Soft palatal melanosis, a simple predictor for neoplasia in the upper aerodigestive tract in Japanese alcoholic men
Funding Information
None.
Abstract
Soft palatal melanosis can be detected by visual inspection during routine physical examination or even personally in a mirror. The aim of this study was to evaluate the association between squamous cell neoplasia in the upper aerodigestive tract (UAT) and soft palatal melanosis. We reviewed digitized records of high-quality endoscopic images of the soft palate of 1786 Japanese alcoholic men who underwent endoscopic screening. Soft palatal melanosis was observed in 381 (21.3%) of the subjects (distinct, 6.3%). Older age, an inactive heterozygous aldehyde dehydrogenase-2 genotype, smoking, and a high mean corpuscular volume were positively associated with the presence of soft palatal melanosis. The age-adjusted odds ratio (95% confidence interval) for UAT neoplasia was 1.92 (1.40–2.64) in the group with melanosis and 2.51 (1.55–4.06) in the group with distinct melanosis, compared with the melanosis-free group. A multivariate analysis showed that the presence of soft palatal melanosis was independently associated with a high risk of UAT neoplasia. We calculated the individual number of risk factors out of four easily identifiable and significant factors: age ≥55 years, current/former alcohol flushing, mean corpuscular volume ≥106 fL, and distinct soft palatal melanosis. Compared with the risk-factor-free condition, the odds ratio (95% confidence interval) values of UAT neoplasia for one, two, three, and four risk factors were 1.49 (0.97–2.30), 3.14 (2.02–4.88), 4.80 (2.71–8.51), and 7.80 (2.17–28.1), respectively. The presence of soft palatal melanosis provides a simple new strategy for identifying heavy drinkers with a high risk for UAT neoplasia.
Melanosis in the UAT (oral cavity, pharynx, larynx, and esophagus) is characterized by flat, dark-pigmented (greenish-black) areas. We previously reported a high prevalence of melanosis in the UAT in Japanese alcoholic men and found that subjects with melanosis have a high risk of esophageal dysplasia and SCC in the UAT.1 The presence of esophageal dysplasia with a diameter of 5 mm or more is a good predictor of a high risk of the future development of SCC in the UAT.2
As melanosis of the soft palate can be detected by ocular inspection using a lighting apparatus during physical or dental examinations, and even personally by looking in a mirror, it may be useful as a marker for identifying individuals with a high risk of neoplasia in the UAT.
Genetic polymorphisms of ADH1B (rs1229984) and ALDH2 (rs671) modify the elimination of ethanol and acetaldehyde, alcohol flushing responses, and susceptibility to alcoholism in East Asians.3 The inactive heterozygous ALDH2 encoded by ALDH2*1/*2 and the slow-metabolizing ADH1B encoded by ADH1B*1/*1 increase the risk of esophageal dysplasia and SCC in the UAT in East Asian drinkers.4-7
Current or former facial flushing after a small amount of alcohol, as evaluated using a simple flushing questionnaire that we previously devised, is a good marker of inactive ALDH2 and is useful for predicting the individual risk of UAT cancer.2, 8 Marked macrocytosis of the MCV ≥106 fL and age (>55 years) have been reported to be high-risk markers for alcoholism-related SCC in the UAT.2, 9
This study was designed to: (i) attempt to identify factors that may contribute to the development of soft palatal melanosis; and (ii) evaluate the association between UAT neoplasia and the degree of soft palatal melanosis in combination with the ADH1B and ALDH2 genotypes, the results of a flushing questionnaire, an MCV ≥106 fL, and other risk factors in Japanese alcoholic men.
Materials and Methods
At the Kurihama Medical and Addiction Center, we routinely use a regimented cancer-screening program consisting of endoscopy combined with oropharyngolaryngeal inspection and esophageal iodine staining in alcoholic men. The screening program and diagnostic procedure used in the present study have been described in our previous report.7 The proposal for this study was reviewed by the ethics committee of the center, and written informed consent was obtained from all participating subjects.
