Impacts of coprophagic foraging behaviour on the avian gut microbiome
ABSTRACT
Avian gut microbial communities are complex and play a fundamental role in regulating biological functions within an individual. Although it is well established that diet can influence the structure and composition of the gut microbiota, foraging behaviour may also play a critical, yet unexplored role in shaping the composition, dynamics, and adaptive potential of avian gut microbiota. In this review, we examine the potential influence of coprophagic foraging behaviour on the establishment and adaptability of wild avian gut microbiomes. Coprophagy involves the ingestion of faeces, sourced from either self (autocoprophagy), conspecific animals (allocoprophagy), or heterospecific animals. Much like faecal transplant therapy, coprophagy may (i) support the establishment of the gut microbiota of young precocial species, (ii) directly and indirectly provide nutritional and energetic requirements, and (iii) represent a mechanism by which birds can rapidly adapt the microbiota to changing environments and diets. However, in certain contexts, coprophagy may also pose risks to wild birds, and their microbiomes, through increased exposure to chemical pollutants, pathogenic microbes, and antibiotic-resistant microbes, with deleterious effects on host health and performance. Given the potentially far-reaching consequences of coprophagy for avian microbiomes, and the dearth of literature directly investigating these links, we have developed a predictive framework for directing future research to understand better when and why wild birds engage in distinct types of coprophagy, and the consequences of this foraging behaviour. There is a need for comprehensive investigation into the influence of coprophagy on avian gut microbiotas and its effects on host health and performance throughout ontogeny and across a range of environmental perturbations. Future behavioural studies combined with metagenomic approaches are needed to provide insights into the function of this poorly understood behaviour.
I. INTRODUCTION
The microbiota of an individual's gastrointestinal tract, comprising microbes, their genes, and their functional traits, is fundamental to host digestion (McWhorter, Caviedes-Vidal & Karasov, 2009), detoxification (Kohl et al., 2016), development (Parfrey, Moreau & Russell, 2018), immune function (Broom & Kogut, 2018), protection from pathogenic bacteria (Roggenbuck et al., 2014; Mendoza et al., 2017), and cognitive performance (Davidson, Raulo & Knowles, 2020a). Gastrointestinal microbiota are thus critical to the maintenance of organismal health and have been suggested to play a significant role in host adaptation to novel and changing environments (Alberdi et al., 2016; Shapira, 2016). Given the importance of the gut microbiome to host health and performance, it is important to understand what shapes the composition and dynamics of these communities, including their establishment, development, maintenance, and any punctuated changes throughout the life of an organism.
Dynamics of an individual's gut microbiota include fluctuations in microbial species composition and abundance, with ensuing changes to gut function and signalling (Waite & Taylor, 2014; Teyssier et al., 2018). These changes may play out over a wide range of timescales, from predictable daily fluctuations reflecting feeding–fasting rhythms (Risely et al., 2021) to cyclical seasonal fluctuations (Wienemann et al., 2011; Bodawatta et al., 2021b), ontogenic changes (van Dongen et al., 2013; Teyssier et al., 2018; Maraci et al., 2022a), and even shifts during senescence (Bosco & Noti, 2021). Animals may also experience unpredictable punctuated fluctuations in microbiota composition associated with shifts in resource availability as a result of habitat destruction, land-use change and urbanization (Fuirst et al., 2018; Murray et al., 2020; San Juan et al., 2020; Maraci et al., 2022b), and exposure to environmental pollutants, including antimicrobials (Ward et al., 2019; Ruuskanen et al., 2020). Similarly, animals entering captivity (Lamberski et al., 2003; Oliveira et al., 2020; Sun et al., 2022; West et al., 2022) or being released after rehabilitation or captive rearing also experience changes in their microbiota (Diaz & Reese, 2021). While predictable changes in microbiota are thought to support host adaptation to altered intrinsic (within-host) or extrinsic (external) environments (Alberdi et al., 2016; Mendoza et al., 2017; Troha & Ayres, 2020), unanticipated disruptions to the microbiota have the potential to alter host development (Kohl et al., 2018), health, and performance (Davidson et al., 2020b). Given the critical role gut microbiomes can play in modulating host ontogeny, behaviour, and health, it is important to understand the mechanisms that allow animals to form and reshape their microbiota in the face of intrinsic and extrinsic change.
