE gels for electrophoresis and transferred to PVDF membranes. The membranes were blocked in 5% fat-free milk for 1 h at room temperature and incubated in primary Abs at 4°C overnight. The membranes were then incubated with HRP-conjugated secondary Abs (1:4000) (Cell Signaling Technology) for 1 h at room temperature and detected with Immobilon Western substrate (Millipore). The anti-TRIM37, anti-MMP-9, anti-N-cadherin, anti-Bcl-xl, anti-Bax, anti-Bcl-2, anti-cleaved caspase-3, and anti-cleaved PARP Abs were purchased from Abcam. The anti-TRAF2, anti-P-IKB, anti-IKB, anti-P-IKK, anti-IKK Abs were purchased from Cell Signaling Technology. All primary Abs were monoclonal and applied at a dilution of 1:1000. The relative protein expression levels were normalized to the GAPDH (Abcam) level. All experiments were repeated three times.
2.8 Transwell assay
Cell migration and invasion were detected by Transwell assay. Briefly speaking, in the migration assay, transfected cells were resuspended in 200 μl FBS-free medium and were implanted into the upper chambers (purchased from BD Biosciences) of 24-well plates at a density of 105 cells. For the invasion assay, the upper chambers were embedded with 50 μl Matrigel (BD Biosciences). The lower chambers were loaded with RPMI-1640 with 20% FBS. Forty-eight hours later, the cells were fixed with 4% paraformaldehyde, stained with 1% crystal violet, and rinsed in PBS three times. The migrated or invaded cells were counted under an inverted microscope. Five visual fields were randomly selected for each chamber. All experiments were repeated in triplicate.
2.9 Immunoprecipitation
The transfected cells were lysed with lysis buffer for 20 min and placed into the ultrasonic crusher. The cell lysate was centrifuged at 12,000 g for 30 min at 4°C. Cell lysate (500 μl) and 5 μg Ab of target proteins were added into a new Eppendorf tube. At the same time, nonspecific homologous Ab was set as the control group. The Eppendorf tubes were mixed gently and incubated at 4°C overnight. Protein A and Protein G (5 μl each) were added into tubes and mixed gently for 3 h. After centrifugation, the supernatant was discarded and the precipitate was retained and washed with Wash Buffer. Lysate was resuspended with 20–40 μl loading buffer and heated at 95°C for 5 min. Western blot analysis was undertaken to detect the protein.
2.10 Ubiquitination detection
Tumor necrosis factor-α (10 ng/ml) was added to transfected cells. The TRAF2 plasmid and pCMV-HA-Ub plasmid were cotransfected into cells. After 24 h, proteasome inhibitor MG-132 (20 μmol/L) was added. The protein was collected and immunoprecipitated with anti-TRAF2 Ab. The level of ubiquitination was detected by western blot.
2.11 Statistical analysis
SPSS 16.0 (IBM) was used to analyze the data. The quantitative data were expressed as mean ± SD. Comparisons between multiple groups were undertaken by one-way ANOVA. Comparisons between two groups were undertaken by two-tailed Student’s t-test, and comparisons of two constituent ratios in the classification data was carried out by χ2-test. p < 0.05 was considered statistically significant.
3 RESULTS
3.1 Expression level of TRIM37 was significantly higher in epithelial ovarian cancer than in normal ovarian tissues
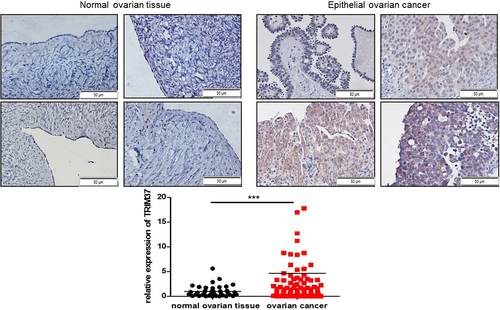
To compare the expression level of TRIM37 between epithelial ovarian cancer tissues and normal ovarian tissues, we collected 60 cases of epithelial ovarian cancer tissues and 30 samples of normal ovarian tissues in our hospital. Immunohistochemistry was used to detect the expression level of TRIM37. We found that the expression level of TRIM37 was significantly higher in epithelial ovarian cancer tissues than in normal ovarian tissues (p < 0.001) (Figure 1).
