Mesenchymal stem cell-derived CCN2 promotes the proliferation, migration and invasion of human tongue squamous cell carcinoma cells
Funding information
Natural Science Foundation of Guangdong Province, China, (Grant/Award Number: ‘2016A030313196‘) Science and Technology Planning Project of Guangdong Province, China, (Grant/Award Number: ‘2014A020212625‘)
Abstract
Recent studies have demonstrated that mesenchymal stem cells (MSC) exhibit a tropism to tumors and form the tumor stroma. In addition, we found that MSC can secrete different types of factors. However, the involvement of MSC-derived factors in human tongue squamous cell carcinoma (TSCC) growth has not been clearly addressed. The CCN family includes multifunctional signaling molecules that affect the initiation and development events of various tumors. In our study, we report that CCN2/connective tissue growth factor (CTGF) was the most highly induced among the CCN family members in MSC that were co-cultured with TSCC cells. To evaluate the relationship between CCN2 and TSCC growth, we downregulated MSC-derived CCN2 expression with shRNA targeting CCN2 and found that MSC-secreted CCN2 promotes TSCC cell proliferation, migration and invasion. We also confirmed that MSC-derived CCN2 partially accelerated tumor growth in vitro. Taken together, these results suggest that MSC-derived CCN2 contributes to the promotion of proliferation, migration and invasion of TSCC cells and may be a possible therapy target in the future.
Tongue squamous cell carcinoma (TSCC) is the most common type of oral cancer and is characterized by its high rate of proliferation and lymph nodal metastasis.1 Despite tremendous improvements in surgery, radiotherapy and chemotherapy over the past decade, the prognosis for patients with TSCC has remained relatively unchanged for the past 3 decades because patients succumb to metastatic disease at regional and distant sites.2 The tumor microenvironment (TME) is a functional ecosystem of tumor and stromal elements that regulate tumor progression. Therefore, we sought to determine the interaction mechanisms of TME elements of TSCC to better understand tumor progression.
Mesenchymal stem cells (MSC) are multipotent stromal cells that exhibit marked tropism for tumors. Cytokines and growth factors secreted by tumor cells together with endocrine factors of inflammatory tissues attract MSC to the tumor stroma.3, 4 Studies suggest that MSC can secrete a variety of growth factors that are known to influence tumor proliferation, migration and angiogenesis, to form a tumor's fibrovascular network, and to differentiate into tumor-associated fibroblasts (TAF) and vascular pericytes.5, 6 MSC have been shown to promote invasion and metastasis in various cancers, such as breast, colon and lymphatic cancers, whereas other studies have reported that MSC suppress tumor growth.7-10 However, the impact and the role of MSC in the TME and the mechanisms of their potential effects on different tumors remain controversial.
The CCN family is a family of six extracellular matrix-associated proteins. The acronym CCN originated from the names of the three-first discovered members:11 cysteine-rich protein 61 (CYR61; CCN1),12 connective tissue growth factor (CTGF; CCN2)13 and nephroblastoma-overexpressed (NOV; CCN3).14 The other three members include WISP1 (also known as CCN4), WISP2 (CCN5) and WISP3 (CCN6).15 CCN proteins have been demonstrated to have not merely a structural role; rather, they also modulate cell–ECM interactions in different manners that accordingly influence the TME.16-20 Among the CCN family, CCN2/CTGF is the most well-known member. As research has become more complex, CCN2 has been found to mediate cell adhesion, aggregation and migration in a large variety of cell types, including vascular endothelial cells, fibroblasts, epithelial cells, aortic smooth muscle cells and pluripotent stem cells.21 Studies have suggested that CCN2 acts as an oncoprotein in glioblastoma growth.22 Moreover, CCN2 enhances the cellular motility of breast cancer.23 In contrast, tumor-suppressive effects of CCN2 have previously been demonstrated in lung cancer, colon cancer and ovarian cancer.24, 25 In HNSCC, CCN2 mRNA and protein are overexpressed,26 whereas elevated expression of CCN2 has been reported to induce the local progression but reduce the invasiveness of HNSCC.27
Although CCN2 has attracted the attention and investigations of increasing numbers of researchers, the source of the elevated CCN2 (MSC or tumor cells) remains unknown. Battula VL et al. demonstrate that MSC express high levels of CCN2 in leukemia and regulate themselves in terms of differentiation.28 We first found that CCN2 is overexpressed in MSC culture medium after stimulation with TSCC cells. Thus, we examined CCN2 expression in MSC and TSCC cells and found that CCN2 expression in MSC was significantly increased compared with that in TSCC cells. Then, we identified CCN2 expression in TSCC tissues and further used CCN2-knockdown MSC to confirm that MSC-derived CCN2 promoted the proliferation, migration and invasion of TSCC cells.
Materials and Methods
Materials
CCN2 was purchased from Protein-Specialists (Brunswick, NJ, USA). The DyNAmo ColorFlash SYBR Green qRT-PCR Kit was purchased from Thermo Fisher Scientific (Rutherford, NJ, USA). Quantitative RT-PCR (qRT-PCR) primers were designed using Oligo 7 and synthesized by Invitrogen (Carlsbad, CA, USA). Neutralizing anti-TGF-β antibody was purchased from R&D Systems (Minneapolis, MS, USA). CCK-8 assay kits were purchased from Dojin Laboratories (Kumamoto, Kyushu, Japan). Antibodies specific for CCN2, Ki67, PCNA and vimentin were purchased from Santa Cruz Biotechnology. Antibodies specific for E-cadherin, N-cadherin, twist, MMP-2 and MMP-9 were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-Fibroblast activation protein antibody was purchased from Abcam (Cambridge, MA, USA).
