Landscape heterogeneity shapes bird phylogenetic responses at forest–matrix interfaces in Atlantic Forest, Brazil
Abstract
enAgricultural intensification is one of the major factors driving biodiversity loss. However, most studies in human-dominated landscapes have used taxonomic diversity in their analysis, ignoring evolutionary relationships. Consequently, the relationship between landscape structure and phylogenetic diversity is not well understood. Here, we tested the hypothesis that landscape heterogeneity is positively related to bird phylogenetic indexes of diversity and structure, leading to over-dispersed phylogenies in very heterogeneous landscapes. We analyzed phylogenetic responses in interfaces between forest edges and anthropogenic matrices (forest–pasture and forest–eucalyptus) using generalized linear mixed models. We also compared these indexes between land covers to assess which one best preserves the phylogenetic history of communities. We used both traditional phylogenetic indexes and those corrected for species richness. Our results showed that phylogenetic diversity varied significantly between land cover types, but this did not occur when we removed effects associated with species richness, suggesting that all land covers preserve similar levels of evolutionary history. Additionally, our best models showed a positive relationship between landscape heterogeneity and bird phylogenetic indexes of diversity and structure, but the strength of these relationships may be land cover dependent. In summary, our work highlights the influence of landscape heterogeneity on the phylogenetic diversity and structure of bird communities, reinforcing the need for its incorporation into conservation-based studies.
RESUMO
ptA intensificação agrícola é uma das principais causas relacionadas à perda de biodiversidade. Entretanto, a maioria dos estudos realizados em paisagens dominadas pelo homem costuma usar apenas índices taxonômicos, ignorando as relações evolutivas entre as espécies. Consequentemente, a relação entre estrutura da paisagem e diversidade filogenética não é bem compreendida. Nesse estudo, testamos a hipótese de que a heterogeneidade da paisagem é positivamente relacionada aos índices filogenéticos de diversidade e estrutura das comunidades de aves, o que induz a presença de filogenias dispersas em paisagens muito heterogêneas. Nós analisamos as respostas filogenéticas em interfaces entre bordas florestais e matrizes antrópicas (floresta-pasto e floresta-eucalipto) a partir de modelos lineares generalizados mistos. Nós também comparamos estes índices entre diferentes coberturas de terra para avaliar qual destas melhor preserva a história filogenética das comunidades. Nós utilizamos tanto índices filogenéticos tradicionais, quanto aqueles corrigidos para a riqueza de espécies. Nossos resultados mostraram que a diversidade filogenética variou significativamente entre as coberturas de terra, mas isso não aconteceu quando removemos os efeitos associados à riqueza de espécies, o que sugere que todas as diferentes coberturas de terra preservam níveis similares de história evolutiva. Adicionalmente, nossos melhores modelos mostraram uma relação positiva entre a heterogeneidade da paisagem e os índices filogenéticos de diversidade e estrutura das comunidades de aves. Entretanto, a força dessas relações pode ser dependente da cobertura florestal. Em síntese, nosso trabalho destaca a influência da heterogeneidade da paisagem na diversidade e estrutura filogenética das comunidades de aves, o que reforça a necessidade de sua incorporação em estudos conservacionistas.
1 INTRODUCTION
The expansion of agricultural frontiers associated with landscape homogenization is considered one of the main causes of biodiversity loss worldwide (Johnson et al., 2017; Newbold et al., 2014). As a result, extensive efforts have been made to understand how landscape heterogeneity determines patterns of species distribution and modifies community composition in those natural habitats that remain, as well as in surrounding anthropogenic matrices (Harlio et al., 2019; Loos et al., 2014; Santana et al., 2017). There is evidence that both compositional and configurational components of landscape heterogeneity can have distinct effects on patterns of taxonomic diversity (Fahrig et al., 2010; Santana et al., 2017; Steckel et al., 2014). High values of compositional heterogeneity (i.e., higher number and more equitable proportion of different land cover forms; Fahrig et al., 2010) are related to high values of taxonomic diversity, due to the greater variety of resources available for species with different ecological requirements (Fabian et al., 2013). On the other hand, configurational heterogeneity (spatial arrangement and shape of land covers; Fahrig et al., 2010) influences several ecological processes, including spillover movement and metapopulation dynamics (Boesing et al., 2017; Moraes et al., 2018; Silva et al., 2015). Such impacts become more acute as structural contrasts between matrix and forest patches increase (Benítez-Malvido et al., 2014; Laurance et al., 2006; Ries et al., 2004).