Subjects
The reference population consisted of 1821 Japanese alcoholic men (aged 40 years or older) with no history of cancer of the UAT who had undergone endoscopic screening at the center for the first time between 2006 and 2011. After excluding 35 subjects for whom digitized records of high-quality endoscopic imaging of the palate were not available, 1786 patients were selected as subjects in the present study. Information regarding the patients’ drinking and smoking habits was obtained from the patients themselves and, when available, their partners. Daily alcohol consumption during the preceding year was expressed in grams of ethanol per day using a standard conversion for alcohol beverages. Beer was assumed to be 5% ethanol (v/v); wine, 12%; sake, 16%; shochu, 25%; and whiskey, 40%. All the alcoholics met the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria for alcohol dependence.10
Endoscopic assessment of melanosis in the soft palate
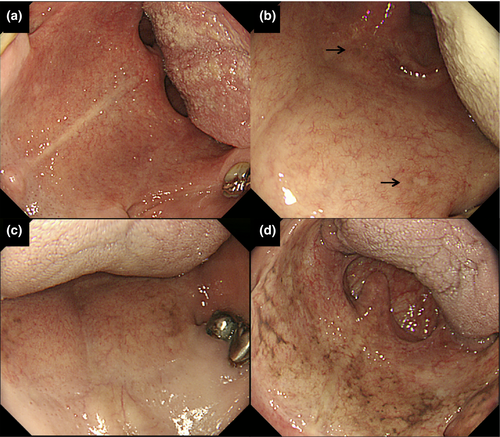
The endoscopy examinations were carried out using an Olympus XQ230, Q240, Q240Z, or Q260 panendoscope (Olympus Optical, Tokyo, Japan). The endoscopic screening and histologic diagnosis procedures for neoplasia in the UAT have been described elsewhere.7 Before insertion of the endoscope into the pharynx, we asked each patient to open his mouth wide and the soft palate was examined using the endoscope. Careful attention was given to the right and left lateral zones of the soft palate adjacent to the hard palate, the most frequent sites of melanosis, and a digitized record of high-quality endoscopic images of the soft palate was made using the medical imaging communication system. A retrospective assessment of the endoscopic images of the soft palate was determined in a joint review by four expert endoscopists (K.H., A.Y., R.N., and T.O.), and the degree of palate melanosis (Fig. 1) was classified as follows: (−), absent; (1+), mild melanosis; (2+), distinct but localized melanosis; and (3+), distinct and diffuse melanosis.
ADH1B and ALDH2 genotyping
ADH1B and ALDH2 genotyping using PCR-RFLP methods was undertaken using lymphocyte DNA samples from 1532 subjects.7
Simple flushing questionnaire
Each subject was asked to complete a simple questionnaire concerning his flushing response to alcohol:8 (i) Do you have a tendency to flush in the face immediately after drinking a glass of beer? (yes, no, or unknown); and (ii) Did you have a tendency to flush in the face immediately after drinking a glass of beer during the 1–2 years after you started drinking? (yes, no, or unknown). Individuals who answered “yes” to question (i) were designated as “current flushing,” while those who answered “no” or “unknown” to question (i) and “yes” to question (ii) were designated as “former flushing.” The remaining subjects were classified as “never flushing.”
Mean corpuscular volume
During each subject's initial visit to the center for the treatment of alcoholism, we measured MCV using the electrical impedance method with an autoanalyzer (CELL-DYN 3500; Abbott, North Chicago, IL, USA). We dichotomized the results into an MCV <106 fL group and an MCV ≥106 fL group, as macrocytosis with an MCV value ≥106 fL was found to be associated with an increased risk of SCC in the UAT in our previous studies in alcoholics.2, 9
Statistical analysis
Values were expressed as mean and standard deviation or percentage. P-values for categorical data were calculated using the Cochran–Mantel–Haenszel test for homogeneity for smoking and for the trends in other variables. The P-values for trends in the mean values were calculated using a general linear model. Age-adjusted and multivariate odds ratios (ORs) and the 95% confidence interval (CI) were calculated using multivariate models. Statistical significance was defined as a P-value of <0.05. All the statistical analyses were carried out using the SAS software program (version 9.4; SAS Institute, Cary, NC, USA).