Cell culture
Mesenchymal stem cells (isolated from human bone marrow aspirates) were obtained from Sixin Biotechnology (Shanghai, China). The culture medium was low-glucose DMEM (Hyclone, Logan, UT, USA) supplemented with 10% FBS (Invitrogen), 100 IU/mL penicillin (Hyclone) and 100 μg/mL streptomycin (Hyclone).
The human TSCC cell lines (TSCCA and CAL27 cells) were purchased from American Type Culture Collection (ATCC; Rockville, MD, USA). The TSCCA cells were maintained in RPMI-1640 culture medium supplemented with 10% FBS, and the CAL27 cells were maintained in high-glucose DMEM supplemented with 10% FBS. All culture media were maintained at 37°C under 5% CO2 in humidified air.
To generate conditioned medium (CM), 5 × 105 MSC or TSCC cells were seeded into 6-well plates in high-glucose DMEM supplemented with 10% FBS and cultured for 48 h.
RNAi transfection
CCN2 expression was knocked down in MSC through the transduction of a lentiviral vector expressing a shRNA with the sequence 5′-TCG AGA AAA AAA CAA CTG TCC CGG AGA CAA TGT GAC AGG AGG CAT TGT CTC CGG GAC AGT TGT CA-3′. Lentiviruses were obtained by transfection of 293T cells using Lipofectamine 2000 (Invitrogen). MSC were seeded into 6-well plates and transfected with CCN2 shRNA using X-tremeGENE HP reagent (Roche, Indianapolis, IN, USA).
Quantitative RT-PCR analysis
Total mRNA from tumor tissues or cells was extracted using an RNeasy Mini Kit (Qiagen, Dusseldorf, NRW, GER) and complementary DNA (cDNA) was synthesized using a QuantiTtect Reverse Transcription Kit (Qiagen) according to the manufacturers’ protocols. qRT-PCR was performed using a DyNAmo ColorFlash SYBR Green qRT-PCR kit as described by the manufacturer with the following primer pairs: human CCN2, 5′-CAG CAT GGA CGT TCG TCT G-3′ (forward) and 5′-AAC CAC GGT TTG GTC CTT GG-3′ (reverse); human Ki67, 5′-CTT CCA GCA GCA AAT CTC A-3′ (forward) and 5′-ACA ATC AGA TTT GCT TCC GA-3′ (reverse); human PCNA, 5′-AGG CAC TCA AGG ACC TCA TCA-3′ (forward) and 5′-GAG TCC ATG CTC TGC AGG TTT-3′ (reverse); human E-cadherin, 5′-CAC AGC CTG TCG AAG CA-3′ (forward) and 5′-CTC TTT GAC CAC CGC TCT-3′ (reverse); human N-cadherin, 5′-CAA ACA GCA ACG ACG G-3′ (forward) and 5′-ATT TTC TGC AGC AAC AGT-3′ (reverse); human vimentin, 5′-GAG AAC TTT GCC GTT GAA GC-3′ (forward) and 5′-GCT TCC TGT AGG TGG CAA TC-3′ (reverse); human twist, 5′-CGT GTC CAG CTC GCC AGTC-3′ (forward) and 5′-TCG TCG CCG CCT CCG AT-3′ (reverse); human MMP-2, 5′-TCT CCT GAC ATT GAC CTT GGC-3′ (forward) and 5′-CAA GGT GCT GGC TGA GTA GAT C-3′ (reverse); and human MMP-9, 5′-TTG ACA GCG ACA AGA AGT GG-3′ (forward) and 5′-GCC ATT CAC GTC GTC CTT AT-3′ (reverse). The thermocycling protocol for all experiments was 40 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 10 s, and extension at 72°C for 30 s (β-actin was used as the internal control).
Elisa
Human CCN2/CTGF ELISA Kits (LifeSpan BioSciences, Seattle, WA, USA) were used to measure the CCN2 secretion into the culture medium. The cells were plated and incubated at 37°C for 36 h. Next, equal volumes of cell culture supernatants were collected. CCN2 protein levels were quantified according to the manufacturer's protocol. The concentrations of CCN2 in the culture media were determined at 450 nm using a microplate reader (Tecan Trading AG, Männedorf, Zürich, Switzerland).
Patients and samples
Primary TSCC samples were obtained from formalin-fixed, paraffin-embedded (FFPE) tissue blocks of previously untreated patients hospitalized at Sun Yat-sen Memorial Hospital between 2008 and 2010. All patients diagnosed with untreated primary TSCC with follow-up data available were eligible for our study. Patient characteristics are summarized in Table1. Classification and stage were determined according to the UICC classification.
| Parameter | n | CCN2 expression | χ2 | P | |
|---|---|---|---|---|---|
| Low | High | ||||
| Age | |||||
| <55 | 40 | 22 | 18 | 0.131 | 0.287 |
| ≥55 | 50 | 33 | 17 | ||
| Sex | |||||
| Male | 52 | 30 | 22 | 0.606 | 0.436 |
| Female | 38 | 25 | 13 | ||
| Pathological differentiation | |||||
| Well | 49 | 32 | 17 | 0.796 | 0.372 |
| Moderate–poor | 41 | 23 | 18 | ||
| T status | |||||
| T1 + T2 | 59 | 36 | 23 | 0.001 | 0.980 |
| T3 + T4 | 31 | 19 | 12 | ||
| Clinical stage | |||||
| I–II | 40 | 27 | 13 | 1.237 | 0.266 |
| III–IV | 50 | 28 | 22 | ||
| N status | |||||
| Positive (+) | 36 | 16 | 20 | 4.370 | 0.037 |
| Negative (−) | 54 | 36 | 18 | ||
Immunohistochemistry
Four-micrometer-thick sections were cut from the FFPE tissue blocks for immunohistochemistry (IHC). After being deparaffinized in xylene and dehydrated with ethanol, the sections were incubated with 0.3% H2O2 to block the endogenous peroxidase activity. Antigen retrieval was performed by autoclave heating treatment in citrate buffer (pH 6.0) at 120°C for 10 min. Then the sections were incubated with a goat anti-human polyclonal antibody against CCN2, a mouse anti-human polyclonal antibody against vimentin and a rabbit anti-human polyclonal antibody against Ki67 (1:50, 1:200, and 1:150 dilutions, respectively; Santa Cruz Biotechnology) at 4°C overnight followed by incubation with secondary antibodies conjugated to HRP (anti-goat, Nichirei, Tokyo, Japan; or anti-mouse/rabbit, Envision + Dual Link Kit, Dako, Carpinteria, CA, USA) at 37°C for 30 min. The immunoreactions were developed using a peroxidase-labeled secondary antibody followed by 3,3′-diaminobenzidine tetrahydrochloride using the Envision System (Dako), and counterstaining was performed with hematoxylin (Zymed Laboratories, San Francisco CA, USA). The samples were mounted for examination.