3.2 High level of TRIM37 correlated with high level of Ki-67 and MMP-9
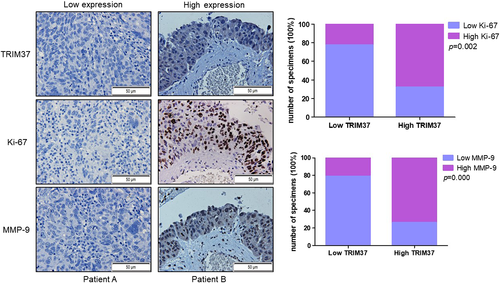
To investigate whether the level of TRIM37 was correlated with tumor proliferation marker Ki-67 and invasion marker MMP-9, we detected the expression of Ki-67 and MMP-9 simultaneously in continuous ovarian cancer tissues of patients. Data showed that the expression of TRIM37 was positively with the level of Ki-67 (p = 0.002) and MMP-9 (p = 0.000) (Figure 2).
3.3 Overexpression of TRIM37 promoted proliferation of epithelial ovarian cancer cell lines
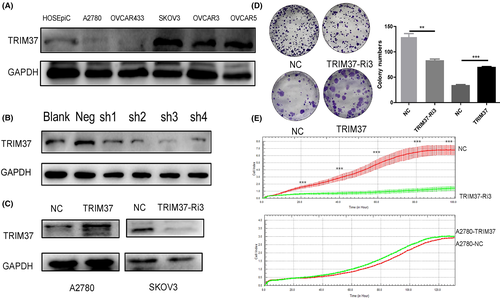
Five common ovarian cancer cell lines, A2780, OVCAR433, SKOV3, OVCAR3, and OVCAR5, were selected to detect the expression of TRIM37 by western blot analysis. As indicated in Figure 3, TRIM37 expression is lowest in A2780 cells and highest in SKOV3. To verify the biological role of TRIM37 in epithelial ovarian cancer, we constructed a TRIM37-overexpressing A2780 cell line and TRIM37-knockdown epithelial ovarian SKOV3 cancer cell line. Colony formation test (Figure 3D) and RTCA proliferation test (Figure 3E) showed that overexpression of TRIM37 promoted the proliferation of epithelial ovarian cancer cell lines while the knockdown of TRIM37 inhibited that compared with respective controls (p < 0.05) (Figure 3).
3.4 Overexpression of TRIM37 promoted migration and invasion of epithelial ovarian cancer cell lines
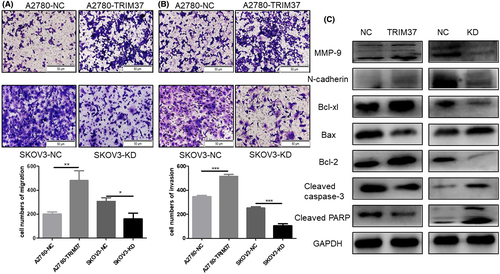
Transwell assay was undertaken to clarify the influence of the expression of TRIM37 on the migration and invasion of epithelial ovarian cancer cell lines. Overexpression of TRIM37 obviously stimulated the migration and invasion of ovarian cancer cell lines, whereas the knockdown of TRIM37 inhibited migration and invasion (p < 0.05) (Figure 4A,B). Next, we detected apoptosis- and invasion-associated proteins in TRIM37-overexpressing or TRIM37-knockdown epithelial ovarian cancer cell lines. Results from western blot analysis showed that the expression of MMP-9 and N-cadherin were upregulated after TRIM37 was overexpressed. Additionally, the antiapoptosis proteins Bcl-xl and Bcl-2 were upregulated in TRIM37-overexpression cells while cleaved caspase-3 and cleaved PARP were downregulated (Figure 4C). To sum up, TRIM37 played an initial role in the proliferation and metastasis of epithelial ovarian cancer. In our previous study, we found that metformin inhibited the proliferation, migration, and invasion.4, 17 We wondered whether the antitumor effect of metformin might partially depend on the regulation of TRIM37.
3.5 Nuclear factor-κB signaling pathway activated by TRIM37 in epithelial ovarian cancer
It was reported that TRIM37 promoted the progression of lung cancer by activating the NF-κB signaling pathway. In this study, we found that overexpression of TRIM37 stimulated the NF-κB signaling pathway (Figure 5A). Furthermore, PDTC (50 μM), an inhibitor of the NF-κB signaling pathway, reversed the upregulated N-cadherin, MMP-9, Bcl-xl, and Bcl-2 and downregulated cleaved PARP (Figure 5B). In contrast, LPS (100 ng/ml), an activator of the NF-κB signaling pathway, played opposite roles (Figure 5C). We drew a preliminary conclusion that TRIM37 can activate the NF-κB pathway in epithelial ovarian cancer, and promote the malignant biological behavior of epithelial ovarian cancer partly depending on this pathway.
