Proton beam therapy for bone sarcomas of the skull base and spine: A retrospective nationwide multicenter study in Japan
Funding information
Japan Agency for Medical Research and Development, Practical Research for Innovative Cancer Control(15ck0106034 h0102), Translational Research Network Program (C33)
Abstract
We conducted a retrospective, nationwide multicenter study to evaluate the clinical outcomes of proton beam therapy for bone sarcomas of the skull base and spine in Japan. Eligibility criteria included: (i) histologically proven bone sarcomas of the skull base or spine; (ii) no metastases; (iii) ≥20 years of age; and (iv) no prior treatment with radiotherapy. Of the 103 patients treated between January 2004 and January 2012, we retrospectively analyzed data from 96 patients who were followed-up for >6 months or had died within 6 months. Seventy-two patients (75.0%) had chordoma, 20 patients (20.8%) had chondrosarcoma, and four patients (7.2%) had osteosarcoma. The most frequent tumor locations included the skull base in 68 patients (70.8%) and the sacral spine in 13 patients (13.5%). Patients received a median total dose of 70.0 Gy (relative biological effectiveness). The median follow-up was 52.6 (range, 6.3–131.9) months. The 5-year overall survival, progression-free survival, and local control rates were 75.3%, 49.6%, and 71.1%, respectively. Performance status was a significant factor for overall survival and progression-free survival, whilst sex was a significant factor for local control. Acute Grade 3 and late toxicities of ≥Grade 3 were observed in nine patients (9.4%) each (late Grade 4 toxicities [n = 3 patients; 3.1%]). No treatment-related deaths occurred. Proton beam therapy is safe and effective for the treatment of bone sarcomas of the skull base and spine in Japan. However, larger prospective studies with a longer follow-up are warranted.
Abbreviations
-
- BED10
-
- biologically effective dose, alpha/beta ratio of 10.0 Gy
-
- BS
-
- bone sarcoma
-
- CI
-
- confidence interval
-
- CS
-
- chondrosarcoma
-
- LC
-
- local control
-
- OS
-
- overall survival
-
- PBT
-
- proton beam therapy
-
- PFS
-
- progression-free survival
-
- PS
-
- performance status
-
- RBE
-
- relative biological effectiveness
-
- SB
-
- skull base
Bone sarcomas (BSs) are extremely rare, accounting for <0.2% of newly diagnosed malignant tumors in the United States each year.1 The primary definitive treatment for BSs is surgical resection. However, BSs of the skull base (SB) and spine are often difficult to resect completely. Radiotherapy is an option for unresectable or partially resectable tumors, although the majority of BSs are resistant to conventional photon radiotherapy. Therefore, photon radiotherapy has traditionally been used in a neoadjuvant or adjuvant setting.2, 3
The efficacy of proton beam therapy (PBT) for BSs (primarily chordomas and chondrosarcomas [CSs]) of the SB and spine has been reported since the 1980s.4-28 Photons emit maximal energy near the body surface; this energy gradually decreases at deeper points in the body. In contrast, charged particles (e.g., protons and carbon ions) deposit a relatively low-dose near the body surface and emit their maximum energy just before they stop inside the body (the Bragg peak effect). The Bragg peak effect may be spread out according to the location and size of the tumor,29, 30 making it possible to deliver high-dose radiation to the tumor, whilst limiting the dose delivered to the organs at risk. The biological effects of protons are almost identical to the biological effects of photons (relative biological effectiveness [RBE], 1.1).31
Much evidence concerning the effectiveness of PBT for BSs of the SB and spine has been reported from Western countries, whereas only a limited number of small studies7, 17, 22, 28 have been published from Japan, even Asia. As of March 2012, six PBT centers treated BSs in Japan since. These include the Hyogo Ion Beam Medical Center, University of Tsukuba, Shizuoka Cancer Center Hospital, National Cancer Center Hospital East, Southern Tohoku General Hospital, and Fukui Prefectural Hospital.
We conducted a retrospective, nationwide multicenter study to evaluate the clinical outcomes of PBT for BSs of the SB and spine in Japan.
Materials and Methods
Study design and patients
We conducted a retrospective, nationwide multicenter study across six PBT centers in Japan. All patients provided written informed consent. The study protocol was approved by the appropriate Institutional Review Board committee of each center. Research was conducted in accordance with the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013). Eligibility criteria included: (i) histologically proven BSs of the SB or spine; (ii) no metastases; (iii) ≥20 years of age; and (iv) no previous radiotherapy. Of the 103 patients treated between January 2004 and January 2012, we retrospectively analyzed data from 96 patients (93.2%) who were followed-up for >6 months or had died within 6 months.