However, most of our knowledge in this field is based on taxonomic diversity (species richness, abundance, and composition; Benton et al., 2003; Fahrig et al., 2010). Consequently, a gap remains in understanding how landscape heterogeneity changes the phylogenetic relatedness of the species that remain. Including phylogenetic diversity (Webb, 2000), the total evolutionary history shared across species within communities, in conservation programs may reduce the chances of losing unique phenotypic and ecological traits (Jetz et al., 2014), and determine whether the evolutionary relationships of a community are related to ecological processes and ecosystem functioning (Cadotte et al., 2012; Webb, 2000). Importantly, phylogenetic diversity could mirror patterns of species coexistence, reflecting mechanisms of niche-based assembly rules or neutral processes (Cavender-Bares et al., 2009; Webb, 2000), which in turn, may predict whether phylogenies are clustered (mostly composed of closely related species), over-dispersed (mostly composed of distantly related species), or random (no pattern of relatedness; Arroyo-Rodríguez et al., 2012; Hubbell, 2001; Si et al., 2017).
Previous studies have shown that community phylogenetic diversity and structure may respond to matrix composition and landscape simplification (Edwards et al., 2015; Frishkoff et al., 2014), the latter being frequently associated with low levels of landscape heterogeneity (Landis, 2017). Therefore, there may be a trend in which the phylogenetic diversity of communities becomes lower in simplified landscapes (low heterogeneity; Frishkoff et al., 2014), which may occur due to the low variability of resources and stressful climate that may exclude entire lineages from those communities. Over the years, this lineage exclusion had been constantly assigned to environmental filtering where abiotic factors should select particular species as a result of their capacity to survive and persist in a given space (Kraft et al., 2015), resulting in clustered patterns of functional and phylogenetic structure. However, it is currently known that species absences from a community do not reflect just the results of environmental filtering processes, but also mechanisms such as competition and dispersal limitation (Cadotte & Tucker, 2017). Despite this, no study to date had directly evaluated whether landscape heterogeneity and land cover influences the phylogenetic history of communities, particularly at forest–matrix interfaces, a vegetation form very common within human-dominated landscapes (Martello et al., 2016).
In this study, we aimed to quantify the relative contribution of compositional heterogeneity and forest–matrix interfaces in human-dominated landscapes to observed bird phylogenetic responses. We compared phylogenetic diversity and community structure across two types of forest–matrix interfaces: forest edge–pasture and forest edge–eucalyptus plantations. In addition, we assessed whether compositional heterogeneity and land cover influenced these phylogenetic indexes. We expected that forest edges would harbor higher phylogenetic diversity than eucalyptus plantations, which in turn, would result in higher phylogenetic diversity than in pastures. We also expected to find a positive relationship between landscape heterogeneity and the phylogenetic indexes of diversity and structure, where bird phylogenies go from clustered (closely related species) to over-dispersed (distantly related species) with increasing landscape heterogeneity (Data S1: Figure S1).
2 METHODS
2.1 Study region
We conducted the study in the Cantareira–Mantiqueira mountain corridor in the southeast part of Atlantic Forest (dense ombrophilous forest type), State of São Paulo, Brazil (Data S1: Figure S2). The regional climate is Cwa (Alvares et al., 2013) under the Köppen classification, and elevation varies between 700 and 1,700 meters a.s.l. (Oliveira-Filho & Fontes, 2000). Originally, the Atlantic Forest covered an area of 1.6 million km2, distributed along the Atlantic coast (Muylaert et al., 2018), but intense forest loss, fragmentation and land-use intensification reduced the total area by 72% (Rezende et al., 2018). Moreover, most of the remaining forests are small in size and suffer with different levels of anthropogenic disturbances (Ribeiro et al., 2009). In the study region, these forest remnants are surrounded by different land cover systems, including a variety of agricultural uses (sugarcane, maize, peaches, and grapes), silvicultural (pine and eucalyptus), and pasture (Barros et al., 2019).
2.2 Study sites and landscape metrics
We created land-use maps for this study area using satellite images of high resolution (ArcGIS 10.3 base map imagery, Digital Globe satellites 2010–2011, 1:4,000; Data S1: Figure S2). We made this map with manual digitization based on visual interpretation of patch differences in color, texture, and shapes. We considered 14 different land covers or human land uses: old-growth forest, pasture, eucalyptus plantations, second-growth, wetland, cropland (mainly maze), sugarcane, water bodies, urban areas, rural homesteads, urban or suburban homesteads, paved roads, buildings, and bare soil (for more details, see Barros et al., 2019). Based on this map, we chose 34 sample landscapes to represent the regional gradient of compositional landscape heterogeneity. Of these, 16 had their points located across forest–eucalyptus interfaces and 16 across forest–pasture interfaces. The eucalyptus plantations were, in general, homogenous, lacking a native vegetation understory, while the pastures were not exposed to intensive grazing. In addition, we used two control landscapes in continuous forest areas so as to include forest specialist species in the phylogenetic tree. This allowed the inclusion of forest species that originally inhabited our study area but which are now only seen in large forest blocks.