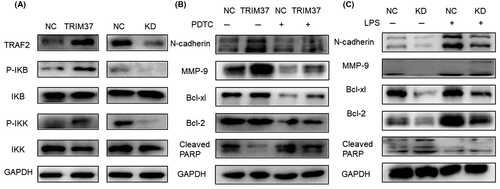
3.6 Metformin inhibited the NF-κB signaling pathway by downregulating TRIM37
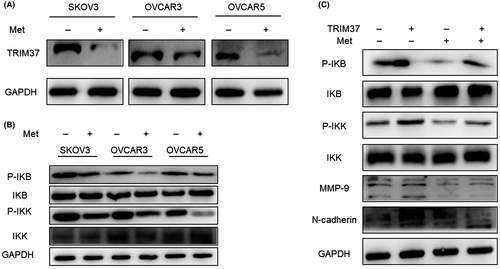
To explore whether metformin could regulate TRIM37, western blot analysis was undertaken and we found that metformin (20 mM) downregulated TRIM37 significantly (Figure 6A). Furthermore, results showed that metformin inhibited NF-κB signaling pathway in SKOV3, OVCAR3, and OVCAR5 ovarian cancer cell lines (Figure 6B). Next, metformin was added to TRIM37-overexpressing cells and we found that metformin reversed the NF-κB signaling pathway, MMP-9, and N-cadherin, which were upregulated by TRIM37 (Figure 6C).
3.7 Metformin inhibited ubiquitination of TRAF2 induced by TRIM37 overexpression
It was reported that TRIM37 contains TRAF fragments, which can be combined with the same fragment on homologous or heterogenous protein.14 An important protein in the NF-κB pathway activated by TNF-α, TRAF2 contains a fragment of TRAF. Immunoprecipitation assay was used to detect the binding of TRIM37 and TRAF2 in epithelial ovarian cancer cell lines. The results showed that TRAF2 was detected in the pull-down proteins of TRIM37-overexpression cells; TRIM37 was also detected in the pull-down proteins of TRAF2-overexpression cells (Figure 7A). Therefore, we concluded that TRIM37 bound to TRAF2 in epithelial ovarian cancer cell lines.
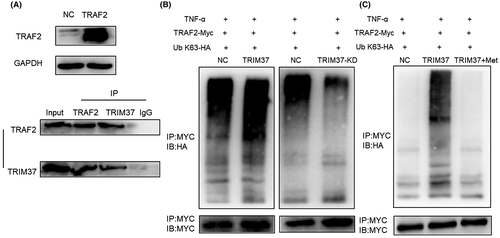
Tumor necrosis factor receptor-associated factor 2 and RIP could be rapidly recruited to form a complex and labeled by ubiquitin when TNF-α stimulates the NF-κB pathway, which is a key step in the activation of NF-κB pathway. Tripartite motif-containing 37 is a member of the ubiquitin ligase family and can bind TRAF2. Therefore, we speculated whether TRIM37 is involved in the ubiquitination of TRAF2. To verify this idea, we cotransfected pCMV-HA-Ub plasmid and TRAF2-overexpression plasmid into TRIM37-overexpression and TRIM37-knockdown epithelial ovarian cancer cell lines, respectively. Tumor necrosis factor-α was added to stimulate the NF-κB pathway and MG132 was added to inhibit protein degradation. Results showed that after overexpression of TRIM37, the ubiquitination level of TRAF2 increased. On the contrary, after interfering with TRIM37, the ubiquitination level of TRAF2 decreased (Figure 7B). Can metformin affect the ubiquitination of TRIM37 on TRAF2? Metformin was added to the pCMV-HA-Ub and TRAF2-overexpression plasmid cotransfection system of TRIM37-overexpression cell lines. We found that metformin significantly inhibited TRAF2 ubiquitination induced by TRIM37 (Figure 7C). Through the above experiments, we concluded that metformin inhibited the ubiquitination degradation of TRAF2 induced by TRIM37, and then inhibited epithelial ovarian cancer.