Wild birds are among the most mobile of all animals. This mobility, together with extreme morphological diversity, has enabled this taxon to occupy every habitat on every continent (Cooney, Seddon & Tobias, 2016; Navalón et al., 2019; Pigot et al., 2020). However, the mobility afforded by flight also presents an energetic and nutritional trade-off (Scanes, 2020), potentially necessitating mechanisms for enhancing the adaptability of their microbiomes (Grond et al., 2018; Bodawatta et al., 2021b). Adaptation to flight has also resulted in birds having intestines with smaller surface area and volume compared to similar-sized mammals (Caviedes-Vidal et al., 2007; McWhorter et al., 2009). Microbiota may therefore be especially important for such taxa to access adequate nutrition and energy.
An expanding body of research into avian microbiomes has demonstrated profound differences in the gut microbiota associated with different host species (Kohl, 2012; Capunitan et al., 2020), host habitats (Gillingham et al., 2019; Berlow, Phillips & Derryberry, 2021), and host diet (Fuirst et al., 2018; Loo et al., 2019; Bodawatta et al., 2021a; Góngora, Elliott & Whyte, 2021). Influential aspects of host diet include feeding guild (Wang et al., 2019; Bodawatta et al., 2021c; Ingala, et al., 2021), dietary breadth, (Drovetski et al., 2019; Schmiedová et al., 2022) and macronutrient composition (Coogan et al., 2017). However, drivers of microbiota dynamics in wild birds have been less explored than those of wild mammals and domestic birds (Grond et al., 2018; Matheen, Gillings & Dudaniec, 2022). Several recent reviews have therefore called for further research to gain a more comprehensive understanding of the impact of environmental drivers on avian gut microbiota and how it influences avian behaviour, ecology, pathogen transmission, and conservation (Grond et al., 2019; Davidson et al., 2020b).
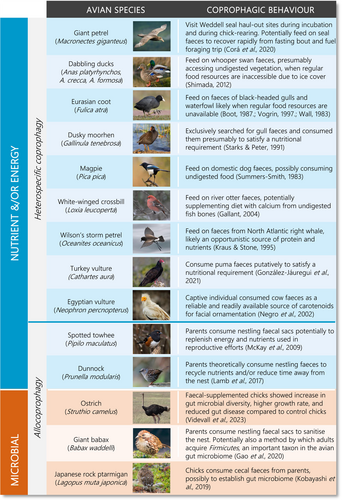
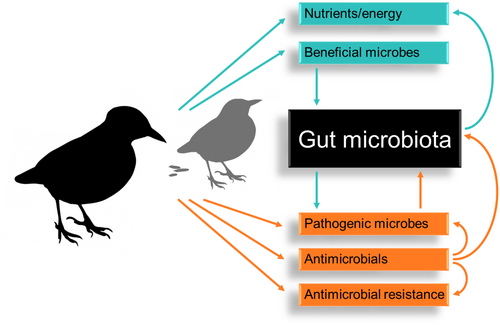
Foraging behaviour (including all activities involved in searching for, procuring or capturing, and processing or handling of diet items prior to ingestion, as well as the choice of food items for consumption), presents a range of potential mechanisms that support dynamic changes in avian microbiota (Vispo & Karasov, 1997; Pigot et al., 2020). However, in contrast to the well-established role of diet composition on vertebrate gut microbiota, the role of foraging behaviour has received less attention. One particular foraging behaviour that could have a profound influence on gut microbiota composition and dynamics is coprophagy. Coprophagy is defined as the ingestion of faeces, whether they are an animal's own (autocoprophagy), from a different individual of the same species (allocoprophagy), or from a distinct species (heterospecific coprophagy) (Hirakawa, 2001). This behaviour is considered a natural physiological phenomenon in many wild and domestic species (Soave & Brand, 1991; Flachowsky, 1997). Coprophagy has been particularly well-documented in mammals where it is considered a means by which animals gain energetic or nutritional supplementation, especially in herbivores (Hörnicke & Björnhag, 1980; Soave & Brand, 1991; Hirakawa, 2001; Waggershauser et al., 2022). Although coprophagic behaviour is considered commonplace in poultry (Line, Hiett & Colan, 2008), our understanding of the importance of coprophagic behaviour in wild birds is in its infancy. In contrast to insects and mammals, avian species reported to practice coprophagy are not limited to herbivores; this behaviour is also seen in avian omnivores, piscivores, and carnivores (Fig. 1). Moreover, although most studies on avian coprophagy have inferred energetic and nutritional benefits of these readily available ‘resources’ (Fig. 2), there is growing evidence to suggest that coprophagy may be a mechanism by which birds augment and dynamically modify their gut microbiome (Kobayashi et al., 2019; Gao, et al., 2020). Coprophagy may therefore be beneficial to birds during development as well as during times of seasonal fluctuation, facilitating the adaptation of an individual's gut microbiota to its surrounding environment (Fig. 3). Here, we review the potential for coprophagy to influence the establishment and adaptability of wild avian gut microbiomes. We draw particular attention to what may drive the propensity for birds to engage in this behaviour, which may play a unique, yet unassessed role in microbiome dynamics. We go on to demonstrate that these behaviours may be beneficial to the overall health of an individual, by promoting adaptation of an individual's microbiota to available food resources or other environmental conditions but may also entail detrimental effects.