CCN2 immunostaining was scored by the percentage of intercellular substance staining: cases with immunopositivity <5% were given a score of 0, 5%–25% received a score of 1, 25%–50% received a score of 2, and cases with >50% received a score of 3. Scores of 0 and 1 were defined as low expression, and scores of 2 and 3 were defined as high expression.
Ki67 is expressed in the nucleus, and vimentin is primarily expressed in the intercellular substance and partly expressed in tumor cells. The Ki67/vimentin index represents the number of Ki67/Vimentin-positive tumor cells divided by the total number of tumor cells × 100% and was determined by counting the number of tumor cells in three randomly selected high-power fields.29
Western blot analysis
Protein concentration was determined using the Thermo Scientific Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Proteins were separated by 10% SDS-PAGE and transferred onto a PVDF membrane. The membranes were blocked in 5% skim milk diluted in TBSB followed by incubation with appropriate primary antibodies (anti-CCN2, vimentin, E-cadherin, N-cadherin, MMP-2 and MMP-9; Santa Cruz Technology and Cell Signaling Technology) overnight at 4°C. After washing thrice with TBST, the membranes were incubated for 1 h with an HRP-conjugated secondary antibody (Cell Signaling Technology) at 37°C. GAPDH was used as an internal control. The blots were visualized with an enhanced chemiluminescence (Millipore, Bedford, MA, USA).
Cell counts
Tongue squamous cell carcinoma cells were treated as indicated in the text and counted every day for 5 days. After collection by centrifugation, TSCC cells were washed once in PBS and resuspended in 200 μL of PBS. Then, the cells were stained with an equal volume of 0.4% trypan blue for 5 min at room temperature. Viable cells (unstained) were counted using a hemocytometer.
Cell viability assay
Cell viability was determined using a CCK-8 assay kit. TSCC cells were plated at a density of 2 × 105 cells/mL on 96-well plates in culture medium (100 μL/well). After 4 h, 10 μL of CCK-8 solution was added to the cells in 96-well plates and incubated at 37°C for 0.5 to 4.0 h. The absorbance in each well was quantified at 450 nm using a microplate reader (Tecan Trading AG). Cell viability was calculated according to the manufacturer's instructions. At least three experiments were performed, and each experiment was tested in triplicate.
Cell colony-formation assay
Tongue squamous cell carcinoma cells were plated in 6-well plates (200 cells/well) and coated in conditioned media (CM) from the control, MSCNTC or MSCshCCN2 supplemented with 10% FBS. The medium was replaced every 3 days, and the cells were fixed in with 4% formaldehyde and stained with Giemsa (Sigma, St. Louis, MO, USA) after 14 days. Colonies larger than 1 mm (>50 cells/clone) in diameter were counted.
Cell motility assay
Tongue squamous cell carcinoma cells seeded on 35-mm glass bottom dishes were placed in a stage top incubator at 37°C with 5% CO2 + 95% air (model: TIZ; Tokai Hit, Shizuoka-ken, Japan). TSCC cell motility was monitored under the 40× oil immersion objective lens with a time-lapse video microscope system (the Nikon TiE 300 inverted epifluorescence microscope with perfect focus) and MetaMorph software (Molecular Devices, Sunnyvale, CA, USA). Time-lapse DIC images were acquired in 20-min intervals for 60 h either under serum-free medium control conditions or in the presence of MSCNTC or MSCshCCN2. Images were analyzed using ImageJ software (National Institute of Health, USA) and cell tracking was performed using the Manual Tracking plugin. The total distance traveled was determined by tracking the movement of the cell gravity center, and its coordinates were used to calculate the distances.
Cell invasion assay
Cell invasion was measured using Matrigel-precoated transwell inserts (Millipore) according to the manufacturer's instructions. Briefly, TSCC cells in serum-free medium were plated into the upper chamber. Then the lower chamber of the transwell was filled with 500 μL DMEM (10% FBS) and either of additives-free (control), 1 × 105 MSCNTC cells (MSCNTC), 1 × 105 MSCshCCN2 cells (MSCshCCN2) or 1 μg/mL CCN2 (CCN2) respectively. After a 48-h incubation, the TSCC cells that migrated over to the lower chamber were fixed with 100% methanol and stained with 1% crystal violet. The cell counts are expressed as the average number of cells per field of view. The counts of invasive cells were quantified from at least 10 randomly selected fields from three independent experiments at a magnification of 200× by three independent observers.
Xenograft studies
The effect of MSC-secreted CCN2 on TSCC proliferation in vivo was evaluated using a subcutaneous xenograft tumor model that was established via the injection of TSCC cells mixed with or without MSC or shCCN2-MSC (1:1, 2 × 106 each) into the right flanks of 8-week-old female SCID mice. Three mice were used in each group. Tumor volumes were calculated using the formula: tumor volume (mm3) = 0.5 × width2 × length. After 4 weeks, the mice were sacrificed, and the tumors were resected for weighing and experimentation. All animal studies were performed according to the guidelines of the Sun Yat-sen University Institutional Animal Care and Use Committee.
