Population genetic structure of the insular Ryukyu flying fox Pteropus dasymallus
Associate Editor: Ferry Slik
Handling Editor: Kim McConkey
Abstract
Small isolated populations are vulnerable to both stochastic events and the negative consequences of genetic drift. For threatened species, the genetic management of such populations has therefore become a crucial aspect of conservation. Flying foxes (Pteropus spp, Chiroptera) are keystone species with essential roles in pollination and seed dispersal in tropical and subtropical ecosystems. Yet many flying fox species are also threatened, having experienced dramatic population declines driven by habitat loss and hunting. The insular Ryukyu flying fox (Pteropus dasymallus) ranges from the Ryukyu Archipelago of Japan through Taiwan to the northern Philippines and has undergone precipitous population crashes on several islands in recent decades. To assess the population genetic structure and diversity in P. dasymallus, and its likely causes, we analyzed mitochondrial and microsatellite DNA. Both markers showed significant genetic differentiation among most island populations, with mitochondrial haplotypes showing some mixing across the regions, likely reflecting historical colonization and/or dispersal events. In contrast, microsatellite markers showed an overall pattern of isolation by distance; however, this pattern appeared to be driven by the presence of deep ocean trenches between geographically distant populations. Thus, the current distribution of P. dasymallus and its subspecific diversity appear to have arisen through vicariance coupled with a long history of restricted gene flow across oceanic barriers. We conclude that isolated island subgroups should be managed separately, with efforts directed at reducing further declines in genetic diversity.
1 INTRODUCTION
Small and isolated populations are vulnerable to stochastic events and the effects of genetic drift, potentially leading to the loss of diversity, reduced reproductive fitness, and increased risk of extinction (Ellstrand & Elam, 1993; Frankham, 2010; Jordan et al., 2016). The genetic management of such populations should therefore be considered an essential aspect of conservation, particularly for endangered and threatened species. With detailed genetic information, we can effectively estimate the loss of genetic diversity and quantify demographic parameters such as population size fluctuation, admixture, and gene flow, all of which contribute to our understanding of long-term species survival (Allendorf et al., 2010; Shafer et al., 2015). Genetic, phenotypic, and environmental considerations can therefore be integrated to enhance the efficiency of conservation programs and further formulate appropriate management strategies (Frankham et al., 2017; Hoban et al., 2013; Polechová & Barton, 2015).
Old World fruit bats (Chiroptera: Pteropodidae) are keystone species that play essential roles in pollination and seed dispersal in tropical and subtropical ecosystems (Cox et al., 1991; Fujita & Tuttle, 1991). Aside from promoting long-distance seed dispersal needed for forest restoration (Nyhagen et al., 2005; Shilton & Whittaker, 2009), these species also pollinate a number of economically important plants, including durian (Sheherazade et al., 2019). However, despite being major contributors to ecosystem services, pteropodids face a variety of threats. The combined impacts of habitat loss, forest degradation, and hunting have led to severe and rapid population declines over the past few decades (Mickleburgh et al., 2002), with wide-ranging negative ecological and other impacts (Cox & Elmqvist, 2000; Florens et al., 2017; McConkey & Drake, 2006). Of the nearly 200 recognized pteropodid species, over one-third are of conservation concern, including ~ 30 species of Pteropus species (IUCN, 2020).
Larger-bodied pteropodids are generally considered to be strong and capable fliers with extensive home ranges. For example, genetic analyses of P. scapulatus and Eidolon helvum have suggested that gene flow can occur over thousands of kilometers, across mainland Australia and Africa, respectively (Peel et al., 2013; Sinclair et al., 1996). In addition, long-distance interisland movements have been recorded and/or inferred indirectly from genetic data in several Pteropus species, for example, P. niger, P. tonganus, and P. vampyrus (Larsen et al., 2014; McConkey & Drake, 2007; Tsang et al., 2018). In such taxa, bats that occur on multiple adjacent islands can appear as a single panmictic population. However, in other flying fox species, for example, P. livingstonii, P. mariannus, and P. samoensis, genetic structure is observed among island groups, indicative of restricted gene flow (Brown et al., 2011; Ibouroi et al., 2018; Russell et al., 2016). These contrasting scenarios require different conservation management strategies; in the former case, island groups might be managed as a single entity, while in the latter case, island populations might be better treated as distinct evolutionarily significant units (ESUs) and thus managed separately (Epstein et al., 2009; Oleksy et al., 2019).