Results
Melanosis of the soft palate was observed in 381 (21.3%) of the 1786 subjects. Almost all the subjects with soft palatal melanosis had a pigmented area in at least one lateral zone of the soft palate adjacent to the hard palate. The degree of soft palatal melanosis was classified as 1+ (mild) in 268 subjects (15.0%), 2+ (distinct but localized) in 93 subjects (5.2%), and 3+ (distinct and diffuse) in 20 subjects (1.1%). Soft palatal melanosis was positively associated with older age, smoking, enlarged MCV, an inactive heterozygous ALDH2 genotype, and current or former flushing (Table 1).
| Total | Soft palatal melanosis | P-value | ||||
|---|---|---|---|---|---|---|
| Absence | 1+ | 2+ | 3+ | |||
| n (%) | 1786 | 1405 (78.7) | 268 (15.0) | 93 (5.2) | 20 (1.1) | |
| Age, years, n (%) | ||||||
| 40–49 | 506 | 422 (83.4) | 62 (12.3) | 19 (3.8) | 3 (0.6) | 0.0100 |
| 50–59 | 599 | 467 (78.0) | 90 (15.0) | 37 (6.2) | 5 (0.8) | |
| 60–69 | 467 | 346 (74.1) | 85 (18.2) | 28 (6.0) | 8 (1.7) | |
| ≥70 | 214 | 170 (79.4) | 31 (14.5) | 9 (4.2) | 4 (1.9) | |
| Mean ± SD | 56.7 ± 10.1 | 56.4 ± 10.3 | 57.6 ± 9.4 | 57.2 ± 8.8 | 60.5 ± 10.3 | 0.0270 |
| Alcohol consumption, g ethanol/day (n = 1777) | ||||||
| Mean ± SD | 121.9 ± 80.0 | 121.9 ± 79.9 | 120.1 ± 71.5 | 129.6 ± 107.6 | 114.4 ± 38.8 | 0.8200 |
| Smoking, cigarettes/day, n (%) | ||||||
| Never-smoker | 182 | 163 (89.6) | 9 (4.9) | 9 (4.9) | 1 (0.5) | <0.0001 |
| Ex-smoker (≥5 years ago) | 227 | 194 (85.5) | 28 (12.3) | 4 (1.8) | 1 (0.4) | |
| 1–19 | 422 | 330 (78.2) | 66 (15.6) | 22 (5.2) | 4 (0.9) | |
| ≥20 | 955 | 718 (75.2) | 165 (17.3) | 58 (6.1) | 14 (1.5) | |
| Mean ± SD | 16.3 ± 12.9 | 15.7 ± 13.0 | 18.6 ± 12.0 | 18.8 ± 12.5 | 18.9 ± 11.0 | 0.0003 |
| MCV, fL, n (%) | ||||||
| <106 | 1286 | 1038 (80.7) | 178 (13.8) | 58 (4.5) | 12 (0.9) | 0.0006 |
| ≥106 | 500 | 367 (73.4) | 90 (18.0) | 35 (7.0) | 8 (1.6) | |
| Mean ± SD | 101.5 ± 8.5 | 101.0 ± 8.4 | 102.7 ± 8.9 | 103.8 ± 7.9 | 104.5 ± 10.3 | <0.0001 |
| ADH1B genotype, n (%) (n = 1532) | ||||||
| *1/*1 | 423 | 326 (77.1) | 69 (16.3) | 23 (5.4) | 5 (1.2) | 0.6200 |
| *1/*2 | 510 | 407 (79.8) | 72 (14.1) | 26 (5.1) | 5 (1.0) | |
| *2/*2 | 599 | 460 (76.8) | 94 (15.7) | 36 (6.0) | 9 (1.5) | |
| ALDH2 genotype, n (%) (n = 1532) | ||||||
| *1/*1 | 1293 | 1031 (79.7) | 189 (14.6) | 61 (4.7) | 12 (0.9) | <0.0001 |
| *1/*2 | 239 | 162 (67.8) | 46 (19.2) | 24 (10.0) | 7 (2.9) | |
| Flushing questionnaire, n (%) (n = 1776) | ||||||
| Never flushing | 1435 | 1145 (79.8) | 204 (14.2) | 71 (4.9) | 15 (1.0) | 0.0380 |
| Current or former flushing | 341 | 253 (74.2) | 62 (18.2) | 21 (6.2) | 5 (1.5) | |
- P-values for categorical data were determined using the Cochran–Mantel–Haenszel test for homogeneity for smoking and for trends in other variables. P-values for the trends of mean values were determined using a general linear model. Melanosis classification: 1+, mild melanosis; 2+, distinct but localized melanosis; 3+, distinct and diffuse melanosis. ADH1B; alcohol dehydrogenase-1B; ALDH2, aldehyde dehydrogenase-2; MCV, mean corpuscular volume.