Statistical analyses
All experiments were performed at least three separate times. The measurement results were provided as the mean ± SEM. The statistical analyses between pairs of samples were performed using Student's t-test. The associations between CCN2 expression and the clinicopathological variables were assessed with χ2-tests. Statistical comparisons involving more than two groups were performed using one-way analysis of variance (anova). Cell number and tumor volume curves were evaluated using repeated-measures anova followed by post hoc tests. In all cases, P < 0.05 was considered significant.
Results
CCN2 is highly produced in mesenchymal stem cells following interaction with tongue squamous cell carcinoma cells
Mesenchymal stem cells in the TME play both pro-tumoral and anti-tumoral roles, and CCN2, which has a regulatory role in the TME, is highly expressed in MSC. Accordingly, we hypothesized that there may be some interaction between these roles. To test this hypothesis, we determined the influence of MSC on CCN members. Thus, we cultured MSC in the absence or presence of conditioned medium (CM) from TSCCA or CAL27 TSCC lines and then screened CCN family mRNA levels in MSC. As illustrated in Figure 1(a) and (b), CCN1, CCN2 and CCN3 expression increased in the MSC treated with TSCCA or CAL27 CM compared with the control MSC. Among these genes, the expression of CCN2 exhibited the greatest increase. This result further increased our interest in the interaction between CCN2 and MSC in the TME.
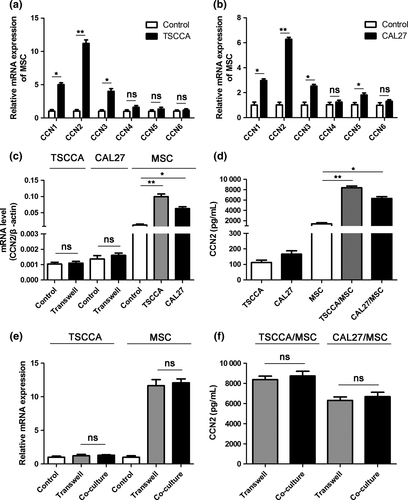
Next, we studied the interactions in the TSCC cells cultured with MSC. As illustrated in Figure 1(c), CCN2 expression was unchanged in the TSCC cells but increased in MSC after co-culture for 36 h. The CCN2 mRNA expression, which was normalized to that of β-actin mRNA, was dramatically different between the MSC and TSCC cells. The increased CCN2 mRNA levels in the MSC supported the notion that MSC are the main source of CCN2. For validation, we assessed CCN2 protein secretion in the group illustrated in Figure 1(c) using a human CCN2 kit and acquired similar results, which suggests that CCN2 was induced in the MSC following the interaction with the TSCC cells, and the secretion of CCN2 by the MSC was considerably increased compared with the secretion of the TSCC cells (Fig. 1d). Furthermore, we co-cultured GFP-expressing MSC and TSCC cells in a direct co-culture system or a transwell system to investigate whether direct contact between cells affected TSCC cell-induced upregulation of CCN2 expression in MSC. After 36 h, the MSC in the direct contact system were sorted by flow cytometry. Then, CCN2 expression in each group was measured by qRT-PCR. The results indicated no increase in CCN2 expression in TSCC cells in a direct contact system. In the other group, CCN2 expression in MSC after co-culture increased equally both in the direct contact system and the transwell system (Fig. 1e). In addition, ELISA revealed no significant differences in CCN2 secretion into the culture medium between the direct contact system and the transwell system (Fig. 1f).
It is known that CCN gene expression is strongly regulated by TGF-β, which is ubiquitously expressed by a lot of cancers.30 In particular, CCN2 is highly induced by TGF-β in various cell systems.31, 32 To test whether TGF-β induced CCN2 expression in MSC, we used neutralizing antibody targeting TGF-β in the media when MSC was co-cultured with TSCC cell lines. The results showed that CCN2 mRNA in MSC was dramatically reduced by TGF-β neutralizing antibody (Fig. S1a,c). A similar result was acquired from CCN2 ELISA assay (Fig. S1b,d). Both these results confirmed that TGF-β played an important role in CCN2 expression, and might be the main soluble factor produced by TSCC cells to induce CCN2 expression in MSC.
CCN2 is overexpressed in tongue squamous cell carcinoma patient tissues
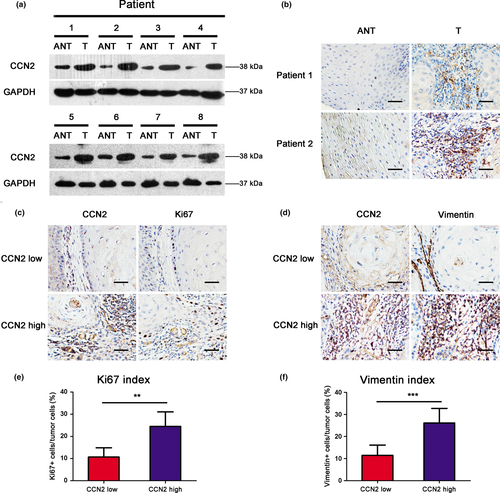
To investigate the correlation between TSCC progression and CCN2 expression levels, western blot was used to analyze CCN2 protein expression in 8 TSCC patients (Fig. 2a). CCN2 expression was markedly increased in cancerous tissue compared with adjacent normal tissue (ANT). The immunohistochemistry results showed that the cells with high expression of CCN2 were spindle-shaped, and were positive for mesenchymal marker FAP (Fig. S2). Furthermore, resected tissue specimens from 90 TSCC cases (Table 1) were immunostained with an anti-CCN2 antibody. Representative immunostaining of TSCC tissues is presented in Figure 2(b). The correlations between CCN2 protein expression levels and clinicopathological variables are summarized in Table2. From the IHC analysis, no significant correlation was noted between CCN2 expression and patient age, sex, pathological differentiation T status and clinical TNM staging. However, CCN2 expression was associated with the lymph node metastasis, suggesting that the lymph node-positive cases tend to exhibit increased CCN2 expression compared with lymph node-negative cases (Table2).