The Ryukyu flying fox (Pteropus dasymallus) is distributed from the Ryukyu Archipelago of Japan through Taiwan to the northern Philippines (Kinjo & Nakamoto, 2015; Figure 1). Five subspecies are recognized, with populations from the Ryukyu Archipelago classified into four subspecies based on their respective island group ranges (Daito flying fox, P. d. daitoensis; Erabu flying fox, P. d. dasymallus; Orii's flying fox, P. d. inopinatus; and Yaeyama flying fox, P. d. yayeyamae). The fifth subspecies, from Taiwan, is classified as the Formosan flying fox (P. d. formosus) (Mickleburgh et al., 1992; Yoshiyuki, 1989), while a population on some of the Batanes and Babuyan Islands in the Philippines was discovered more recently and has yet to be named formally as a subspecies (Heaney et al., 1998).
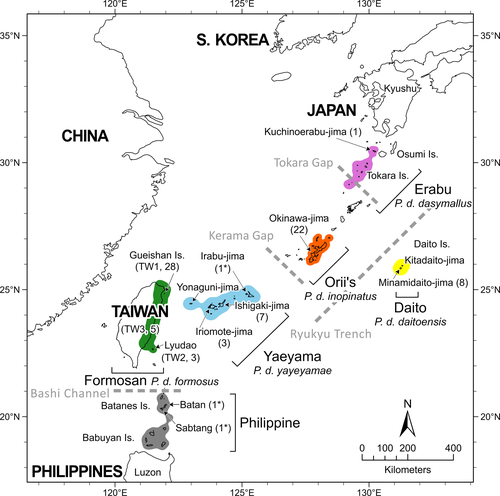
P. dasymallus is currently listed as Vulnerable on the IUCN Red List (IUCN, 2020); however, the local conservation status differs among the six subspecies (including the unnamed Philippine subspecies) (Vincenot et al., 2017). Three (Orii's, Yaeyama, and the Philippines subspecies) are more common and are not classified as locally threatened (Heaney et al., 1998; Saitoh et al., 2015). The remaining three (Daito, Erabu, and Formosan subspecies) are all characterized by small populations of ~ 50–300 individuals (Saitoh et al., 2015; Wu, 2010) and are protected by national laws (Daito is a “National Endangered Species”; Daito and Erabu are “Natural Monuments” of Japan; and Formosan is an “Endangered Species” in Taiwan). Of these, the Daito flying fox is geographically restricted to only two small islands (Minamidaito-jima and Kitadaito-jima, Figure 1), where most natural habitat has been converted into farmland and where typhoons remain a major threat (Saitoh et al., 2015). The Erabu flying fox is found on the Osumi Islands and Tokara Islands, representing the northern limit of this species (Yoshiyuki, 1989). In Taiwan, the Formosan flying fox was once abundant on Lyudao (Green Island), 30.6 km off the southeastern coast of Taiwan; however, intensive hunting and habitat loss in the 1970s and 1980s led to its near extinction (Lin & Pei, 1999). In 2004, an additional small population of the Formosan flying fox was recorded for the first time on Gueishan Island (Turtle Island), 9.7 km off the northeastern coast of Taiwan, and some individuals have occasionally been found on the main island of Taiwan since 2006 (Wu, 2010), although the origin of these populations is unclear. Other indirect evidence of movements across water comes from the population census of Orii's flying foxes (Nakamoto et al., 2011) and from inferred gene flow from the Yaeyama flying fox (Taki et al., 2020).
To date, the phylogenetic relationships and levels of population divergence among the subspecies of P. dasymallus are poorly known but are likely to reflect the geography of the Ryukyu Archipelago. This island chain comprises approximately 150 islands, extending for 1,200 km between the main islands of Japan and Taiwan, some of which were connected by land bridges during the Last Glacial Maximum (LGM) (Ota, 1998). Genetic studies of reptiles and amphibians have revealed divergence between populations from the Northern, Central, and Southern Ryukyus (Ota, 1998, 2000), regions that remained separated during the LGM by two deep tectonic straits: the Tokara Gap and the Kerama Gap (Figure 1) (Nakamura et al., 2013). Both of these gaps formed geographical barriers, promoting genetic drift and divergence between isolated populations (Lin et al., 2002; Toda et al., 1997). Taiwan is geographically closest to the Southern Ryukyus (110 km), and more similarities in species and genetic diversity of some reptile and amphibian species have also been found among Taiwan and the Southern Ryukyus than among the other regions (> 270 km) (Ota, 2000; Tominaga et al., 2015). The two oceanic archipelagoes of the Batanes and Babuyan Islands were formed approximately three million years ago (late Pliocene), with the Batanes closer to Taiwan (150 km) than to Luzon (200 km) (Bellwood & Dizon, 2013). The genetic structure of mammals on the island chain has seldom been addressed, likely due to logic difficulties in conducting research here.