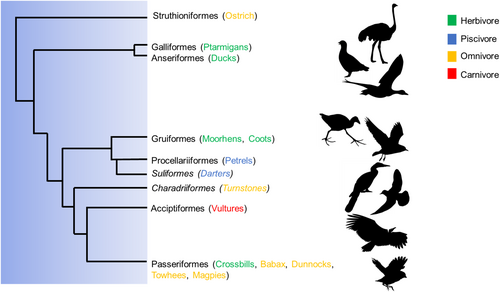
II. INFLUENCE OF EARLY COPROPHAGY ON GUT MICROBIOME ESTABLISHMENT
Like mammals, where colonization of the gut microbiome begins during and after birth by maternally acquired bacteria (de Goffau et al., 2019; Kuperman et al., 2020), initial acquisition of microbiota in birds occurs after hatching, as the egg generally remains sterile (Grond et al., 2017; Zhou et al., 2020a). Although bacteria from the oviduct and/or cloaca of the female parent may penetrate the egg and inoculate the growing foetus (Ballou et al., 2016; Ilina et al., 2016; Ding et al., 2017; Broom & Kogut, 2018; Dietz et al., 2019; Lee et al., 2019b; Těšický et al., 2023) this may only occur when the bacteria are pathogenic, rather than commensal (Ilina et al., 2016). The vast majority of colonization and recruitment therefore involves post-hatch exposure to microbes acquired through the environment (Grond et al., 2019) and foraging (Grond et al., 2017; Kobayashi et al., 2019). Foraging strategy and developmental trajectory therefore play a profound role in shaping the establishment of avian gut microbiomes.
Prior to fledging, developmental trajectories of avian young range from altricial to precocial. Altricial chicks are underdeveloped at hatching and therefore completely dependent on their parents for food and protection for an extended period, ranging from 30 days to 4 months (Schekkerman, Visser & Blem, 2001; Perrig et al., 2017; Martin et al., 2018; Matsubayashi et al., 2021). Precocial chicks, on the other hand, are more developed at hatching and able to leave the nest and forage directly from their environment (Naef-Daenzer & Grüebler, 2016; Grond et al., 2018), with parents providing some protection and guidance to suitable resources (Naef-Daenzer & Grüebler, 2016). Parents of altricial chicks can provide food at a much higher rate than precocial chicks are able to self-source, and as a result, altricial young show a faster growth rate (Naef-Daenzer & Grüebler, 2016). Parents of altricial young are also likely to provision them with microbes by salivary transfer during feeding (Grond et al., 2018; Chen et al., 2020). In addition, extended occupation of the nest increases opportunities for microbial seeding from the nest environment, particularly parental faecal matter (Somers et al., 2023; Grond et al., 2018; Dietz et al., 2019; Diez-Méndez et al., 2022; Maraci et al., 2022a). By contrast, precocial chicks establish their microbiota from the environment, within as little as 2 days after hatching (Ballou et al., 2016; Grond et al., 2017).
The stark difference in microbial sources between these developmental trajectories plays a fundamental role in the composition and dynamics of the gut microbial communities of birds. Altricial species have been shown to undergo profound changes in the abundance of different bacterial phyla early in their development, with a progressive increase in the abundance of Firmicutes up to 21 days post hatching, and a concomitant decrease in Proteobacteria (Grond et al., 2017; Zhou et al., 2020a; Xu et al., 2022). These compositional shifts were accompanied by shifts in metabolic function, which have been found to facilitate the digestion efficiency, rapid body mass gain and development typically seen in altricial species (Caviedes-Vidal et al., 2007; Evans et al., 2017; Teyssier et al., 2018).