| Number | Sex | Age | TNM status | TNM staging | Differentiation | ||
|---|---|---|---|---|---|---|---|
| T | N | M | |||||
| 1 | F | 79 | 2 | 0 | 0 | II | High |
| 2 | F | 66 | 2 | 1 | 0 | III | Middle |
| 3 | M | 70 | 1 | 0 | 0 | I | Middle |
| 4 | F | 58 | 4 | 0 | 0 | IV | High |
| 5 | M | 31 | 4 | 2 | 0 | IV | High |
| 6 | F | 66 | 2 | 1 | 0 | III | High |
| 7 | M | 54 | 1 | 2 | 0 | IV | High |
| 8 | M | 37 | 2 | 0 | 0 | II | Middle |
| 9 | M | 56 | 3 | 0 | 0 | III | Middle |
| 10 | M | 65 | 2 | 0 | 0 | II | High |
| 11 | M | 47 | 2 | 2 | 0 | IV | Middle |
| 12 | M | 59 | 4 | 1 | 0 | IV | High |
| 13 | F | 50 | 2 | 0 | 0 | II | Middle |
| 14 | F | 45 | 2 | 0 | 0 | II | Middle |
| 15 | F | 47 | 1 | 0 | 0 | I | High |
| 16 | M | 64 | 4 | 2 | 0 | IV | Middle |
| 17 | M | 56 | 4 | 1 | 0 | IV | High |
| 18 | F | 54 | 2 | 0 | 0 | II | High |
| 19 | M | 45 | 4 | 1 | 0 | IV | Middle |
| 20 | F | 70 | 4 | 0 | 0 | IV | High |
| 21 | M | 55 | 4 | 0 | 0 | IV | High |
| 22 | F | 36 | 1 | 0 | 0 | I | Middle |
| 23 | F | 47 | 4 | 2 | 0 | IV | High |
| 24 | M | 67 | 1 | 0 | 0 | I | High |
| 25 | F | 56 | 2 | 0 | 0 | II | High |
| 26 | M | 57 | 2 | 0 | 0 | II | High |
| 27 | F | 47 | 1 | 0 | 0 | I | High |
| 28 | M | 56 | 4 | 2 | 0 | IV | Middle |
| 29 | M | 73 | 3 | 0 | 0 | III | High |
| 30 | M | 55 | 3 | 0 | 0 | III | Middle |
| 31 | F | 52 | 2 | 0 | 0 | II | Low |
| 32 | M | 38 | 1 | 0 | 0 | I | High |
| 33 | M | 64 | 2 | 2 | 0 | IV | Middle |
| 34 | M | 62 | 2 | 0 | 0 | II | Middle |
| 35 | F | 49 | 1 | 0 | 0 | I | High |
| 36 | M | 42 | 2 | 2 | 0 | IV | High |
| 37 | F | 52 | 3 | 2 | 0 | IV | High |
| 38 | M | 53 | 2 | 0 | 0 | II | High |
| 39 | F | 47 | 4 | 2 | 0 | IV | High |
| 40 | M | 59 | 2 | 0 | 0 | II | Middle |
| 41 | M | 22 | 2 | 1 | 0 | III | Middle |
| 42 | F | 73 | 3 | 2 | 0 | IV | High |
| 43 | F | 59 | 3 | 0 | 0 | III | Middle |
| 44 | M | 44 | 2 | 1 | 0 | III | Middle |
| 45 | F | 61 | 2 | 0 | 0 | II | High |
| 46 | M | 72 | 4 | 2 | 0 | IV | High |
| 47 | M | 62 | 2 | 0 | 0 | II | Middle |
| 48 | M | 45 | 2 | 2 | 0 | IV | High |
| 49 | F | 80 | 4 | 1 | 0 | IV | High |
| 50 | M | 50 | 2 | 1 | 0 | III | High |
| 51 | M | 55 | 2 | 2 | 0 | IV | High |
| 52 | F | 49 | 4 | 0 | 0 | IV | Middle |
| 53 | M | 73 | 3 | 0 | 0 | III | High |
| 54 | F | 62 | 3 | 0 | 0 | III | High |
| 55 | F | 56 | 1 | 0 | 0 | I | High |
| 56 | M | 22 | 2 | 2 | 0 | IV | High |
| 57 | F | 61 | 2 | 2 | 0 | IV | Middle |
| 58 | F | 53 | 4 | 2 | 0 | IV | Low |
| 59 | F | 79 | 1 | 0 | 0 | I | Middle |
| 60 | M | 44 | 3 | 2 | 0 | IV | Middle |
| 61 | F | 67 | 2 | 1 | 0 | III | High |
| 62 | M | 56 | 2 | 1 | 0 | III | Middle |
| 63 | F | 77 | 3 | 0 | 0 | III | Low |
| 64 | F | 58 | 1 | 0 | 0 | I | Middle |
| 65 | M | 82 | 2 | 0 | 0 | II | High |
| 66 | M | 64 | 4 | 1 | 0 | IV | High |
| 67 | M | 46 | 4 | 2 | 0 | IV | Low |
| 68 | M | 48 | 2 | 0 | 0 | II | Middle |
| 69 | M | 58 | 2 | 0 | 0 | II | Middle |
| 70 | M | 63 | 1 | 0 | 0 | I | High |
| 71 | F | 41 | 2 | 0 | 0 | II | High |
| 72 | F | 52 | 2 | 2 | 0 | IV | Middle |
| 73 | M | 26 | 2 | 0 | 0 | II | Middle |
| 74 | F | 58 | 1 | 0 | 0 | I | High |
| 75 | M | 41 | 2 | 0 | 0 | II | Middle |
| 76 | M | 60 | 1 | 0 | 0 | I | High |
| 77 | F | 28 | 2 | 0 | 0 | II | Low |
| 78 | M | 52 | 2 | 0 | 0 | II | Low |
| 79 | M | 59 | 4 | 0 | 0 | IV | High |
| 80 | M | 47 | 2 | 0 | 0 | II | High |