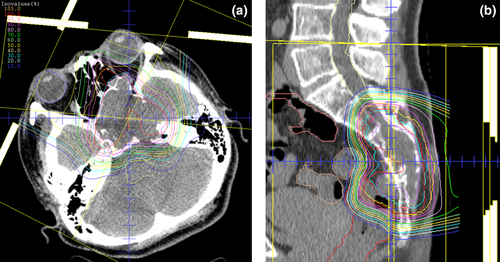
The representative PBT planning procedure was as follows. Radiation treatments were planned using a computed tomography-based three-dimensional treatment planning system. Each patient was immobilized using a custom-made thermoplastic cast and computed tomography and magnetic resonance imaging were performed. The target volumes and organs at risk were delineated on computed tomography-magnetic resonance imaging fusion images. The clinical target volume was defined as the gross tumor volume plus a 5.0-mm basic margin, and the adjacent structures were included in selected patients. The planning target volume was defined as the clinical target volume plus a setup margin and an internal margin where necessary.
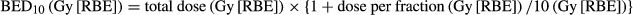
The following are examples of dose constraints in a 32-fraction protocol: brainstem, optic nerve, and spinal cord (cauda equina not included), maximum dose of ≤48.0 Gy (RBE); small intestine, maximum dose of ≤52.0 Gy (RBE); large intestine, maximum dose of ≤57.0 Gy (RBE); and rectum, volume receiving ≥65.0 Gy (RBE) of ≤17.0% and volume receiving ≥40.0 Gy (RBE) of ≤35.0%.
Representative treatment plans for PBT in patients with BSs of the SB and spine are represented in Fig. 1.
Toxicities were evaluated using the Common Terminology Criteria for Adverse Events, version 4.0.
Statistical analyses
Continuous variables are presented as medians and ranges and categorical variables are presented as frequencies and percentages. Overall survival (OS), progression-free survival (PFS), and local control (LC) curves were estimated using the Kaplan–Meier method and compared by the log-rank test. Variables with a P < 0.05 from the univariate analysis were included in the multivariate analysis, using a Cox proportional hazards model. All statistical analyses were conducted using Statistical Package for the Social Sciences for Windows, software version 23 (IBM Corp., Armonk, NY, USA). A two-sided P < 0.05 was considered statistically significant.
Results
Patients
Patient characteristics are summarized in Table 1. Seventy-two patients (75.0%) had chordoma, 20 patients (20.8%) had CS, and four patients (4.2%) had osteosarcoma. The most frequent tumor location was the SB in 68 patients (70.8%), followed by the sacral spine in 13 patients (13.6%). Therefore, the most frequent combinations of histological subtypes and tumor locations were chordoma of the SB (n = 53 patients; 55.2%), CS of the SB (n = 15 patients; 15.6%), and chordoma of the sacrum (n = 12 patients; 2.5%). Pre-PBT, 68 patients (70.8%) underwent surgical resection. Fifty-five (80.9%) of 68 patients with a tumor of the SB and 11 (73.3%) of 15 patients with a tumor of the spine underwent surgical resection, whereas only two (15.4%) of 13 patients with a tumor of the sacrum underwent surgical resection. Four patients (4.2%; osteosarcoma [n = 2 patients], CS [n = 1 patient], and chordoma [n = 1 patient]) received chemotherapy pre-PBT.