A multivariate analysis showed that older age, inactive heterozygous ALDH2 genotype, smoking, and an MCV ≥106 fL were positive determinants for the presence of soft palatal melanosis (OR [95% CI] = 1.20 [1.05–1.36], 1.70 [1.17–2.49], 1.21 [1.10–1.33], and 1.36 [1.03–1.78], respectively; Table 2).
| Soft palatal melanosis, presence versus absence | ||
|---|---|---|
| OR (95% CI) | P-value | |
| Age, per +10 yearsa | 1.20 (1.05–1.36) | 0.0070 |
| ADH1B | ||
| *2 carrier | 1.00 (ref.) | |
| *1/*1 | 1.14 (0.87–1.51) | 0.3500 |
| ALDH2 | ||
| *1/*1 | 1.00 (ref.) | |
| *1/*2 | 1.70 (1.17–2.49) | 0.0060 |
| Flushing questionnaire | ||
| Never flushing | 1.00 (ref.) | |
| Current or former flushing | 0.98 (0.68–1.40) | 0.9000 |
| Alcohol consumption, per +22 g ethanol/daya | 1.00 (0.96–1.03) | 0.7900 |
| Smoking, per +10 cigarettes/daya | 1.21 (1.10–1.33) | 0.0001 |
| MCV | ||
| <106 fL | 1.00 (ref.) | |
| ≥106 fL | 1.36 (1.03–1.78) | 0.0280 |
- a An increment of 10 years in age, 22 g of ethanol/day, and 10 cigarettes/day increased the risk by 1.20-fold, 1.00-fold, and 1.21-fold, respectively. The multivariate odds ratios (OR) were estimated using a logistic regression model, where all the variables were entered into the model. CI, confidence interval; MCV, mean corpuscular volume; ref., reference.
Table 3 shows the detection of neoplasia in the esophagus and oropharyngolarynx according to the degree of soft palatal melanosis. Esophageal distinct iodine-unstained lesions (EDIUL) ≥5 mm were observed in 246 subjects (13.8%). Targeted biopsies of EDIULs ≥5 mm were performed in 219 subjects. The main reasons for not performing a biopsy were the presence of a bleeding tendency and/or liver cirrhosis. The biopsy specimens from 18 subjects were diagnosed as “inadequate specimens,” “indefinite for dysplasia,” or “non-dysplasia.” Esophageal dysplasia without esophageal SCC was observed in 142 subjects (8.0%) and esophageal SCC was observed in 59 subjects (3.3%). Oropharyngolaryngeal SCC was observed in 20 subjects (1.1%), four of whom had esophageal dysplasia, and seven of whom had esophageal SCC. Altogether, SCC in the UAT was observed in 72 subjects (4.0%), and neoplasia in the UAT was observed in 210 subjects (11.8%). When the 1531 subjects without an EDIUL ≥5 mm or SCC in the UAT were used as a control group, the degree of soft palatal melanosis was associated with esophageal dysplasia, esophageal SCC, SCC in the UAT, and neoplasia in the UAT.