| 81 | M | 55 | 4 | 0 | 0 | IV | Middle |
| 82 | M | 70 | 1 | 0 | 0 | I | High |
| 83 | F | 45 | 2 | 0 | 0 | II | High |
| 84 | M | 82 | 4 | 1 | 0 | IV | Low |
| 85 | M | 70 | 1 | 0 | 0 | I | Middle |
| 86 | F | 52 | 2 | 1 | 0 | III | High |
| 87 | M | 68 | 1 | 1 | 0 | III | Middle |
| 88 | M | 42 | 1 | 0 | 0 | I | High |
| 89 | F | 53 | 3 | 0 | 0 | III | High |
| 90 | M | 57 | 2 | 1 | 0 | III | Low |
We also evaluated the correlations of CCN2 expression with the Ki67 index and the vimentin index in the human TSCC tissues with immunohistochemical staining (Fig. 2c,d). The Ki67 indexes were 10.67 ± 8.99% in the tissues with low CCN2 expression levels and 24.50 ± 13.59% in the tissues with high CCN2 levels (Fig. 2e). The vimentin indexes were 11.47 ± 7.62% in the tissues with low CCN2 expression levels and 21.65 ± 9.62% in the tissues with high CCN2 levels (Fig. 2f). A Student's t-test analysis demonstrated that CCN2 expression was positively correlated with the Ki67 and vimentin indexes (P = 0.001; P < 0.001), with suggests that CCN2 may play a role in cancer proliferation and the epithelial–mesenchymal transition.
Mesenchymal stem cell-secreted CCN2 enhances human tongue squamous cell carcinoma cell proliferation
To determine the influence of the MSC-secreted CCN2 on TSCC cell proliferation, we explored the effect of CCN2 knockdown on cultured MSC. qRT-PCR assays and western blotting indicated that CCN2 mRNA and protein levels were decreased in MSC transfected with a vector expressing a short hairpin (inhibitory) RNA (shRNA) targeting CCN2 (MSCshCCN2) compared with normal MSC (MSCNTC) (Fig. 3a).
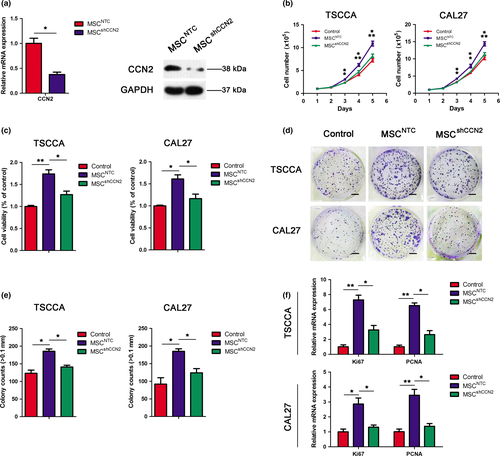
Then, to determine whether CCN2 secreted by MSC is involved in TSCC cell proliferation, we explored the effect of CCN2 knockdown in MSC on TSCC cell proliferation. TSCC cells were cultured in the presence of CM from normal MSC or shCCN2-MSC. CM from shCCN2-MSC promoted a substantially smaller increase in TSCC cell numbers compared with the normal MSC (Fig. 3b). TSCC cell data on viability (obtained by CCK-8 assays) and colony-forming ability (Fig. 3c–e) exhibited a consistent effect with the cell count results and the mRNA levels of the proliferation markers Ki67 and PCNA (Fig. 3f).
Mesenchymal stem cell-secreted CCN2 promotes human tongue squamous cell carcinoma cell migration and invasion
To address whether MSC-secreted CCN2 promoted TSCC cell migration, we then observed the random cell movement of TSCC cells cultured in the presence of CM from normal MSC or shCCN2-MSC. Figure 4(a) illustrates the random moving traces of 5 representative cells under the control condition, revealing minimal motility. In the presence of shCCN2-MSC, TSCC cell motility increased, whereas the TSCC cells in the presence of normal MSC exhibited the greatest motility among the three groups. The total movement distance and average movement speed data are summarized in Figure 4(b), and these results are consistent with the motility outcome.
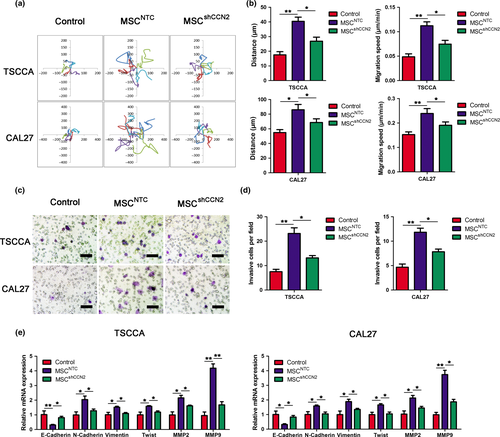
The involvement of MSC-secreted CCN2 in TSCC cell invasion was investigated using transwell invasion assays. These assays revealed that MSCNTC had a substantially greater effect on TSCC cells invasion ability compared with control conditions. However, the invasion-stimulating effect was decreased by knockdown of CCN2 in MSC (Fig. 4c,d).