Landscape sites were defined using 1.2-km buffers around the centroid of the two sampling sites (one at forest edge, the other in pasture, or at eucalyptus edge). We chose this spatial scale based on previous evidence from multiscale analysis of bird responses to landscape structure (Barros et al., 2019). We also defined a 2-km minimum distance between sampling landscapes to avoid recounting the same individuals in different landscapes.
For each landscape, we calculated the compositional landscape heterogeneity (Fahrig et al., 2010) via a Shannon diversity index using Fragstats v.4 software (McGarigal et al., 2002). This index is based on the number of physical land cover types or human land uses and their evenness within the landscape. When the value of the Shannon index is zero, the landscape contains only one patch (i.e., homogeneous landscape). Landscape heterogeneity increases as the number of different patch types increases and/or the proportional distribution of area among patch types becomes more equitable.
2.3 Bird sampling
We used point counts, with a 50-m survey radius, to sample bird communities (Sutherland, 2006). We selected paired forest–matrix ecotones as sample sites in each landscape, one in forest edge and other in the adjacent pasture or eucalyptus plantation. The paired sampling sites were located around 70–100 m from the edge (140–200 m from each other, Data S1: Figure S2), while control sampling sites consisted of a single point at each location, situated in the interior of continuous forests, and at least 1 km from the edge. We sampled each site for ten minutes, three times, on different days during the first three hours after sunrise, during two consecutive breeding seasons, totaling 30 min per site. We sampled half of sampling sites from September 2014 to January 2015, and the remainder from October to December 2015. Only birds obviously using the habitats were recorded (e.g., birds flying overhead were not included). For each sampling site, data recorded from the three replicates were combined into a single community database, except for the abundance data. We set the abundance data of each sampling site as the highest value recorded in one single day. This is a conservative value adopted to avoid overestimated data.
2.4 Bird phylogenetic trees
We considered all species recorded in the 34 landscapes as the regional pool of species. Then, to construct the phylogenetic tree, we used the phylogeny database of bird species built by Jetz et al. (2014), available for download at http://birdtree.org/, using the Hackett All Species option as the source of trees. From the 1,000 trees pruned for our full set of species, we created a consensus tree using the Mesquite 2.75 program (Maddison & Maddison, 2018).
2.5 Phylogenetic metrics
To evaluate the phylogenetic metrics for each community, we used the “picante” package (Kembel et al., 2010) in R statistical software (R Development Core Team, 2018). We chose six phylogenetic metrics to represent the phylogenetic diversity and structure of sampled bird communities.
2.5.1 Phylogenetic diversity (PD)
Described as the total sum of phylogenetic history, this is measured through the total branch length of a phylogeny representing the species in a community (Faith, 1992).
2.5.2 Standard effect size of PD (SES.PD)
PD of communities may be correlated with their species richness (SR, or number of species; Swenson, 2014). Thus, the effect of SR can be removed by comparing the PD values of studied communities with that of communities of equal species richness generated by null models drawn randomly from the regional species pool.
2.5.3 Mean nearest taxon distance (MNTD) abundance-weighted
MNTD is weighted by abundance and represents the average phylogenetic distance between an individual and the most closely related non-conspecific individual (Webb, 2000; Webb et al., 2002); high MNTD indexes indicate the co-occurrence of distantly related species within communities (phylogenetic over-dispersion), while low levels indicate the co-occurrence of closely related species (phylogenetic clustering).
2.5.4 Standard effect size of MNTD (SES.MNTD) abundance-weighted
MNTD may also be correlated with SR. Communities with higher than expected MNTD values for a given SR indicate the co-occurrence of distantly related species (phylogenetic over-dispersion or SES.MNTD> +1.5), while low values indicate the co-occurrence of closely related ones (phylogenetic clustering or SES.MNTD <−1.5). The SES.MNTD index is a better metric for limiting similarity relationships (phylogenetic over-dispersion).
2.5.5 Mean pairwise distance (MPD)
Represents the average phylogenetic distance between all pairwise species combinations present in a community, and it is influenced by relationships in deep evolutionary time (Webb et al., 2002). High MPD indexes indicate the co-occurrence of distantly related species within communities (phylogenetic over-dispersion), while low levels indicate the co-occurrence of closely related species (phylogenetic clustering).
2.5.6 Standard effect size of MPD (SES.MPD)
MPD may be correlated with SR. Communities with higher than expected MPD values for a given SR indicate co-occurrence of distantly related species (phylogenetic over-dispersion or SES.MPD> +1.5), while low values indicate co-occurrence of closely related ones (phylogenetic clustering or SES.MPD <−1.5). The SES.MPD index is a better indicator of environmental filter relationships (phylogenetic clustering; Kraft et al., 2007; Webb, 2000).