4 DISCUSSION
As early as 1986, Sen and Baltimore18 detected for the first time a nuclear protein factor from B lymphocyte extracts that was able to specifically bind to the Igκ light chain gene enhancer κB sequence. The factor was called NF-κB. Studies have shown that after NF-κB is activated, it can regulate the transcription of genes related to immunity, inflammation, and cell proliferation.19, 20 Persistently high expression of NF-κB could be found in a variety of malignancies, including diffuse large-cell lymphoma21 and non-small-cell lung cancer.22 In addition, NF-κB activation is associated with tumor metastasis and promotes the transcriptional expression of a variety of factors, including vascular endothelial growth factor, urokinase plasminogen activator, and MMP.23, 24 E3 ubiquitin ligase plays a key role in the ubiquitin proteasome system. E3 isolated by differential display is a ubiquitin ligase of the HECT family that manifests as mutation or overexpression in several tumors, including ovarian cancer. Highly expressed EDD is positively associated with recurrence in patients with serous ovarian cancer.25 Knocking down EDD in ovarian cancer cells can induce apoptosis and increase the sensitivity of ovarian cancer cells to cisplatin.26
The NF-κB pathway plays an important role in tumor generation and progression, and almost every step of activation of this pathway is regulated by ubiquitin modifications.27, 28 When the NF-κB pathway is activated by TNF-α, TRAFs and RIPs are rapidly ubiquitinated, which causes the recruitment and activation of TGF-β-activated kinase 1 (TAK1) and IKK complexes.29, 30 IκBα is then phosphorylated by the activated IKK complex. Phosphorylated IκBα is degraded by ubiquitination which promoted nuclei translocation and activation of NF-κB.31, 32 In the whole process of activation of the NF-κB pathway, ubiquitination of TRAF2 is the most critical step.33, 34 Tada et al.35 constructed TRAF2 and TRAF5 double KO mice and found that TNF- but not interleukin-1-induced nuclear translocation of NF-κB was severely impaired in MEFs derived from double KO mice. Double KO MEFs were more susceptible to TNF-induced cytotoxicity than TRAF2 KO MEFs. These results proved TRAF2 plays a pivotal role in TNF-induced activation of the NF-κB pathway and protection from cell death. By generating TRAF2 and TRAF3 KO mice, Vallabhapurapu et al.33 found that TRAF2 and TRAF3 in a ubiquitination cascade activates NIK-dependent alternative NF-κB signaling. A member of the TRIM protein family, TRIM37, has the function of ubiquitin ligase and has been reported to play a procancer role in a variety of tumors, including colorectal cancer36 and liver cancer.37 However, the clinical significance and molecular mechanism of TRIM37 in ovarian cancer are not well understood.
In this experiment, we showed that TRIM37 is highly expressed in ovarian cancer tissues compared with normal ovarian tissues. High levels of TRIM37 were positively correlated with expression levels of Ki-67 and MMP-9. Overexpression of TRIM37 promoted ovarian cancer cell line migration and invasion. In subsequent experiments, we found TRIM37 could activate the NF-κB pathway and influence the expression of apoptosis-, invasion-related proteins partially depending on the NF-κB pathway. Studies have shown that metformin can inhibit tumor by changing epigenetic modification. It was reported that AMPK could phosphorylate histone methyltransferase EZH2 and influence with the interaction between EZH2 and PRC2, thus blocking the methylation of histone H3.38 As a result, PRC2-targeted tumor suppressor genes were upregulated. Metformin is a confirmed activator of AMPK. Therefore, metformin can inhibit cancer through this mechanism.
To further explore whether metformin inhibits ovarian cancer by modulating TRIM37 and thereby inhibiting the NF-κB pathway, we found that metformin downregulated TRIM37. Metformin treatment of TRIM37-overexpressing ovarian cancer cells reversed the activation of the NF-κB pathway and downregulated invasion-related proteins induced by TRIM37 overexpression. Considering that ubiquitination of TRAF2 is a critical step in the activation of the NF-κB pathway, we speculated whether TRIM37 can interact with TRAF2 and thus ubiquitinate TRAF2. We cotransfected ubiquitin and TRAF2 in TRIM37-overexpressing and TRIM37-downreguled epithelial ovarian cancer cell lines and, using immunoprecipitation technology, found that TRIM37 interacted with TRAF2. After TRIM37 overexpression, the ubiquitination level of TRAF2 increased, while after interfering with TRIM37, the ubiquitination level of TRAF2 decreased. To verify whether metformin regulates the NF-κB pathway by influencing the ubiquitination of TRAF2, metformin was added to TRIM37-overexpressed cells and we found that metformin significantly reduced the ubiquitination level of TRAF2 induced by TRIM37.
In summary, this present study revealed that TRIM37 was overexpressed in ovarian cancer and was significantly associated with metastasis. In addition, TRIM37 could promote ovarian cancer cell invasion and metastasis by activation of the NF-κB pathway. Metformin inhibited ovarian cancer by depressing the ubiquitination of TRAF2 induced by TRIM37.
AUTHOR CONTRIBUTIONS
Hong Sun conceived and designed the experiments. Ya Zheng and Haiyan Zhang completed the experiments and explained the experimental data. Ya Zheng and Haiyan Zhang participated in the drafting of the manuscript, integrated all experimental data for data analysis and statistical analysis, and calibrated the publication of the final version. All authors confirmed the authenticity of all the raw data and have read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by the Science and Technology Commission of Shanghai Municipality (No. 16411963600) to Hong Sun.
ETHICAL APPROVAL
Approval of the research protocol by an institutional review board: N/A.
Informed consent: N/A.
Registry and registration no. of the study/trial: N/A.
Animal studies: N/A.
DISCLOSURE
None.