Here, we examined genetic diversity and population genetic structure in P. dasymallus using mitochondrial DNA (mtDNA) and microsatellite markers. We aimed to: (a) quantify the genetic diversity of P. dasymallus across the island groups; (b) assess whether genetic differentiation occurs among island groups, corresponding to their subspecies designations; and (c) characterize the pattern of population genetic structure, including determining how the recently recorded Formosan flying fox populations from Taiwan's main island and its offshore Gueishan Island and the Philippines are related to the other populations. Our hypotheses were as follows: First, we hypothesized that the Daito, Erabu, and Formosan subspecies would have lower genetic diversity than the other three subspecies due to their relatively small population sizes. Second, we hypothesized that genetic differentiation exists in P. dasymallus and corresponds to their respective subspecies identities. Third, similar to many other species in this region, this differentiation can be accounted for by regional deep-sea trenches: the Tokara and Kerama Gaps in the Ryukyu Archipelago and the Bashi Channel between Taiwan and the northern Philippines. Finally, we hypothesized that the newly recorded Formosan individuals would show closest genetic affiliations with bats from the nearest Yaeyama Islands, consistent with colonization from the latter. An understanding of the genetic structure and degree of gene flow among different island groups of P. dasymallus, along with insights into whether these patterns are ancient or recent, can inform conservation management decisions, including whether or not to treat small populations separately or whether to translocate isolated vagrants.
2 METHODS
2.1 Sampling
We obtained samples of P. dasymallus opportunistically over a period of 10 years (2009–2019) from wild-caught individuals and carcasses found in the wild, as well as rescued and/or captive individuals. All samples originated from eight different Taiwanese and Ryukyu islands and were classified into the five subspecies based on their geographical source following Yoshiyuki (1989), Mickleburgh et al. (1992), and Kinjo and Nakamoto (2015). A total of 77 samples were analyzed for this study after removing three duplicate samples and three putative first-degree relatives, as outlined below. Sample sizes per subspecies were 36 Formosan, 10 Yaeyama, 22 Orii's, 1 Erabu, and 8 Daito. Formosan flying fox samples were further divided into three groups denoted as TW1 (Gueishan Island), TW2 (Lyudao), and TW3 (Taiwan's main island) according to the islands from which they originated. Yaeyama samples originated from Iriomote-jima and Ishigaki-jima, Orii's samples from Okinawa-jima, Erabu sample from Kuchinoerabu-jima, and Daito samples from Minamidaito-jima (Figure 1, Table S1).
Samples ranged from wing membrane biopsies, blood, and frozen muscle tissue to fecal samples. Wing membrane samples were collected with a 3-mm biopsy punch and placed in 99.5% ethanol, Allprotect Tissue Reagent (Qiagen), or silica beads until extraction. For blood, a volume of 0.5 cc was taken by a professional veterinarian and preserved in an EDTA anticoagulant tube. Frozen tissue obtained from specimens and fresh feces were stored in 99.5% ethanol or RNAlater (Qiagen).
2.2 DNA extraction and amplification
To extract genomic DNA from wing membrane, blood, and frozen muscle samples, we used DNeasy Blood and Tissue Kit (Qiagen). For fecal samples, we used the QIAamp Investigator Kit or QIAamp Fast DNA Stool Mini Kit (Qiagen).
We amplified a section of the mtDNA control region using the primers BovL 14,987 (5'-CGC-ATA-TGC-AAT-CCT-ACG-A-3') and BovR 15,967 (5'-GCG-GGT-TGC-TGG-TTT-CAC-3'), which we designed for this study. PCR was carried out in a total volume of 15 μl, containing 20–100 ng of template DNA, 0.375 μl of 10 μM of each primer, and 7.5 μl of Quick Taq HS DyeMix (TOYOBO). Amplification was performed with the following profile: 2 min at 94ºC followed by 35 cycles of 30 s at 94ºC, 30 s at annealing temperature (55℃), 50 s at 68ºC, and a final extension of 10 min at 68ºC. PCR products were run on an ABI 3730XL DNA Analyzer (Applied Biosystems). Chromatograms were edited and aligned in the SeqMan and MegAlign (DNASTAR) programs. We also obtained three published P. dasymallus partial control region sequences from GenBank for one Yaeyama flying fox from Irabu-jima (NC_002612) (Nikaido et al., 2000a, 2000b) and two individuals collected from the Batanes Islands, with one from Batan Island and the other from Sabtang Island (MN477630 and MN477629, respectively), representing the Philippine population (Tsang et al., 2020a, 2020b).