To determine whether SES values differed from the community expected by chance, we compared observed values between individuals to expected SES values for 999 communities using an independent swap algorithm (Gotelli, 2000). Additionally, to assess whether such traits were conserved across the phylogeny (Data S1), we calculated the phylogenetic signal (Blomberg et al., 2003) for six bird functional traits. For this, we conducted a phylogenetically independent contrast analysis using the “aotf” function in Phylocom software (Webb et al., 2008).
2.6 Statistical analysis
To assess whether the phylogenetic diversity indexes and species richness differed among all land covers, we used one-way ANOVA, and to compare differences among matrices and forest edges, we used paired-sample t tests (Rezende & Diniz-Filho, 2012). To compare only matrices (pasture and eucalyptus plantations), we performed simple t tests. Results were interpreted via P-values where p < .05 indicates significant differences between land covers. Statistical analyses were performed using R Statistical Software (R Development Core Team, 2018).
We used generalized linear mixed models (Table 1) to analyze the relationship between phylogenetic and landscape metrics (Glmm; function “lmer,” package “lme4”; Bates et al., 2015; Bolker et al., 2009). For each phylogenetic metric of diversity (PD and SES.PD) and structure (MPD, MNTD, SES.MPD, and SES.MNTD), we produced simple and additive models using spatial heterogeneity and land cover as predictive variables. We fitted these models using the landscape identification (a code for each landscape) as a random effect to account for spatial dependence of the sampling points present in the same landscape. In some models, we also tested, as random effects, the landscape heterogeneity and land cover. Next, we ranked these models and, using the Akaike information criterion corrected for small samples (AICc), estimated which ones best predicted the phylogenetic diversity and structure of bird communities. We considered best models to be those with the lowest AICc values. Models with ΔAICc differences less than two were considered as equally plausible to explain observed patterns (Martensen et al., 2012). For each model, we also calculated the Akaike weights wi (range from 0 to 1; the highest values were considered the most plausible models). We calculated all these indexes using the AICctab function of the “bbmle” package (Bolker, 2017).
| Phylogenetic metrics | Models | AICc | ∆AICc | df | weight | DHARMa Moran's I test | |||
|---|---|---|---|---|---|---|---|---|---|
| Observed | Expected | p-value | |||||||
| Diversity | PD | Heterogeneity + Land +(1|Landscape) | 856.9 | 0 | 7 | 0.9918 | 0.15 | −0.02 | 0.01986 |
| Land+(1|Landscape) | 866.6 | 9.6 | 6 | 0.0082 | |||||
| Heterogeneity+(1|Landscape)+(1|Land) | 900 | 43.1 | 5 | <0.001 | |||||
| 1+(1|Landscape)+(1|Land) | 909.7 | 52.8 | 4 | <0.001 | |||||
| Heterogeneity+(1|Landscape) | 976.3 | 119.4 | 4 | <0.001 | |||||
| 1+(1|Landscape) | 987.3 | 130.3 | 3 | <0.001 | |||||
| SES.PD | Heterogeneity+(1|Landscape) | 156.1 | 0 | 4 | 0.645 | 0.43 | −0.02 | < 2.2e−16 | |
| Heterogeneity+(1|Landscape)+(1|Land) | 158.4 | 2.4 | 5 | 0.199 | |||||
| 1+(1|Landscape) | 159.7 | 3.6 | 3 | 0.104 | |||||
| 1+(1|Landscape)+(1|Land) | 162 | 5.9 | 4 | 0.033 | |||||
| Heterogeneity + Land+(1|Landscape) | 163.4 | 7.3 | 7 | 0.017 | |||||
| Land+(1|Landscape) | 167.9 | 11.8 | 6 | 0.002 | |||||
| Structure | MNTD | Heterogeneity + Land+(1|Landscape) | 601 | 0 | 7 | 0.9969 | 0.24 | −0.02 | 6.23E−08 |
| Land+(1|Landscape) | 612.6 | 11.5 | 6 | 0.0031 | |||||
| Heterogeneity+(1|Landscape)+(1|Land) | 626.2 | 25.1 | 5 | <0.001 | |||||
| 1+(1|Landscape)+(1|Land) | 638.4 | 37.4 | 4 | <0.001 | |||||
| Heterogeneity+(1|Landscape) | 645.5 | 44.5 | 4 | <0.001 | |||||
| 1+(1|Landscape) | 660.6 | 59.5 | 3 | <0.001 | |||||