Western blotting and qRT-PCR were used to investigate the alteration in the abundance of epithelial to mesenchymal transition (EMT) markers. CCN2 knockdown increased the mRNA level of epithelial markers, including E-Cadherin, in TSCCA and CAL27 cells, whereas the mRNA levels of mesenchymal markers, including N-cadherin, Vimentin and Twist, MMP2 and MMP9, were significantly reduced (Fig. 4e,f). Correspondingly, the protein expressions of these markers were consistent with the results of the mRNA levels (Fig. S3). These results indicated that MSC-derived CCN2 might contribute to EMT progression in TSCCA and CAL27 cells and, thus, aid migration and invasion.
CCN2 stimulates human tongue squamous cell carcinoma cell proliferation, migration and invasion
In addition to assessing the effects of MSC-secreted CCN2 on tumor cells, we also evaluated the influence of CCN2 alone on TSCC cell proliferation, migration and invasion. As shown in Figure 5(a), the number of TSCC cells conditioned with CCN2 increased substantially compared with those exposed to negative control medium, and similar cell viability and colony-forming ability results were verified by CCK-8 assays (Fig. 5b) and colony-formation assays (Fig. 5c), respectively. In addition, the mRNA expression levels of proliferating cell nuclear antigen (PCNA) and Ki67, which are markers of cell proliferation, were increased in TSCC cells conditioned with CCN2 compared with the control group (Fig. 5d). Figure 5(e) presents the results of transwell assays that illustrated that CCN2 enhanced the invasion ability of TSCC cells.
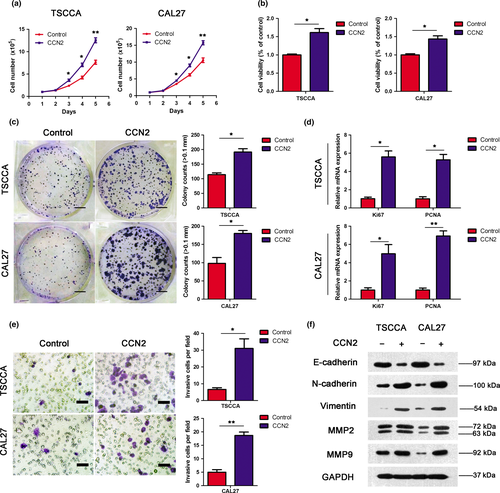
Furthermore, we detected the different expression of certain classic EMT markers in TSCC cells from CCN2-treated medium or negative control medium. As illustrated in the western blotting results (Fig. 5f), CCN2 increased the expressions of mesenchymal markers (i.e. N-cadherin, vimentin, MMP2 and MMP9). By contrast, E-cadherin expression was decreased in the CCN2-treated medium. Correspondingly, the mRNA expression levels of these markers (Fig. S4) were consistent with the protein expression level results from western blotting. In addition, cell motility of TSCC medium conditioned with or without exogenous CCN2 was recorded as before during a 20-min recording period over 6 h (Fig. S5). These results indicated that exogenous CCN2 might play a positive role in EMT progression in TSCCA and CAL27 cells, and aid migration and invasion.
Mesenchymal stem cells promote tumor growth via the secretion of CCN2 in vivo
To further test whether MSC promote tumor proliferation in vivo via CCN2 secretion, we subcutaneously injected 2 × 106 TSCC cells or a mixture of 2 × 106 TSCC cells and 2 × 106 MSC (parental or shCCN2-expressing) into SCID mice. The tumors were measured every 4 days for 28 days, and the tumor weights were collected after the mice were sacrificed (Fig. 6). The tumors formed from TSCC cells co-injected with MSC were larger than those formed from TSCC cells alone, whereas the tumors formed under the effect of MSCshCCN2.
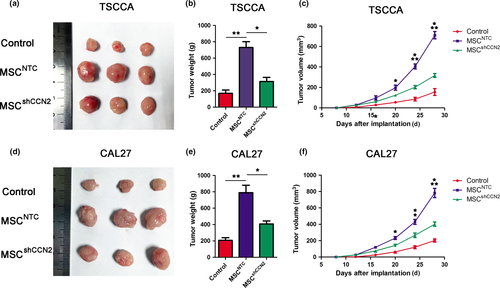
To find out whether the sizes of tumor were influenced by MSC proliferation, we examined MSC proliferation both in vitro and in vivo. First, results of CCK-8 assay and cell number counting indicated no statistic difference between MSCNTC and MSCshCCN2 proliferation (Fig. S6a,b). We used flow cytometry to count MSC number in xenograft tumors derived from the mixture of TSCC cells and GFP-labeled MSC. The result showed no statistic difference for GFP+ cell numbers between MSCNTC and MSCshCCN2 coinjected with TSCC cell lines (Fig. S6c,d). All these results indicated that MSC proliferation was not changed by knockdown of CCN2. Thus, the different volumes of tumors were the result of decreased cell growth of TSCC cells.
Discussion
In the present study, we detected the expression levels in MSC and TSCC cells before and after co-culture, and the results indicated that MSC expressed CCN2 at high levels following stimulation with TSCC cells. We further identified the silencing effects of CCN2 on the proliferation, migration and invasion of TSCC cells by knocking down CCN2 in MSC, and the results confirmed that CCN2 knockdown in MSC similarly reduced the proliferation, migration and invasion of TSCC cells. In addition, we found that reducing CCN2 expression in MSC slowed the TSCC xenograft tumor growth in the SCID mice. Consistent with these results, we demonstrated that in co-cultures of MSC and TSCC cells, the CCN2 secreted by MSC was dominantly involved in the promotion of the proliferation, migration and invasion of TSCC.