| Total | Soft palatal melanosis | P-valuea | ||||
|---|---|---|---|---|---|---|
| Absence | 1+ | 2+ | 3+ | |||
| No EDIUL ≥5 mm and no SCC in the UAT | 1531 | 1232 (80.5) | 215 (14.0) | 71 (4.6) | 13 (0.8) | Ref.a |
| Esophageal dysplasia | 142 | 95 (66.9) | 29 (20.4) | 14 (9.9) | 4 (2.8) | <0.0001 |
| Esophageal SCC | 59 | 40 (67.8) | 12 (20.3) | 4 (6.8) | 3 (5.1) | 0.0030 |
| Oropharyngolaryngeal SCC | 20 | 14 (70.0) | 3 (15.0) | 2 (10.0) | 1 (5.0) | 0.0670 |
| SCC in the UAT | 72 | 49 (68.1) | 14 (19.4) | 6 (8.3) | 3 (4.2) | 0.0020 |
| Neoplasia in the UAT | 210 | 142 (67.6) | 43 (20.5) | 18 (8.6) | 7 (3.3) | <0.0001 |
- a P-values are for the trend versus no esophageal distinct iodine-unstained lesion (EDIUL) ≥5 mm and no squamous cell carcinoma (SCC) in the upper aerodigestive tract (UAT). Of the 20 subjects with oropharyngolaryngeal SCC, four had esophageal dysplasia and seven had esophageal SCC. Melanosis classification: 1+, mild melanosis; 2+, distinct but localized melanosis; 3+, distinct and diffuse melanosis. Ref., reference.
We obtained age-adjusted ORs of 2.69 (1.54–4.67) for esophageal dysplasia, 2.47 (1.07–5.69) for esophageal SCC, 2.60 (1.23–5.48) for SCC in the UAT, and 2.51 (1.55–4.06) for neoplasia in the UAT in the group with 2+/3+ melanosis, compared with the melanosis-free group, while the corresponding ORs in the group with 1+/2+/3+ melanosis were slightly lower (OR [95% CI] = 1.98 [1.36–2.88], 1.88 [1.07–3.30], 1.87 [1.12–3.12], and 1.92 [1.40–2.64], respectively; Table 4). When the group with absence/1+ melanosis was used as a reference group, the group with 2+/3+ melanosis showed age-adjusted ORs of 2.43 (1.41–4.19) for esophageal dysplasia, 2.39 (1.14–4.97) for SCC in the UAT, and 2.27 (1.41–3.65) for neoplasia in the UAT (Table 4).
| Soft palatal melanosis | Results of endoscopic screening, age-adjusted odds ratio (95% CI), P-value | ||||
|---|---|---|---|---|---|
| Esophageal dysplasia | Esophageal SCC | Oropharyngo-laryngeal SCC | SCC in the UAT | Neoplasia in the UAT | |
| 1+/2+/3+ vs. absence | 1.98 (1.36–2.88), 0.0003 | 1.88 (1.07–3.30), 0.0280 | 1.70 (0.65–4.48), 0.2800 | 1.87 (1.12–3.12), 0.0170 | 1.92 (1.40–2.64), <0.0001 |
| 2+/3+ vs. absence | 2.69 (1.54–4.67), 0.0005 | 2.47 (1.07–5.69), 0.0340 | 2.98 (0.84–10.6), 0.0920 | 2.60 (1.23–5.48), 0.0120 | 2.51 (1.55–4.06), 0.0002 |
| 2+/3+ vs. absence/1+ | 2.43 (1.41–4.19), 0.0010 | 2.24 (0.98–5.09), 0.0550 | 2.92 (0.84–10.19), 0.0930 | 2.39 (1.14–4.97), 0.0200 | 2.27 (1.41–3.65), 0.0007 |
- The age-adjusted odds ratio was estimated using a logistic regression model. Melanosis classification: 1+, mild melanosis; 2+, distinct but localized melanosis; 3+, distinct and diffuse melanosis. CI, confidence interval; SCC, squamous cell carcinoma; UAT, upper aerodigestive tract.