| MPD | Heterogeneity + Land+(1|Landscape) | 583 | 0 | 7.000 | 0.91 | 0.14 | 0.14 | 0.00074 | |
| Land+(1|Landscape) | 587.6 | 4.6 | 6 | 0.09 | |||||
| Heterogeneity+(1|Landscape)+(1|Land) | 607.6 | 24.6 | 5 | <0.001 | |||||
| 1+(1|Landscape)+(1|Land) | 612.3 | 29.3 | 4 | <0.001 | |||||
| Heterogeneity+(1|Landscape) | 630.4 | 47.4 | 4 | <0.001 | |||||
| 1+(1|Landscape) | 635.8 | 52.8 | 3 | <0.001 | |||||
| SES.MNTD | Heterogeneity+(1|Landscape) | 160 | 0 | 4 | 0.638 | 0.40 | 0.02 | < 2.2e−16 | |
| Heterogeneity+(1|Land)+(1|Landscape) | 161.8 | 1.8 | 5 | 0.258 | 0.40 | −0.02 | < 2.2e−16 | ||
| Heterogeneity + Land+(1|Landscape) | 165.1 | 5.1 | 7 | 0.049 | |||||
| 1+(1|Landscape) | 166 | 6 | 3 | 0.032 | |||||
| 1+(1|Land)+(1|Landscape) | 167.1 | 7.2 | 4 | 0.018 | |||||
| Land+(1|Landscape) | 169.6 | 9.6 | 6 | 0.005 | |||||
| SES.MPD | 1+(1|Landscape) | 147.6 | 0 | 3 | 0.5716 | ||||
| 1+(1|Land)+(1|Landscape) | 149.8 | 2.3 | 4 | 0.183 | |||||
| Heterogeneity+(1|Landscape) | 149.9 | 2.4 | 4 | 0.1741 | |||||
| Heterogeneity+(1|Land)+(1|Landscape) | 152.3 | 4.7 | 5 | 0.0538 | |||||
| Land+(1|Landscape) | 155.4 | 7.9 | 6 | 0.0113 | |||||
| Heterogeneity + Land+(1|Landscape) | 156.6 | 9.1 | 7 | 0.0061 | |||||
Note
- Landscape = identification code for the sites; Heterogeneity = Landscape compositional heterogeneity was measured using the extent of land-use diversity (Shannon diversity index); Land = physical land type or human land-use (i.e., forest, pasture, and eucalyptus plantations) where sampling points were located.
After selecting the most plausible models (ΔAICc < 2, wi > 0.1), we tested for spatial autocorrelation in the residual distribution using Moran's I test (“DHARMa” package in R). Because of spatial dependence, we used spatial regression models that included the spatial effect (function “fitme,” package “spam”; Rousset & Ferdy, 2014; Table 2). In these models, we excluded landscape identification as a random effect as these regression models already took into account the spatial pattern present in the data. Finally, we visually checked the model fits and residual distributions and selected the best ones that controlled the spatial effect (Data S1: Figure S3). All these analyses were performed using the R statistical software package (R Development Core Team, 2018).
| Phylogenetic metrics | Models | Predictors | Fixed effects of heterogeneity | Random effects | |||
|---|---|---|---|---|---|---|---|
| Coefficient Estimate | SE | Variance parameters (“lambda”) | |||||
| Latitude + Longitude | Land | ||||||
| Diversity | PD | Heterogeneity + Land+(1|Latitude + Longitude) | Heterogeneity | 102.9 | 173.12 | 1.347 | |
| Eucalypt | 698.8 | 110.84 | |||||
| Forest–Eucalypt | 1,140.7 | 91.74 | |||||
| Forest–Pasture | 1,287.1 | 95.69 | |||||
| Pasture | 749.8 | 7.83 | |||||
| SES.PD | Heterogeneity+(1 |Latitude + Longitude) | 0.7758 | 0.2949 | 0.5547 | |||
| Structure | MNTD | Heterogeneity + Land+(1|Latitude + Longitude) | Heterogeneity | 37.12 | 12.95 | 880.5 | |
| Eucalypt | 62.97 | 20.22 | |||||
| Forest–Eucalypt | −61.2 | 10.33 | |||||
| Forest–Pasture | −58.8 | 10.96 | |||||
| Pasture | −48.73 | 10.96 | |||||
| MPD | Heterogeneity + Land+(1|Latitude + Longitude) | Heterogeneity | −1.43 | 13.46 | 218.1 | ||
| Eucalypt | 211.14 | 20.11 | |||||
| Forest–Eucalypt | 50.96 | 7.77 | |||||
| Forest–Pasture | 56.9 | 9.81 | |||||
| Pasture | 51.15 | 9.81 | |||||
| SES.MNTD | Heterogeneity+(1|Latitude + Longitude) | Heterogeneity | 1.004 | 0.3078 | 0.5894 | ||
| Heterogeneity+(1|Land)+(1|Latitude + Longitude) | Heterogeneity | 1.013 | 0.3151 | 0.3868 | 0.01878 | ||
Note
- Heterogeneity = Landscape compositional heterogeneity measured using the extent of land-use diversity (Shannon diversity index); Land = physical land type or human land-use (i.e., forest edges adjacent to eucalyptus, forest edges adjacent to pastures, pasture and eucalyptus plantations) where sampling points were located.