For microsatellite DNA analyses, we successfully genotyped 76 samples at 26 polymorphic loci developed for this study (Table S2). PCR was carried out in a total volume of 10 μl, containing approximately 10–50 ng of template DNA, 0.5 μl of 10 μM of each primer, and 5 μl of Quick Taq HS DyeMix. Amplification was performed with the following profile: 2 min at 94°C, followed by 40 cycles of 30 s at 94°C, 30 s at annealing temperature (54°C), 1 min at 68°C, and a final extension of 10 min at 68°C. PCR products were also run on an ABI 3730XL DNA Analyzer, and allele scoring was performed using the software GeneMarker 4.2 (SoftGenetics). Identity and parentage analyses were performed using Cervus 3.0.7 (Kalinowski et al., 2007). Samples with identical genotypes across all loci were determined to be duplicates and removed. Similarly, first-degree descendant relatives were determined on the basis of no allele mismatches, and where potential parent-offspring pairs were inferred, only one individual from each pair was retained.
2.3 MtDNA analysis
Based on mtDNA data, we estimated the number of haplotypes, haplotype diversity (h), nucleotide diversity (π), and average number of pairwise differences for each subspecies. We determined the extent of genetic differentiation by applying an analysis of molecular variance (AMOVA) (Excoffier et al., 1992). Only populations with sample sizes greater than one were included. The total variance was partitioned into variance components attributable to within and among subspecies. To measure the degree of genetic differentiation among subspecies, we calculated ΦST and assessed significance by performing 20,000 random permutations. These analyses were performed using Arlequin 3.5.2.2 (Excoffier & Lischer, 2010).
To determine whether population genetic structure followed a pattern of isolation by distance, we tested for a correlation between pairwise genetic and geographical distances among islands. Pairwise genetic distance was estimated as ΦST/(1–ΦST) following Rousset (1997). For corresponding pairwise geographical distances (km), we took the natural logarithm of the linear Euclidean distance between the centers of pairwise sampling islands, computed based on latitudinal and longitudinal coordinates (see Rousset, 1997). A total of eleven islands were included in the analysis of isolation by distance (Minamidaito-jima, Kuchinoerabu-jima, Okinawa-jima, Irabu-jima, Ishigaki-jima, Iriomote-jima, Gueishan Island, Lyudao, the main island of Taiwan, Batan Island, and Sabtang Island). The significance of the correlation was assessed using a Mantel test with 20,000 permutations in Arlequin 3.5.2.2. To examine whether any detected pattern of isolation by distance remained evident after controlling for the presence of deep-sea trenches between island groups, we ran a partial Mantel test. For this analysis, pairs of populations separated by a deep-sea trench were scored as 1, and pairs not separated by a trench were scored as 0.
To further visualize genetic structure with respect to subspecies, we generated a haplotype network. An unrooted maximum likelihood tree was generated in MEGA X (Kumar et al., 2018) and converted into a haplotype network using Haplotype Viewer (Center for Integrative Bioinformatics Vienna).
2.4 Microsatellite DNA analysis
Deviation from Hardy–Weinberg equilibrium (HWE) at each locus and subspecies and linkage disequilibrium for each pair of loci were tested using the Markov chain method (10,000 dememorization steps, 1,000 batches, and 10,000 iterations per batch). We assessed statistical significance using Bonferroni correction for multiple comparisons. For each locus, we recorded the number of alleles (NA), observed heterozygosity (HO), and expected heterozygosity (HE). For each subspecies, we derived diversity indexes, including the mean number of alleles (Na), allelic richness corrected for unequal sample size (AC), the mean HO and HE, and inbreeding coefficient (FIS). The average pairwise relatedness (RI) of each subspecies was calculated to infer relationships between individuals (Ritland, 1996).
As for mtDNA, we also conducted genetic structure analyses, including AMOVA, pairwise differentiation, estimated by FST, and isolation by distance, for microsatellite data. Eight islands were included here due to a lack of microsatellite data for Irabu-jima, Batan Island, and Sabtang Island. These analyses were performed in GenAlEx 6.51 (Peakall & Smouse, 2006, 2012), Fstat 2.9.4 (Goudet, 2003), Genepop 4.7.3 (Rousset, 2008), and Arlequin 3.5.2.2.
To examine relationships among populations based on multilocus microsatellite genotype data, we inferred the number of genetically distinct clusters using the Bayesian clustering approach implemented in STRUCTURE 2.3.4 (Falush et al., 2003; Pritchard et al., 2000). An admixture ancestry model with correlated allele frequencies was used with a burn-in of 100,000 iterations followed by 1,000,000 Markov chain Monte Carlo (MCMC) repetitions. The number of ancestral populations (K) was set from 1 to 10. To confirm consistency across runs, 10 independent runs for each K were performed with prior information on population origins. The best number of K was determined using the ad hoc statistic ΔK (Evanno et al., 2005) in Structure Harvester (Earl & vonHoldt, 2012). The outputs were generated and visualized with Clumpak 1.1 (Kopelman et al., 2015).