| Characteristic | Patients (n = 96) |
|---|---|
| Age, years | |
| Median (range) | 56 (20–80) |
| <60 | 55 (57.3) |
| ≥60 | 41 (42.7) |
| Sex, n (%) | |
| M | 51 (53.1) |
| F | 45 (46.9) |
| PS, n (%) | |
| 0 | 39 (40.6) |
| 1 | 50 (52.1) |
| 2 | 5 (5.2) |
| 3 | 2 (2.1) |
| Histological subtype, n (%) | |
| CH | 72 (75.0) |
| CS | 20 (20.8) |
| OSA | 4 (4.2) |
| Tumor location, n (%) | |
| SB | 68 (70.8) |
| Cervical spine | 8 (8.3) |
| Lumbar spine | 5 (5.2) |
| Lumbosacral spine | 2 (2.1) |
| Sacral spine | 13 (13.6) |
| Tumor status, n (%) | |
| Primary | 73 (76.0) |
| Recurrent | 23 (24.0) |
| Surgery, n (%) | |
| Pre-PBT | 68 (70.8) |
| Post-PBT | 2 (2.1) |
| None | 26 (27.1) |
| Chemotherapy, n (%) | |
| Pre-PBT | 4 (4.2) |
| Post-PBT | 0 (0.0) |
| None | 92 (95.8) |
| PTV, mL | |
| Median (range) | 72 (9–1,984) |
| ≤70 | 48 (50.0) |
| >70 | 48 (50.0) |
| Radiotherapy, n (%) | |
| PBT alone | 93 (96.9) |
| PBT + photon radiotherapy | 3 (3.1) |
| Total dose, Gy (RBE)a | |
| Median (range) | 70 (50–84) |
| ≤70 | 50 (52.1) |
| >70 | 46 (47.9) |
| BED10, Gy (RBE)a | |
| Median (range) | 86 (60–103) |
| ≤85 | 49 (51.0) |
| >85 | 47 (49.0) |
- a The sums of the photon dose/BED10 and proton dose/BED10 were used for patients treated with PBT + photon radiotherapy.
- BED10, biologically effective dose, alpha/beta = 10 Gy; CH, chordoma; CS, chondrosarcoma; F, female; M, male; OSA, osteosarcoma; PBT, proton beam therapy; PS, performance status; PTV, planning target volume; RBE, relative biological effectiveness; SB, skull base.
Patients received a median total dose of 70.0 Gy (RBE) (BED10, 86.0 Gy [RBE]). Three patients (3.1%) were treated with combined PBT and photon radiotherapy (12.5–44.0 Gy in 5–22 fractions). Accelerated hyperfractionation (60.5–77.4 Gy [RBE] in 50–64 fractions, twice daily) was administered to 20 patients (20.8%).
Survival and local control
The median follow-up was 52.6 (range, 6.3–131.9) months. The 5-year OS, PFS, and LC rates for all 96 patients were 75.3% (95.0% confidence interval [CI]: 65.7%–84.9%), 49.6% (95.0% CI: 38.6%–60.6%), and 71.1% (95.0% CI: 60.1%–82.1%), respectively (Fig. 2, 3). The 5-year OS, PFS, and LC rates for chordoma patients (n = 72) were 75.5% (95.0% CI: 63.9%–87.1%), 45.6% (95.0% CI: 32.7%–58.5%), and 68.4% (95.0% CI: 55.1%–81.7%), respectively. The 5-year OS, PFS, and LC rates for CS patients (n = 20) were 83.5% (95.0% CI: 66.3%–100.0%), 72.2% (95.0% CI: 51.2%–93.2%), and 82.2% (95.0% CI: 63.8%–100.0%), respectively. The 5-year OS, PFS, and LC rates for patients with tumors of the SB (n = 68) were 77.6% (95.0% CI: 66.6%–88.6%), 57.0% (95.0% CI: 44.3%–69.7%), and 76.2% (95.0% CI: 64.4%–88.0%), respectively. The 5-year OS, PFS, and LC rates for patients with tumors of the spine (n = 28) were 70.7% (95.0% CI: 51.7%–89.7%), 30.7% (95.0% CI: 11.1%–50.3%), and 55.6% (95.0% CI: 30.3%–80.9%), respectively. The 5-year OS, PFS, and LC rates for patients with chordoma of the SB (n = 53) were 74.6% (95.0% CI: 61.3%–87.9%), 52.8% (95.0% CI: 38.1%–67.5%), and 73.8% (95.0% CI: 59.9%–87.7%), respectively.