3 RESULTS
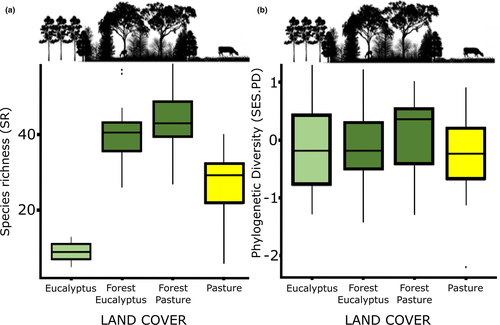
We recorded 206 bird species from 48 families across all sampling landscapes (32 human-modified + 2 control sampling landscapes; N = 3,813 bird contacts). Eight species, all forest specialists, were found exclusively in the control landscapes (Data S1: Table S2). Species richness (SR) differed widely among land covers (ANOVA, F3.60 = 71.35, p < .001; Figure 1a), and both forest edges had significantly higher SR than their respective adjacent matrices (paired-sample t tests pasture15 = 5.35, p < .001; t tests eucalyptus15 = 15.03, p < .001). Between the matrices, pastures had higher SR than eucalyptus plantations (t tests14 = 7.92, p < .001).
3.1 Phylogenetic diversity
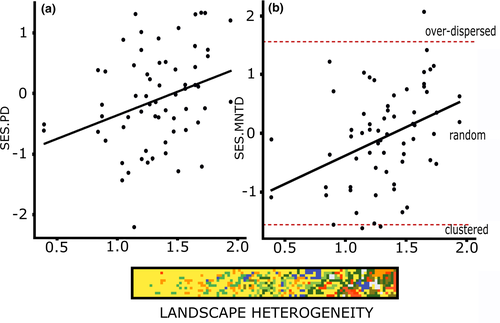
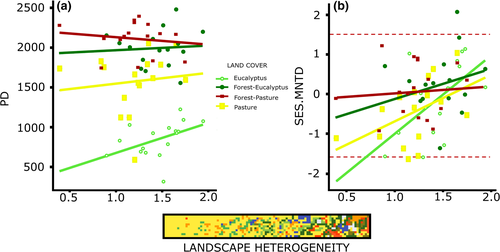
When we compared PD across all land covers, both forest edges had higher PD than their respective matrices (paired-sample t tests pasture15 = 4.67, p = .0003; t tests eucalyptus15 = 12.73, P «0.001). Moreover, pastures had higher PD than eucalyptus plantations (t tests14 = 6.62, P «0.001), but when we removed the species richness effect on PD using SES.PD, we found no difference between land covers (ANOVA, F3.60 = 0.55, p = .6508, Figure 1b). In spatial regression models (Table 1 and 2, Data S1: Figure S3a), we found that landscape heterogeneity had positive effects on both PD and SES.PD (Figure 2a). For PD, taking into account the additive effect of land cover was also important (Figure 3a). Thus, in general, PD increased as landscape heterogeneity increased, but the range and intensity of this variation were land cover dependent.
3.2 Phylogenetic structure
We found that most communities exhibited random phylogenies (94%), with only four of them being non-random. One community exhibited over-dispersed phylogeny in a forest edge adjacent to eucalyptus plantation (higher SES.MNTD), and three exhibited clustered phylogenies in a control landscape, a pasture, and an eucalyptus plantation (lower SES.MNTD). Since most of our communities had random phylogenies, we were not able to infer precisely which families and traits were being extirpated along the heterogeneous gradient. However, the clustered communities of pasture and eucalyptus plantation had only 10 and 7 families, respectively, indicating a low representation of distantly related species.
From the spatial regression models, we found a positive effect of landscape heterogeneity on SES.MNTD. This relationship was evident after using land cover as a random effect (Tables 1 and 2; Figure 2b, Data S1: Figure S3b, e and f). In this model, the strength of landscape heterogeneity effect (regression slope) on SES.MNTD was land cover dependent (Figure 3b). The matrices had the lowest SES.MNTD values in comparison to their forest edges but showed the highest SES.MNTD increment along the landscape heterogeneity gradient. This result suggests that, even when random phylogenies are present, there is a trend for bird community phylogenies to go from clustered to over-dispersed with increasing landscape heterogeneity, and this effect is stronger in communities inhabiting matrices rather than forests (Figures 2b and 3c). For MPD and SES.MPD, none of the models performed well (Tables 1 and 2, Data S1: Figure S3c), indicating that variation observed in MPD and SES.MPD was not related with landscape heterogeneity or land cover.