3 RESULTS
3.1 MtDNA analysis
Analyses of partial mtDNA control region sequences revealed 33 haplotypes from a total of 80 P. dasymallus samples encompassing the five recognized subspecies and the Philippine population. Haplotype diversity, nucleotide diversity, and pairwise difference averaged over all samples were 0.948, 0.012, and 3.556, respectively. A summary of the genetic diversity is presented in Table 1. The Philippine population showed the highest diversity. On the other hand, the Daito flying fox consistently showed the lowest diversity. We excluded the Erabu individual from the subspecies-level analyses given that only one sample was available.
| Subspecies | mtDNA | Microsatellite DNA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | H | h | π | k | N | N a | A C | H O | H E | RI | F IS | |
| Formosan | 36 | 16 | 0.889 | 0.012 | 3.557 | 36 | 4.885 | 3.582 | 0.572 | 0.599 | 0.004* | 0.058 |
| Yaeyama | 11 | 10 | 0.982 | 0.011 | 3.200 | 10 | 3.731 | 3.479 | 0.588 | 0.581 | 0.004 | 0.039 |
| Orii's | 22 | 9 | 0.844 | 0.008 | 2.377 | 22 | 3.846 | 3.351 | 0.542 | 0.575 | 0.029* | 0.081 |
| Erabu | 1 | 1 | – | – | – | 1 | – | – | – | – | – | – |
| Daito | 8 | 3 | 0.679 | 0.007 | 2.214 | 7 | 2.885 | 2.885 | 0.429 | 0.415 | 0.149* | 0.045 |
| Philippine | 2 | 2 | 1.000 | 0.043 | 13.000 | – | – | – | – | – | – | – |
Note
- N: sample size, H: number of haplotypes observed, h: haplotype diversity, π: nucleotide diversity, k: average number of nucleotide differences, Na: mean number of alleles per locus, AC: allelic richness, HO: observed heterozygosity, HE: expected heterozygosity, RI: relatedness, FIS: inbreeding coefficient. The Erabu flying fox was excluded for subspecies-level analyses given that only one sample is available.
- * Statistically significant p < .05.
The AMOVA revealed genetic differentiation among the five analyzed populations (Daito, Orii's, Yaeyama, Formosan, and Philippine populations with sample sizes > 1). The ΦST value was 0.140, which was significantly different from zero (p < .001, Table 2), indicating that ~ 14.0% of the total mtDNA genetic variation was accounted for by differences among subspecies. The magnitude of the mean pairwise differentiation varied markedly with the lowest value in the Formosan-Yaeyama population pair. The Daito and Philippine populations both had relatively high values of genetic differentiation (Table 3).
| Source of variation | mtDNA | Microsatellite DNA | ||
|---|---|---|---|---|
| Variation (%) | p value | Variation (%) | p value | |
| Among subspecies | 13.97 | <.001* | 6.99 | <.001* |
| Within subspecies | 86.03 | 93.01 | ||
Note
- Five subspecies, including the Daito, Orii's, Yaeyama, Formosan, and Philippine subspecies, with sample sizes greater than one are included in the mtDNA analysis. The first four are included in the microsatellite analysis
- * Statistically significant.
| Subspecies | Formosan | Yaeyama | Orii's | Daito | Philippine |
|---|---|---|---|---|---|
| Formosan | 0.000NS | 0.096* | 0.166* | 0.407* | |
| Yaeyama | 0.011NS | 0.079* | 0.158* | 0.361* | |
| Orii's | 0.057* | 0.035* | 0.247* | 0.519* | |
| Daito | 0.136* | 0.141* | 0.161* | 0.439* |
Note
- ΦST, above diagonal based on mtDNA data; FST, below diagonal based on microsatellite data. The Erabu flying fox is excluded. Statistical significance is also provided.
- Abbreviation: NS, nonsignificant.
- * Significant at p < .05.
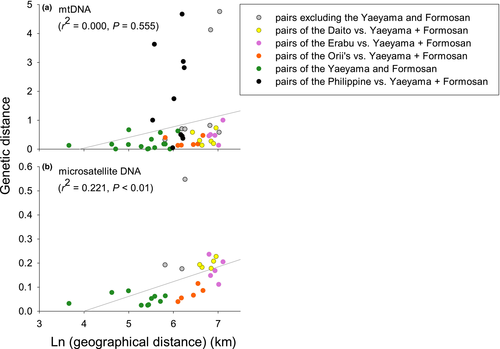
The relationship between pairwise genetic and geographical distances was not significant (r2 = 0.000, p = .555, Mantel test), indicating that genetic differentiation of mtDNA in P. dasymallus across its range did not fit an isolation-by-distance model (Figure 2). Based on pairwise genetic distances, the TW1 Formosan flying fox showed a close relationship with Yaeyama populations from Iriomote-jima and Ishigaki-jima (0.082 and 0.016, respectively), with lower pairwise distances than those recorded between TW1 and both the TW2 and TW3 populations from the same subspecies (0.252 and 0.143, respectively).