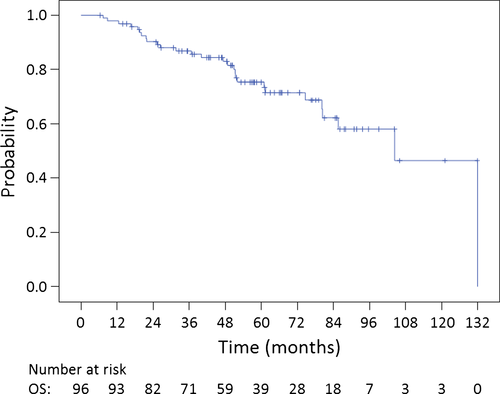
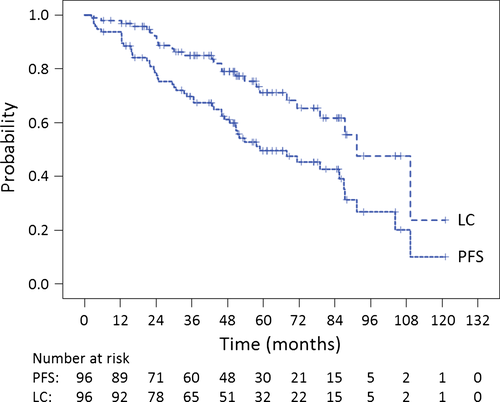
During follow-up, 27 (28.1%) and 19 patients (19.8%) experienced local (in-field) or regional/distant (out-of-field) recurrences, respectively. Frequent sites of out-of-field recurrence included regional (n = 8 patients; 8.3%) and bone metastases (n = 4 patients; 4.2%).
A performance status (PS) of 0–1 was associated with a significantly longer OS (log-rank test, P < 0.001; Table 2). PS (0–1; P < 0.001), tumor location (SB; P = 0.019), and planning target volume (≤70.0 mL; P = 0.026) were associated with a significantly longer PFS, while female sex was associated with a significantly improved LC rate (P = 0.041). Histological subtype, surgery, and BED10 were not associated with OS, PFS, or LC.
| Variable | Patients (n = 96) | P-value | ||
|---|---|---|---|---|
| OS | PFS | LC | ||
| Age, years | ||||
| <60 | 55 | |||
| ≥60 | 41 | 0.167 | 0.455 | 0.380 |
| Sex | ||||
| M | 51 | |||
| F | 45 | 0.606 | 0.455 | 0.041a |
| PS | ||||
| 0–1 | 89 | |||
| 2–3 | 7 | <0.001a | <0.001a | 0.066 |
| Histological subtype | ||||
| CH | 72 | |||
| Other | 24 | 0.773 | 0.194 | 0.169 |
| Tumor location | ||||
| SB | 68 | |||
| Spine | 28 | 0.524 | 0.019a | 0.176 |
| Tumor status | ||||
| Primary | 73 | |||
| Recurrent | 23 | 0.393 | 0.077 | 0.067 |
| Surgery | ||||
| Pre-PBT | 68 | |||
| Post-PBT/none | 28 | 0.241 | 0.537 | 0.971 |
| Chemotherapy | ||||
| Pre-PBT | 4 | |||
| Post-PBT/none | 92 | 0.065 | 0.117 | 0.880 |
| PTV, mL | ||||
| ≤70 | 48 | |||
| >70 | 48 | 0.056 | 0.026a | 0.154 |
| Radiotherapy | ||||
| PBT alone | 93 | |||
| PBT + photon radiotherapy | 3 | 0.280 | 0.145 | 0.193 |
| BED10, Gy (RBE) | ||||
| ≤85 | 49 | |||
| >85 | 47 | 0.250 | 0.240 | 0.637 |
- a P < 0.05.
- BED10, biologically effective dose, alpha/beta = 10 Gy; CH, chordoma; F, female; LC, local control; M, male; OS, overall survival; PBT, proton beam therapy; PFS, progression-free survival; PS, performance status; PTV, planning target volume; RBE, relative biological effectiveness; SB, skull base.
The Cox proportional hazards model revealed that only a PS of 0–1 was associated with a significantly longer PFS (P < 0.001), whilst tumor location (SB) exhibited a trend towards a longer PFS (P = 0.053; Table 3).
| Covariate | Patients (n = 96) | PFS | |
|---|---|---|---|
| 95% CI | P-value | ||
| PS | |||
| 0–1 | 89 | ||
| 2–3 | 7 | 0.071–0.441 | <0.001a |
| Tumor location | |||
| SB | 68 | ||
| Spine | 28 | 0.992–3.283 | 0.053 |
| PTV, mL | |||
| ≤70 | 48 | ||
| >70 | 48 | 0.890–2.859 | 0.116 |
- a P < 0.05.
- CI, confidence interval; PFS, progression-free survival; PS, performance status; PTV, planning target volume; SB, skull base.
Toxicities
Grade 3 acute toxicities occurred in nine patients (9.4%). The most frequent toxicity was dermatitis in four patients (4.2%). All patients completed the planned radiotherapy and subsequently recovered from their reactions. No acute toxicities of ≥Grade 4 occurred.