3.3 Phylogenetic signal
Most of the analyzed traits in the regional species pool phylogeny showed a phylogenetic signal (Data S1: Table S1): body mass, forest affinity, clutch size, diet (invertebrates, vertebrates, fruits, nectar, and seed), foraging strata (ground, understory, and canopy), and social behavior (solitary, pair, mixed-flocks, and single-species flocks). This means that certain trait variations are more associated with certain lineages within the phylogeny and may be decisive for species persistence along environmental gradients.
4 DISCUSSION
The negative impacts of land-use intensification and landscape homogenization on taxonomic diversity have been widely acknowledged (Johnson et al., 2017; Lewis et al., 2015; Newbold et al., 2014). Complementing this, our results highlight that landscape modifications also affect the phylogenetic diversity and structure of bird communities. Conversion of forest into eucalyptus plantations or pastures may reduce phylogenetic diversity (PD), but does not affect its standard effect size (SES.PD) corrected for species richness. This suggests that even with species richness reduction or species replacement during landscape changes, preservation of phylogenetic history in these matrices is possible. Additionally, as we expected, there was a positive relationship between landscape heterogeneity and the phylogenetic indexes of diversity (PD and SES.PD) and structure (SES.MNTD), but the magnitude of this effect was land cover dependent. Thus, landscape heterogeneity can increase both the values of phylogenetic diversity and phylogenetic structure, the latter meaning that a trend exists where the bird phylogenetic representation goes from clustered to over-dispersed along a gradient of increasing heterogeneity. However, this effect is limited by land cover, being stronger in eucalyptus plantations than pastures.
4.1 Phylogenetic responses to land cover
The lack of differences in phylogenetic diversity (SES.PD) among land covers may be explained by compensatory dynamics (Morante-Filho et al., 2017), where the phylogenetic impoverishment of forest birds is offset by the phylogenetic enrichment of non-forest birds. This process becomes clear when the families exclusive to each land cover are delimited. Our sampled forest edges had nine exclusive families, which may have been replaced by the seven exclusive families found in pastures. However, these dynamics were absent from eucalyptus plantations where bird communities present were simply a subset of communities inhabiting forest edges (nested pattern; Jacoboski et al., 2016; Wethered & Lawes, 2003). Except for Cariama cristata and Elaenia flavogaster, all species recorded in eucalyptus plantations were also found in forest edges. Thus, species within the same clades may exhibit different responses to forest conversion into eucalyptus plantations—in which they may occur or not—but the clades will still be represented without affecting the SES.PD values. Another potential explanation for the lack of differences in SES.PD involves extirpation of the most sensitive forest species in the region due to the historic forest loss and fragmentation (Prescott et al., 2015). Therefore, only clades with higher plasticity were able to retain species in continuously changing landscapes, resulting in a reduction in forest birds SES.PD values over the time. The forest species seen exclusively in our control landscapes may have suffered from this effect, where families such as Odontophoridae, Rhynocryptidae, and Scleruridae were only recorded in these larger forest fragments.
Although several studies have considered correlations between phylogenetic diversity and ecosystem functions (EF; Cadotte et al., 2009; Cavender-Bares et al., 2009; Srivastava et al., 2012), few have attempted to disentangle the effects of SR. Thus, an increasing number of studies have questioned the strength of phylogenetic diversity indexes as EF predictors (Mazel et al., 2018). Also, recent studies have suggested an integrated use of phylogenetic and functional indexes as a means of providing a better understanding of the impacts of human-induced effects on biodiversity (Chapman et al., 2018; Mazel et al., 2018; Si et al., 2017). Barros et al. (2019), using the same dataset as us, found EF to differ widely between land covers, just like our PD values. However, these differences did not occur in SES.PD values, showing that, in this case, this index may not be a reliable EF predictor.
4.2 Phylogenetic responses to landscape heterogeneity
Although the comparison among different land covers did not reveal differences in the average values of Standard Effect Size of PD (SES.PD), our best model showed a positive relationship between landscape heterogeneity and SES.PD, indicating that communities contain more bird lineages as landscape heterogeneity increases. Thus, the novelty here is that - beyond affecting multiple taxonomic and functional (Benton et al., 2003) facets of bird communities - landscape heterogeneity also affects their phylogenetic diversity, independent of its species richness. These findings suggest that landscape heterogeneity is an important parameter for safeguarding the evolutionary history of bird communities.