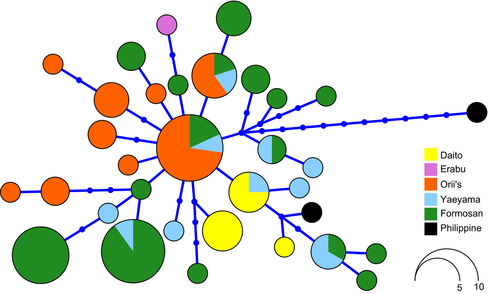
The haplotype network indicated that the Philippine sample from Sabtang Island was most genetically divergent from all the other samples. On the other hand, low levels of haplotype divergence were seen within Japan and Taiwan (Figure 3). Of these, two haplotypes were the most common, shared by 11 and 10 individuals mainly found in Okinawa-jima (Orii's flying fox) and Gueishan Island (TW1 Formosan flying fox), respectively.
3.2 Microsatellite analysis
Genotype analysis based on 26 polymorphic microsatellite loci from 76 P. dasymallus samples revealed a moderate degree of polymorphism across subspecies. The mean HO and HE values were 0.533 and 0.542, respectively, with the Erabu sample excluded. The highest genetic diversity was recorded in the Formosan and Yaeyama flying foxes. In contrast, and in line with the mtDNA results, the Daito flying fox harbored the lowest genetic diversity (Table 1). The FIS values were all not significant, implying no major deviations from HWE. Four locus-population combinations deviated from HWE; however, there was no consistent pattern according to either subspecies or locus. No significant linkage disequilibrium was found among loci. Finally, average pairwise relatedness was significant in all analyzed populations except Yaeyama. The Daito population showed a particularly high value of 0.149.
The AMOVA based on microsatellite data showed significant genetic differentiation among nearly all pairs of subspecies, with a FST value of 0.070 (p < .001, Table 2). The only exception to this was the Formosan and Yaeyama flying foxes, which did not show significant differentiation. We found a pattern of isolation by distance among pairs of islands (r2 = 0.221, p < .01, Figure 2). In addition, while we detected a strong correlation between genetic distance and sea trenches (r2 = 0.320, p < .05, Mantel test), we found that isolation by distance was not significant after controlling for the effect of sea trenches (r2 = 0.001, p = .519, partial Mantel test).
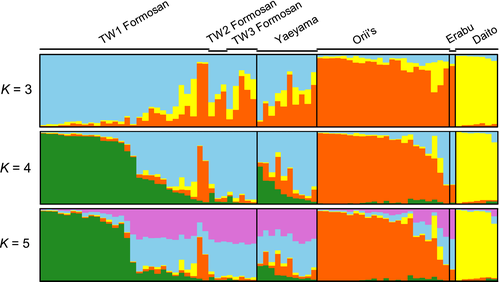
The STRUCTURE analysis revealed a clear substructure between some localities and subspecies identities (Figure 4), with the most likely number of genetic clusters found to be K = 4. All or nearly all of the Daito and Orii's flying fox samples were assigned unambiguously to their own clusters, with the exception of one individual of Orii's flying fox. Several individuals also showed evidence of partial inferred ancestry. Formosan flying foxes from TW2 (Lyudao) and TW3 (Taiwan's main island) and Erabu flying foxes were assigned to a third genetic cluster. In contrast, TW1 Formosan flying foxes (Gueishan Island) and Yaeyama flying foxes showed a greater admixture across different genetic clusters with full or partial membership. For K = 3, the Erabu and Orii's flying foxes were grouped together. The Formosan and Yaeyama flying foxes showed admixtures of different genetic clusters. For K = 5, the Erabu flying fox also had an admixture of membership.
4 DISCUSSION
We examined genetic diversity and structure among the five recognized subspecies of P. dasymallus from the Ryukyu Archipelago of Japan to Taiwan and included published data from two individuals sampled from a poorly known population from Batanes, Philippines. Our analyses based on mtDNA control region sequences and 26 microsatellite markers revealed significant genetic differentiation among island groups, broadly supporting the subspecies identities of the Daito and Orii's flying foxes based on their respective geographical localities of Minamidaito-jima and Okinawa-jima (Figure 4). No significant differentiation was detected between the Yaeyama and Formosan flying foxes, consistent with the apparent mixed ancestry of the latter subspecies.