A multivariate analysis showed that the presence of soft palatal melanosis of grade 1+ (OR [95% CI] = 1.58 [1.05–2.38]) or grade 2+/3+ (2.03 [1.20–3.43]) was independently associated with a higher risk of neoplasia in the UAT (Table 5). A higher risk of neoplasia in the UAT was similarly identified for age (1.34 [1.13–1.60]), ADH1B*1/*1 genotype (2.41 [1.71–3.39]), ALDH2*1/*2 genotype (3.52 [2.47–5.01]), and an MCV ≥106 fL (1.95 [1.39–2.75]).
| Neoplasia in the UAT versus controla | ||
|---|---|---|
| OR (95% CI) | P-value | |
| Age, per +10 years | 1.34 (1.13–1.60) | 0.0007 |
| ADH1B | ||
| *2 carrier | 1.00 (ref.) | |
| *1/*1 | 2.41 (1.71–3.39) | <0.0001 |
| ALDH2 | ||
| *1/*1 | 1.00 (ref.) | |
| *1/*2 | 3.52 (2.47–5.01) | <0.0001 |
| Alcohol consumption, per +22 g ethanol/day | 1.00 (0.95–1.05) | 0.8900 |
| Smoking, per +10 cigarettes/day | 1.10 (0.96–1.25) | 0.1700 |
| MCV | ||
| <106 fL | 1.00 (ref.) | |
| ≥106 fL | 1.95 (1.39–2.75) | <0.0001 |
| Soft palatal melanosis | ||
| Absence | 1.00 (ref.) | |
| 1+ | 1.58 (1.05–2.38) | 0.0280 |
| 2+/3+ | 2.03 (1.20–3.43) | 0.0090 |
- a Control: no esophageal distinct iodine-unstained lesion ≥5 mm and no SCC in the UAT. The multivariate ORs were estimated using a logistic regression model, where all the variables were entered into the model. Melanosis classification: 1+, mild melanosis; 2+, distinct but localized melanosis; 3+, distinct and diffuse melanosis. CI, confidence interval; MCV, mean corpuscular volume; ref., reference.
When the multivariate analysis included the results of the flushing questionnaire and excluded the ADH1B and ALDH2 genotypes, the presence of soft palatal melanosis of grade 1+ (1.51 [1.03–2.21]) or of grade 2+/3+ (2.24 [1.37–3.66]) was similarly associated with a higher risk of neoplasia in the UAT. Increased risks of neoplasia in the UAT were also identified for age (OR = 1.37; 95% CI, 1.17–1.60), current or former flushing (1.57 [1.12–2.21]), and an MCV ≥106 fL (1.80 [1.32–2.45]; Table 6).
| Neoplasia in the UAT versus controla | ||
|---|---|---|
| OR (95% CI) | P-value | |
| Age, per +10 years | 1.37 (1.17–1.60) | <0.0001 |
| Flushing questionnaire | Never flushing | 1.00 (ref.) |
| Current or former flushing | 1.57 (1.12–2.21) | 0.0100 |
| Alcohol consumption, per +22 g ethanol/day | 1.00 (0.96–1.05) | 0.9500 |
| Smoking, per +10 cigarettes/day | 1.08 (0.96–1.22) | 0.1800 |
| MCV | ||
| <106 fL | 1.00 (ref.) | |
| ≥106 fL | 1.80 (1.32–2.45) | <0.0001 |
| Soft palatal melanosis | ||
| Absence | 1.00 (ref.) | |
| 1+ | 1.51 (1.03–2.21) | 0.0340 |
| 2+/3+ | 2.24 (1.37–3.66) | 0.0010 |
- a Control: no esophageal distinct iodine-unstained lesion ≥5 mm and no SCC in the UAT.The multivariate ORs were estimated using a logistic regression model, where all the variables were entered into the model. Melanosis classification: 1+, mild melanosis; 2+, distinct but localized melanosis; 3+, distinct and diffuse melanosis. CI, confidence interval; MCV, mean corpuscular volume; ref., reference.