Late toxicities of ≥Grade 3 occurred in nine patients (9.4%). Grade 3 late toxicities included musculoskeletal and connective tissue disorders in three patients (3.1%; deformity [n = 2] and necrosis [n = 1]), eye disorders in one patient (1.0%; blurred vision and pain), middle ear inflammation in one patient (1.0%), and pain in one patient (1.0%). Grade 4 late toxicities included tissue necrosis in two patients (2.1%) and a brainstem infarction in one patient (1.0%). The patient suffering from a brainstem infarction received a high-dose (maximum, 65.3 Gy [RBE] with a mean of 46.7 Gy [RBE]) to the brainstem. No treatment-related deaths occurred.
Discussion
Our study is the first to evaluate PBT for BSs of the SB and spine on a nationwide multicenter basis in Japan. To the best of our knowledge, this study comprises the largest cohort of patients among reports published from Asia. Our findings are promising given that BSs of the SB and spine are difficult to resect completely. Recently, reports regarding particle therapy, including PBT and carbon ion radiotherapy,32-36 for BSs of the SB and/or spine have been increasing rapidly, especially between 2014 and 2016. The results of recent studies, from 2011 to 2016, are summarized in Table 4. With respect to histological subtype, CS patients were generally associated with more favorable outcomes compared to chordoma patients. Weber et al.25 demonstrated in a multivariate analysis that CS patients had a significantly improved OS and LC rate compared to chordoma patients. In our study, histological subtype was not a significant factor for OS, PFS, or LC. Regarding the comparison between PBT and carbon ion radiotherapy, there appears to be no apparent differences between these two treatment modalities. Mima et al.22 published the results of particle therapy using carbon ions or protons as a definitive treatment for primary sacral chordoma patients and reported that there were no significant differences between the two ion types. Although a randomized controlled trial is needed to validate this finding, a German group is conducting a phase II trial of PBT and carbon ion radiotherapy for chordomas of the SB, CSs of the SB, and sacrococcygeal chordomas.37-39
| Author(s) | Year | Patients | Histological subtype | Tumor location | Therapy | OS | LC |
|---|---|---|---|---|---|---|---|
| Staab et al.18 | 2011 | 40 | CH | Spine | P ± X ± S | 80% (5y) | 62% (5y) |
| Fuji et al. 17 | 2011 | 16 | CH/CS | SB | P ± S | 100% | 100% (CH), 86% (CS) (3y) |
| Matsumoto et al. 32 | 2013 | 47 | CH/CS/OSA/Other | Spine | C ± S | 52% (5y) | 79% (5y) |
| Uhl et al. 33 | 2014 | 155 | CH | SB | C ± S | 85% (5y) | 72% (5y) |
| Uhl et al. 34 | 2014 | 79 | CS | SB | C ± S | 96% (5y) | 88% (5y) |
| DeLaney et al. 20 | 2014 | 50 | CH/CS/Other | Spine | X + P ± S | 84% (5y) | 81% (5y) |
| Deraniyagala et al. 21 | 2014 | 33 | CH | SB | P ± S | 92% (2y) | 86% (2y) |
| Mima et al. 22 | 2014 | 23 | CH | Sacrum | C or P | 83% (3y) | 94% (3y) |
| Rotondo et al. 23 | 2015 | 126 | CH | Spine | X + P ± S | 81% (5y) | 62% (5y) |
| Uhl et al. 35 | 2015 | 56 | CH | Sacrum | C ± IMRT | 52% (5y) | 79% (5y) |
| Holliday et al. 24 | 2015 | 19 | CH/CS | Spine | S + P | 93% (2y) | 58% (2y) |
| Weber et al. 25 | 2016 | 222 | CH/CS | SB | S + P | 86% (5y) | 81% (5y) |
| Imai et al. 36 | 2016 | 188 | CH | Sacrum | C | 81% (5y) | 77% (5y) |
| Feuvret et al. 26 | 2016 | 159 | CS | SB | S + P | 96% (5y) | 95% (5y) |
| Indelicato et al. 27 | 2016 | 51 | CH/CS | Spine | P ± S | 72% (4y) | 58% (4y) |
| Hayashi et al. 28 | 2016 | 19 | CH | SB | S + P | 83% (5y) | 75% (5y) |
| Current study | 2016 | 96 | CH/CS/OSA | SB, Spine | P ± S | 75% (5y) | 71% (5y) |
- C, carbon ion; CH, chordoma; CS, chondrosarcoma; IMRT, intensity modulated radiotherapy; LC, local control; OS, overall survival; OSA, osteosarcoma; P, proton; S, surgery; SB, skull base; X, photon; y, year.