Differences in the patterns of differentiation recovered by both types of markers provide insights into the history of connectivity of these island populations. Notably, the individuals from the five Ryukyu and Taiwanese subspecies showed weak haplotype sorting with respect to their island localities (Figure 3). This pattern suggests past gene flow, either through recurrent dispersal or colonization and admixture, alongside some evidence of isolation and genetic drift in a few cases, likely reflecting small population sizes. The central position of the orange haplotype, which was most abundant in Orii's flying foxes, suggests that this taxon might have served as a source of other populations across the Ryukyu and Taiwanese islands. If this scenario is correct, then it would imply that these other subspecies populations were founded by colonization events, eastward to the Daito Islands and westward to Taiwan, although more sampling is needed to confirm this. While we were only able to examine two individuals from two different islands from the Philippines, they showed evidence of high levels of divergence with respect to each other, with the bat sampled from Sabtang Island also showing clear separation from all other samples. Further work, including sampling of bats from the Babuyan Islands, is needed to assess the likely causes of this apparent deep structure; however, it is plausible that the undersampled Philippine population contains high genetic diversity.
Levels of genetic differentiation obtained from multilocus microsatellite markers were generally lower than those based on mtDNA. Given the contrasting modes of biparental and maternal inheritance of ncDNA and mtDNA, stronger subdivision in the latter is consistent with a system of female philopatry and male-biased gene flow but is also an expected consequence of the smaller effective size of the mitochondrial genome (see Arnold & Wilkinson, 2015; Chen et al., 2008) (Table 2). Microsatellite analyses also revealed a downward cline in genetic diversity from the Formosan and Yaeyama flying foxes to the Daito flying fox (Table 1). It is noteworthy that the Yaeyama flying fox also showed a relatively lower inbreeding coefficient, which, in concert with its high haplotype and nucleotide diversity, could point to a rather large stable population with a long evolutionary history (Grant & Bowen, 1998).
Of all the taxa, the Daito flying fox was seen to form a separate cluster with no admixture across different values of K (Figure 4). The genetic distinctiveness of the Daito flying fox may be explained by the comparatively large geographical distance between the remote easternmost Daito Islands and the other Ryukyu islands (~360 km east of Okinawa-jima) coupled with the absence of islands that could serve as stepping stones for dispersers. Indeed the Daito Islands are uplifted coral islands that are thought to have emerged approximately 1.2–1.6 million years ago in the mid-Pleistocene and have never been connected to a continental landmass (Shiroma et al., 2015). Instead, the Ryukyu Trench (Figure 1), a deep, broad water body that separates the Daito Islands from the Eurasia Plate, has served as a significant geographical barrier to gene flow. We suggest that the low genetic diversity and high differentiation from other subspecies imply that the Daito subspecies arose from a historical event involving long-distance oceanic dispersal coupled with geographical and reproductive isolation. Similar differentiation and restricted gene flow between the Daito Islands and other Ryukyu islands has been reported for the elegant scops owl (Otus elegans) (Hsu, 2005).
We also found differentiation among other populations across the Ryukyu Archipelago based on microsatellite genotypes, notably between the Erabu, Orii's, and Yaeyama flying foxes. The results for Orii's and Yaeyama subspecies were somewhat surprising, especially in light of the mtDNA data. The observed subdivisions based on ncDNA corresponded well with the positions of deep-sea channels, including the Tokara and Kerama Gaps, which separate the Northern, Central, and Southern Ryukyus. Thus, differentiation among these island groups appears to have been driven by their long-term isolation. For Erabu, the inclusion of just one sample strongly limits our interpretations about this population.
In addition to the strong genetic subdivisions detected among populations across Taiwan and the Ryukyu Islands, our results also showed a significant positive correlation between geographical and genetic distances for ncDNA. Isolation by distance is typically considered to be a consequence of migration–drift equilibrium, whereby recurrent gene flow follows a stepping stone model and is thus more likely to occur among neighboring populations (Kimura & Weiss, 1964). Nevertheless, signals of isolation by distance can also arise through a colonization process, in which the contribution of genetic drift outweighs that of gene flow. Isolation by distance might also be more easily detected over greater distances due to the higher probability that barriers to gene flow will naturally occur when considering larger spatial scales (Bossart & Prowell, 1998). Indeed, in our study, it is notable that island groups characterized by stronger genetic differentiation were also more likely to occur on opposite sides of deep-sea trenches (Hutchison & Templeton, 1999), and we found that the trend of isolation by distance was no longer significant after taking this into account.