We also found a positive effect of landscape heterogeneity on the phylogenetic structure (SES.MNTD) of bird communities, but the magnitude of this effect is land cover dependent. This finding indicates that landscape heterogeneity may influence the phylogenetic structure of bird communities, creating a trend from clustered phylogenies in less heterogeneous landscapes to over-dispersed phylogenies in more heterogeneous ones. Moreover, the slope of this effect is more accentuated in the anthropogenic matrices, indicating that landscape heterogeneity management could maintain communities composed of distantly related species. Overall, we think that these trends could be largely explained by the environmental filter process, even though it is difficult to disentangle other mechanisms from it (i.e., competition and dispersal limitation; Cadotte & Tucker, 2017). Therefore, the environment selects against certain species and only those with particular sets of traits are able to coexist in less heterogeneous landscapes (Frishkoff et al., 2014; Gámez-Virués et al., 2015). This assumption is based on the fact that our bird community phylogenies exhibited a correlation with an environmental gradient and most of our analyzed traits contained phylogenetic signals, showing its conservatism across evolutionary time (Blomberg et al., 2003; Cadotte & Tucker, 2017). These traits are ecologically relevant from an evolutionary and adaptive point of view (Barnagaud et al., 2014) and may be sensitive to landscape homogenization (Frishkoff et al., 2014), so they may be strongly influential in maintaining bird lineages in less heterogeneous landscapes.
If we consider that the degree of landscape heterogeneity may reflect land-use changes (Landis, 2017), our results regarding the trend toward environmental filtering are also consistent with simulations made by Frishkoff et al. (2014). They showed that, in human-dominated landscapes, more evolutionarily distinct species experienced greater extirpation rates compared to less evolutionarily distinct ones. Such differences in extirpation rates suggest that, over time, more distinct evolutionarily species would face challenges in maintaining their populations in human-dominated landscapes (Frishkoff et al., 2014), leading to the presence of communities with clustered phylogenies.
Even with this filtering trend, most communities in our study showed random phylogenies (SES.MNTD values between −1.5 and + 1.5). Therefore, while bird lineages may fluctuate in their species richness, no entire clades are being extirpated and no drastic changes in the phylogenetic structure of communities are expected to occur. These results indicate that neutrality may also be operating and obscuring the detection of non-random community phylogenetic patterns, since both neutral and niche processes can jointly affect local communities (Adler et al., 2007; Vellend et al., 2014). Another possibility is that the opposing mechanisms of niche-based assembly rules (environmental filter and competition) are canceling each other out, creating patterns indistinguishable from those that would be caused by random processes (Cavender-Bares et al., 2009; Vamosi et al., 2009). In addition, the random patterns found in our communities could also be associated with the relatively recent history of landscape modification in the study area, which may not have been in operation long enough to promote evolutionarily processes such as in situ speciation and character displacement (Cardillo et al., 2008). Thus, species have not been able to keep up with landscape changes and so have been excluded randomly from the local species pool.
5 CONCLUSIONS
Here, we found that bird phylogenetic diversity differed widely between land cover forms, but this most likely occurred due to differences in their species richness. Therefore, we can infer that pastures, eucalyptus plantations, and their respective forest edges preserve similar levels of phylogenetic diversity, but not the same suites of species. We also found that landscape heterogeneity may act as a phylogenetic environmental filter. As a consequence, habitats embedded in less heterogeneous landscapes are expected to harbor closely related species (clustered phylogenies). Conversely, heterogeneous landscapes are expected to retain a broader range of bird lineages (over-dispersed phylogenies) and higher phylogenetic diversity. Finally, our results suggest that phylogenetic indexes corrected for species richness are not reliable predictors of ecosystem function, since such functions differ widely between land cover forms (Barros et al., 2019). Considering these results, we emphasize that spatial heterogeneity management at the landscape level should be considered whenever the conservation of the full spectrum of lineage diversity is the focus. We also recommend studies relating phylogenetic metrics at landscape scale with different taxa, since most of the existing studies on this topic focus on plants or birds.
ACKNOWLEDGMENTS
We are grateful for all the help provided by ECOFRAG and LEEC staff at the Universidade Federal de Alfenas and Universidade Estadual Paulista (UNESP), respectively. We are also grateful to CAPES for funding. MCR thanks FAPESP (processes #2013/50421-2; #2020/01779-5), CNPq (processes # 312045/2013-1; #312292/2016-3), and PROCAD/CAPES (project # 88881.068425/2014-01) for their financial support. FMB thanks FAPESP (processes 2013/19732-1 and 2016/13576-1) for financial support and Marco Pizo, Julia Assis, IPÊ (Instituto de Pesquisas Ecológicas), AMBEV Guarulhos, Reserva Ibirapitanga e Instituto Florestal/SP for the logistical support.
CONFLICT OF INTEREST
The corresponding author confirms on behalf of all authors that there have been no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated.
Open Research
DATA AVAILABILITY STATEMENT
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.xpnvx0kd5 (Adorno et al., 2020).