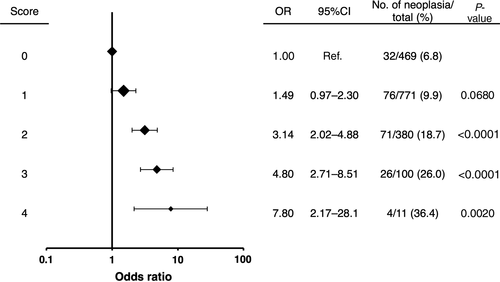
We calculated the individual number of risk factors out of four easily ascertainable and significant factors: age ≥55 years, current/former flushing, MCV ≥106 fL, and soft palatal melanosis of grade 2+/3+ (Fig. 2). The prevalence of neoplasia in the UAT increased as the number of risk factors increased: 6.8% for 0; 9.9% for 1; 18.7% for 2; 26.0% for 3; and 36.4% for 4. The OR (95% CI) for neoplasia in the UAT increased as the number of risk factors increased: 1 for 0; 1.49 (0.97–2.30) for 1; 3.14 (2.02–4.88) for 2; 4.80 (2.71–8.51) for 3; and 7.80 (2.17–28.1) for 4.
Discussion
Many previous East Asian reports have shown that ADH1B and ALDH2 genotyping is a strong tool for identifying drinkers with a high risk of UAT neoplasia, but such knowledge cannot be easily applied for public education or mass-screening at this time. Melanosis of the soft palate was observed in 21.3% of the present alcoholic subjects. The right and left lateral zones of the soft palate adjacent to the hard palate were the most common sites of the melanosis. Mild (1+) melanosis was observed in 15.0% of the subjects, and distinct (2+/3+) melanosis was observed in 6.3%. Soft palatal melanosis was positively associated with UAT neoplasia, and distinct melanosis showed a stronger association. Our earlier small study showed that melanosis of the palate, pharynx, and esophagus is correlated with a high risk of UAT neoplasia,1 but the detection of palate melanosis has a special clinical implication, as the palate can be examined by visual inspection during routine medical or dental examinations, or even by the patient.
A high MCV, which is a traditional biomarker for alcoholism,11 is used as a high-risk marker for UAT cancer among Japanese alcoholics,9 as the MCV of drinkers with inactive ALDH2*2 increases markedly in response to exposure to acetaldehyde; additionally, smoking, a low BMI, and folate deficiency are known to increase the MCV.9 Current or former flushing, which is a surrogate marker for inactive ALDH2*2, is also useful for predicting an individual's risk of UAT cancer,2, 8 although the presence of alcoholism substantially reduces the sensitivity of current or former flushing for detecting inactive ALDH2*2.12 A multivariate analyses undertaken in this study showed that older age, ALDH2*2, MCV ≥106 fL, and smoking were significant determinants of the presence of soft palatal melanosis, suggesting a common etiology for melanosis and UAT neoplasia in these alcoholics. Current or former flushing was not selected as a determinant of the presence of soft palatal melanosis in the model, probably because flushing is a surrogate marker of inactive ALDH2. Furthermore, soft palatal melanosis, especially distinct melanosis, was a strong risk factor for UAT neoplasia independently of the abovementioned risk factors. A combination of distinct soft palatal melanosis with older age, current or former flushing, and an MCV ≥106 fL could provide a simple approach for identifying alcoholics with a very high risk of UAT neoplasia.
Studies of Japanese alcoholics have failed to establish any dose-dependent association between alcohol consumption and UAT neoplasms.2, 7 The same is true for soft palatal melanosis in this study, and the lack of a dose-dependent association may be related to the homogeneity of the study population with regard to their extremely high alcohol consumption. Alcoholics tend to be heavy smokers, and we have reported positive associations between heavy smoking and the risk of UAT neoplasia among Japanese alcoholics.2, 7 Smoking precipitates oral pigmentation,13 and “smoker's melanosis” has been reported to increase with the level of tobacco use.14 Such oral pigmentation may act as a protective barrier against noxious components in tobacco smoke, either through the stimulation of melanin production or the binding of melanin to toxic products, including polycyclic amines and free radicals.13 The results of the present study revealed that smoking is positively associated with “alcoholic's melanosis.” Multivariate analyses selected soft palatal melanosis and an MCV ≥106 fL, but not smoking, as significant determinants for the presence of UAT neoplasia, and these results can be partly explained by the positive association of both melanosis and macrocytosis with smoking.