Recent years have witnessed increased interest in studying the tumor microenvironment (TME) and its contribution to tumor progression, invasion and metastasis. MSC and fibroblasts are the major sources of stromal cells in a tumor. We had used fibroblasts derived from human skin to co-culture with TSCC cell lines and found that fibroblasts’ effect on proliferation and invasion promotion in TSCC cell lines is not as efficient as MSCs’, following examination with CCK-8 assay (Fig. S7a,b), cell number counting (Fig. S7c,d) and transwell invasion assay (Fig. S7e,f). We thus focused on the relation between MSC and TSCC cells. MSC, together with other TME elements, produce extracellular matrix, growth factors, cytokines, proteases and their regulators and, thus, form the TME.33 As an important component of the TME, MSC are recruited to primary tumor sites and the sites of metastasis where they play essential roles in tumor progression.10, 34 In our study, after adding the TSCC supernatant into the MSC culture medium, the CCN2 expression was substantially increased. This outcome provides cause for further investigations of the underlying connection of CCN2, MSC and TSCC cells.
CCN2 has been reported to act as a multifunctional growth factor in different cancers; CCN2 can act either as a tumor promoter or suppressor. In most cases, with the exception being ovarian and lung cancers, CCN2 expression is elevated, including in breast cancer, prostate cancer, glioma, pancreatic cancer, colon cancer, thyroid carcinoma, chondrosarcoma, gallbladder carcinoma and leukemia.20, 35-40 Many of these findings relate CCN2 to cell adhesion and migration in terms of its mechanism of action as a tumor promoter. In accordance with the controversial role of CCN2 in tumors, Takigawa et al. found that the overexpression of CCN2 elicits benign conversions in oral squamous cell carcinoma cells,41 whereas Chang et al. demonstrated that CCN2 in HNSCC promotes local progression but reduces invasiveness by coordinating the expression of the pluripotency genes through c-Jun to promote the MET.27 Our study found that CCN2 is expressed at higher levels in TSCC tissues than in paraneoplastic tissues, and its expression seems to be correlated with lymph node metastasis. Moreover, the experimental results indicated that the CCN2 overexpression enhanced the proliferation, migration and invasion of TSCC cells compared with the TSCC cells subjected to the downregulation of CCN2 expression in vitro. The link between CCN2 and cell adhesion is based on the protein interactions in the extracellular domain, with stimuli causing matrix production and the upregulation of adhesion pathways rather than the adhesive properties of CCN2 itself. These adhesion pathways are triggered by integrins or MAPK activation and result in cell attachment and detachment. Accounting for the CCN2 in cell migration and cancer metastasis processes, studies in recent years have observed significant progression of different malignancies.42 In chondrosarcoma, CCN2 enhances cell migration via matrix metalloproteinase-13 upregulation that is mediated through integrin-αvβ3,43 and in gastric cancer cells, CCN2 enhance cell migration via the downregulation of E-cadherin though the NF-κB pathway. It has been suggested that enhancement of CCN2 expression reconstitutes FOXP1 expression and inhibits oral squamous cell carcinoma migration and invasion.44 The overexpression of CCN2 in esophageal squamous carcinoma cells results in the accumulation and nuclear translocation of β-catenin-TCF/LEF signaling and the upregulation of c-myc and cyclin D1 (two target genes of β-catenin-TCF/LEF signaling).45 Overall, the roles of CCN2 in various cancers seems to be considerably diverse, although the underlying mechanisms have not yet been investigated.
Mesenchymal stem cells exhibit an essential role in the promotion of the invasion and metastasis of various cancers, such as breast, colon and lymphatic cancers.8, 46 However, other studies have reported that MSC have negative effects or no effect on tumor growth and metastasis. The MSC-mediated effects of tumor support or suppression depend on the vascular support, the fibroblast source, the immunomodulatory effects, the metastasis promotion and the secretion of paracrine factors. Wang et al. demonstrated that MSC migrate to the tumor site and differentiate into pericytes, which induce tumor vasculogenesis and promote tumor recurrence.47 In addition, one study confirmed the existence of an MSC-mediated immunosuppression of T cell proliferation through the upregulation of B7-H1 marker triggering by IFN-γ.48 Karnoub et al. report that MSC-secreted CCL5 induces a transient prometastatic effect on breast cancer cells,8 and Chang report a new signal from the CCL5 secreted by BM-MSC that enhances prostate cancer cell invasion.49 In TSCC, it is also well accepted that MSC are involved in tumor progression. For example, Salo et al. found that the interaction between BMMSC and TSCC cells induces cytokine and matrix molecule expression that results in high levels of type I collagen production and, thus, promotes cancer invasion.50 A number of studies have demonstrated such diverse mechanisms of interaction between MSC and TSCC cells. In our study, we clarified a unique interaction by which MSC promote the proliferation, invasion and metastasis of TSCC cells through the notably increased secretion of CCN2. However, the question of how the tissue context is able to determine the effects of CCN2 on tumor development deserves further exploration. Beyond that, there may be many other different mechanisms involved in the interaction between TSCC cells and MSC that remain unknown.
In summary, our study is the first to demonstrate that the CCN2 secreted by MSC in the TME of TSCC promotes cell proliferation, migration and invasion. This breakthrough provides insight into the understanding of the interplay between MSC and CCN2 during cancer progression. Furthermore, our report also provides useful information for establishing a probable future therapeutic strategy involving the suppression of CCN2 or MSC in TSCC.
Acknowledgments
This work was supported by Science and Technology Planning Project of Guangdong Province, China (2014A020212625) and Natural Science Foundation of Guangdong Province, China (2016A030313196).
Disclosure Statement
The authors have no conflict of interest to declare.