Previous studies have reported gene flow among flying fox populations located hundreds to thousands of kilometers apart, although these have tended to focus on movements over land or along coastlines. In our study, similar genetic profiles of populations from Taiwan and Yaeyama support genetic mixing via movements across water, coupled with the formation of a land bridge in the LGM. These two subspecies are geographically closest and least genetically structured. On the other hand, strong differentiation among island populations of flying foxes separated by 200–300 km, such as that between Orii's and Yaeyama, suggests that this distance represents an upper limit for recurrent gene flow in these bats. Supporting this, limited movements were previously reported for both Orii's based on radio tracking data (Nakamoto et al., 2009, 2012) and Yaeyama subspecies based on gene flow within Yaeyama Islands, Japan (Taki et al., 2020). Despite this, our structure-based clustering analyses did reveal a small number of putative migrants, with two individuals recorded in Taiwan (TW1) and one in Yaeyama assigned to the Orii's genetic cluster. Interestingly, such individuals all appear to result from east to west movements, and not the converse, possibly as a result of the prevailing northeasterly winds that prevail during winter in this region (Yang, 2007).
A surprising result of this study was the high recorded genetic diversity in the Formosan flying fox from Gueishan Island (TW1). People living on Gueishan Island before it was designated as a military control area in 1977 claim that no flying foxes were present (Wu, 2010); thus, this population is considered to be newly established via oceanic dispersal. Although this population appears to show a closer relationship with the Yaeyama flying fox from Iriomote-jima than with the other populations on Taiwan (TW2 and TW3), its high diversity likely stems from genetic admixture involving several different genetic clusters (e.g., Comas et al., 2004). Indeed, our results indicate that the TW1 population likely has multiple ancestral origins with putative founders from Yaeyama, TW2 (from Lyudao), TW3 (from Taiwan's main island), and/or the Philippine population.
A combination of one or more explanations would account for the genetic diversity found on Gueishan Island. First, flying foxes might have arrived on Gueishan Island as a result of strong winds associated with seasonal typhoons or the winter northeast monsoon. Second, individual bats may have actively dispersed in search of resources. A third scenario is that active dispersal was driven by population expansion of the Yaeyama flying fox population. Yonaguni-jima of Japan, the westernmost margin of the distribution of the Yaeyama flying fox, is 110 km from Gueishan Island. According to Nakamoto et al. (2011), Yaeyama flying foxes have been presumed to be dispersing eastward across the sea to a new insular habitat approximately 50 km away (from Tarama-jima to Miyako-jima). The flying fox population on Yonaguni-jima or the neighboring islands may also expand westward to Gueishan Island with the help of wind, forming a widely distributed and diverse population.
5 CONCLUSIONS
Our findings from mitochondrial and nuclear markers support the current division of subspecies of P. dasymallus from the Ryukyu Archipelago and Taiwan and, in particular, the distinctiveness of the subspecies of Daito, Orii's, and the Philippine flying foxes. Genetic subdivisions among some island groups appear to reflect a lack of frequent long-distance movements across water, coupled with the presence of deep-sea channels that prevented the formation of land bridges during the LGM. We also find evidence that the recent colonization of Taiwan has involved founders from several distinct clusters. Taken together, our results reveal that highly isolated and genetically distinct populations, such as the Daito and Orii's subspecies and the Philippine bats, should be treated as separate management units. In addition, populations that occur on adjacent islands, such as the Yaeyama and Formosan subspecies—that show strong evidence of recent and frequent gene flow—can be managed as a single population. It is noteworthy that the latter two subspecies are not panmictic but persist their genetic patterns under the effect of drift over a set of insular small populations. The comparatively higher level of divergence between the Philippine sample from Sabtang Island and all the other sampled bats highlights the need for future work to better understand this population. More generally, our results indicate that the evolutionary and ecological forces shaping the pattern of the genetic structure in P. dasymallus are dynamic and ongoing. As a taxon that ranges from the temperate northern Ryukyu Archipelago and subtropical Taiwan to the tropical northern Philippine islands, this species may serve as an excellent model for studying the processes driving island biogeography.
ACKNOWLEDGMENTS
We are grateful to the Okinawa Zoo and Museum Foundation, Hirakawa Zoological Park, University of Ryukyus, Japan, and Taipei Zoo, Taiwan, for granting access to the collections. We thank Han-Chun Lee, Hui-Wen Wu, Ching-Feng Lin, Dr Ching-Lung Lin, Dr Atsushi Nakamoto, Dr Emyo Fujioka, and many assistants for their hard work in the field, Dr Si-Min Lin for valuable suggestions on the manuscript, and Dr Tetsuo Denda for laboratory support. This study was financially supported by the Forestry Bureau, Council of Agriculture (107-9.1-SB-17(1), 108-9.1-SB-30) and Ministry of Science and Technology (MOST 107-2621-B-305-001), Taiwan, and JSPS KAKENHI (Grant-in-aid for Scientific Research on Innovative Areas: No JP16H06542), Japan.
CONFLICT OF INTEREST
No potential conflict of interest was reported by the authors.
ETHICAL APPROVAL
All applicable institutional and national guidelines for the care and use of animals were followed.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the Dryad Digital Repository at https://doi.org/10.5061/dryad.qfttdz0fp.