In the present study, univariate and multivariate analyses revealed that a PS of 0–1 was associated with a significantly longer OS (P < 0.001) and PFS (P < 0.001), whereas female sex was associated with a significantly improved LC rate (P = 0.041). To the best of our knowledge, this is the first report to identify PS as a significant prognostic factor, although most previously published reports did not include PS as a variable in the prognostic analyses. The statistical significance of PS may be due to chance alone since it was highly unbalanced between the two groups (0–1: n = 89 vs. 2–3: n = 7). However, it is logical that PS would affect survival. Staab et al.18 and Mima et al.22 reported that male chordoma patients had a significantly longer OS and PFS than female chordoma patients. Conversely, in the present study comprising 72 chordoma patients (75.0%), female sex was associated with a significantly improved LC rate. Several hypotheses have been proposed to explain the possible influence of sex on the treatment outcome of chordoma patients, as reviewed by Halperin.40 For instance, sex hormone receptors may represent an influential factor in adult chordoma patients and genetic factors may also play a role in determining clinical outcomes. Although not statistically significant overall, a planning target volume of ≤70.0 mL was associated with a significantly longer PFS in the univariate analysis (P = 0.026), but not the multivariate analysis (P = 0.116), and exhibited a trend towards a longer OS (P = 0.056). Several other studies11, 15, 18, 25, 41 have demonstrated that tumor volume is a significant prognostic factor.
Proton beam therapy-related acute and late toxicities appeared to be tolerable. Grade 3 acute toxicities were reversible and did not influence treatment schedules. Nine patients (9.4%) experienced ≥Grade 3 late toxicities with a median follow-up of 52.6 months. However, the tumors were close to the affected organs and the events were considered to be unavoidable in all cases. Grade 4 brainstem infarction occurred in a patient with CS of the SB. Information concerning radiation-associated toxicities is very limited in the literature, most probably because most published series are retrospective studies that extend back several decades to accumulate an adequate number of patients. In such a scenario, two long-term studies of PBT for BSs of the SB or spine20, 25 have been published. DeLaney et al.20 reported Grade 3/4 late toxicities in 10.0% and 13.0% of spinal chordoma, CS, and other sarcoma patients at 5 and 8 years, respectively, whereas Weber et al.25 reported Grade 3/4 late toxicities in 8.1% of SB chordoma and CS patients with a median follow-up of 50.0 months. Our results are comparable with the findings of these two studies. Despite high doses to treatment volumes, accompanying toxicities were relatively low, even though the majority of tumors were located in regions adjacent to the organs at risk (i.e., the brainstem, optic nerve, and spinal cord), because the precise dose distribution of PBT could limit doses to critical structures.
This study has three important limitations. The foremost are its retrospective design and the relatively low impact of the statistical analyses. However, many previously published studies have also used a retrospective design, which is likely related to the difficulty of performing a prospective study given the rarity of the disease. Second, the follow-up period was relatively short (median, 52.6 months) and the major histological subtypes (chordoma and CS) in this study are slow growing with the potential for recurrence 5 years post-PBT. Therefore, we will continue to monitor these patients with the intention of being able to report on follow-up data. Finally, PS was highly unbalanced between the two groups (0–1: n = 89 vs. 2–3: n = 7), and thus, the statistical significance of this variable may have occurred by chance alone. However, we identified no biased estimates with unstable standard errors in our multivariate analysis.
In conclusion, we are the first to demonstrate the safety and efficacy of PBT for BSs of the SB and spine in a retrospective, nationwide multicenter study in Japan. Larger prospective studies with a longer follow-up are required to validate these findings.
Acknowledgments
We thank the Japanese Society for Radiation Oncology for its full cooperation and support for this study. We also thank the members of the Hokkaido University Hospital Clinical Research and Medical Innovation Center (Hokkaido, Japan) for their assistance in study planning and data management. This study was partially supported by grants from the Translational Research Network Program (C33) and Practical Research for Innovative Cancer Control (15ck0106034 h0102) from the Japan Agency for Medical Research and Development.
Disclosure Statement
The authors have no conflict of interest.