The International Agency for Research on Cancer concluded that acetaldehyde associated with alcohol consumption is carcinogenic to humans.15 Acetaldehyde levels in saliva after drinking are strikingly higher than in the blood, and acetaldehyde production by oral microorganisms contributes largely to the acetaldehyde level in saliva.16, 17 The salivary acetaldehyde levels of ALDH2*1/*2 heterozygotes are higher than those of ALDH2*1/*1 homozygotes.17, 18 Alcoholic beverages themselves contain high levels of acetaldehyde.18 As one of the major chemical constituents of tobacco smoke, acetaldehyde dissolves in saliva during active smoking and reaches high concentrations.19 As ALDH2 activity is disproportionately lower than the activity of ADH in the UAT,20, 21 the inefficient degradation of acetaldehyde by tissue ALDH2 in the UAT combined with higher acetaldehyde exposure may be critical for local acetaldehyde accumulation in alcoholics, and this condition may be associated with a higher risk of melanosis and neoplasia in the UAT.
Exposure to high local levels of acetaldehyde may be involved in the etiology of epithelial hyperproliferation in the UAT that has been reported in experimental animals.22 Proliferating keratinocytes may play a regulatory role in melanogenesis through paracrine effects.23 Acetaldehyde-induced epithelial proliferation may be at least partly related to increased melanogenesis. Histological examination of melanosis in the UAT has shown that melanin is produced by proliferating melanocytes located in the basal layer of the epithelium and is transferred by dendrites to surrounding keratinocytes and to macrophages and fibroblasts in the mucosal layer. 24, 25
Other aspects of alcoholics’ lifestyles or hereditary traits may be associated with the development of melanosis and neoplasia in the UAT. Chronic alcohol consumption and metabolism result in the generation of several classes of DNA-damaging molecules, including reactive oxygen species and lipid peroxidation products.26 Melanosis in the UAT may function as an antioxidant defense, because melanogenesis in the epidermis is involved in neutralizing reactive oxygen species and prevents damage to cell lipids, proteins, and DNA.27
The present study has several potential limitations. The prevalence of soft palatal melanosis in this study was even higher than the prevalence in our previous study examining alcoholics.1 In addition to this difference in populations, the improvement of endoscopes and the retrospective review of digitized records of high-quality endoscopic images might have contributed to the high detection rate of palate melanosis. To apply the present risk assessment to public education and screening, the grade of palatal melanosis that is actually visible during simple ocular inspection alone should be evaluated. Furthermore, the reference population in this study consisted of Japanese alcoholic men who had sought treatment for alcoholism. The extent to which the present findings are generally applicable must be investigated in additional studies among various East Asian populations. Follow-up studies will also be important for clarifying whether or to what extent the presence of melanosis influences future cancer development.
Our previous endoscopic follow-up study of cancer-free alcoholic men showed that esophageal dysplasia ≥5 mm is a strong predictor of the future development of SCC in the UAT.2 The cumulative rate for SCC in the UAT within 5 years was estimated as 35% in those with esophageal dysplasia ≥5 mm, compared with 4% in those without an EDIUL ≥5 mm.2 Because esophageal dysplasia is seldom diagnosed without using esophageal iodine staining,7 esophageal iodine staining is useful for predicting the risk of the future development of UAT cancer as well as for improving the rate of detection of early esophageal SCC. Treatment for early SCC in the UAT using an endoscopic or endoscope-guided mucosectomy has become a widespread practice in Japan and has succeeded in improving the outcome of this high-mortality cancer.28, 29
A new risk assessment strategy using palatal melanosis might be helpful for persuading high-risk individuals to undergo endoscopic screening or for convincing them to change their lifestyle choices so as to avoid or prevent neoplasms.
Acknowledgments
We thank Sachiko Hara and Minori Tsukahara (Kurihama Medical and Addiction Center) for their expert technical assistance.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
-
- ADH1B
-
- alcohol dehydrogenase-1B
-
- ALDH2
-
- aldehyde dehydrogenase-2
-
- CI
-
- confidence interval
-
- EDIUL
-
- esophageal distinct iodine-unstained lesion
-
- MCV
-
- mean corpuscular volume
-
- OR
-
- odds ratio
-
- SCC
-
- squamous cell carcinoma
-
- UAT
-
- upper aerodigestive tract