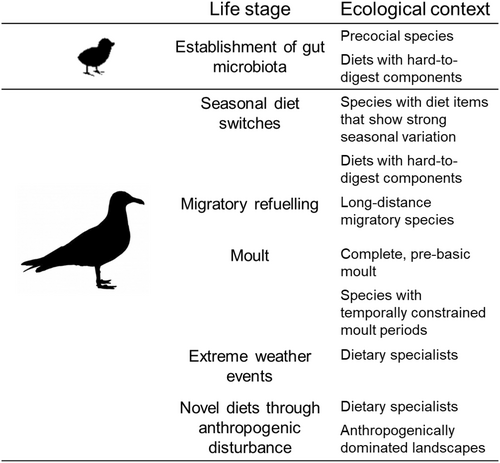
By contrast, precocial chicks are highly mobile and forage independently on various food items in addition to any microbiota seeding from their parents. As a result, their gut microbiome is expected to be more dynamic during ontogeny, fluctuating in response to changes in their diet and surrounding environment (Grond et al., 2019). For example, in dunlin (Calidris alpina) and semipalmated sandpiper (Calidris pusilla) chicks, bacterial diversity and abundance was highest at 3 days of age with Clostridia and Gammaproteobacteria being the most abundant, however from 3 to 10 days of age bacterial abundance and diversity stabilized with Clostridia and Bacilli becoming the most dominant classes (Grond et al., 2017). In coprophagic Japanese rock ptarmigans (Lagopus muta japonica) Coriobacteriia and Clostridia were consistently the most abundant class at all growth stages and there was a considerable variability in microbiota colonization during development (Kobayashi et al., 2019). Coprophagy may therefore allow precocial chicks to seed their microbiota with microbes suited to efficient digestion of the available food items (Kobayashi et al., 2019) and may also benefit from a microbial structure that reduces susceptibility to disease (Videvall et al., 2023). Indeed, captive precocial species like ostrich (Struthio camelus) show a high frequency of the behaviour in their early life stages (Fericean et al., 2021). Indeed, recent experimental research assessing faecal-supplemented ostrich chicks has shown that coprophagy is associated with distinct gut microbial composition, phylogenic structure, and taxonomic abundance compared to control chicks, with microbiomes that progressed more rapidly to that of adult ostriches (Videvall et al., 2023). Coprophagy-treatment chicks also had higher growth rates, with lower rates of gut disease (Videvall et al., 2023), suggesting coprophagy is not only important for establishing the gut microbiota of the chicks, but that this foraging behaviour plays a foundational role in chick growth, development, and health. This may, in part, relate to the herbivorous diet of adult ostriches. In early life, young ostriches supplement their diet with insects after hatching, before progressing to the herbivorous diet of adulthood. Vispo & Karasov (1997) suggest this may be because chicks lack the microbiota needed to assist with utilization of a plant-based diet in their infancy. Although this has not been definitively established, the experimental study of Videvall et al. (2023) provides an insight into this possibility. Given the paucity of species and environmental contexts in which these observations have been made (Fig. 1), the limited number of studies of allocoprophagy (Fig. 2), and the lack of mechanistic investigations into the causes and benefits of this behaviour (Fig. 2), further studies into the importance of coprophagy in seeding the gut microbiota of avian young are sorely needed. Experimental manipulations controlling energy and nutrient intake would improve our understanding of the importance of coprophagy to microbiota ontogeny. Detailed data on avian microbial communities throughout development, and their functional profiles, would be especially valuable in quantifying how coprophagy can assist with dietary changes during ontogeny (Fig. 4).
III. ROLE OF COPROPHAGY ON ADULT GUT MICROBIOME STABILIZATION
Once a bird's microbiota is established, rapid changes in gut microbiota may still be necessary to adapt dynamically to different diets, exposure to toxins (Kohl et al., 2016) and pathogenic bacteria (Roggenbuck et al., 2014; Mendoza et al., 2017), and for the ongoing maintenance of immune function (Broom & Kogut, 2018). Dynamic shifts in microbiota could be particularly important in birds with temporally varying diet composition. Research has shown that changes in diet can alter gut microbiota composition within 24 h (Wu et al., 2011) and that the relative abundance of microbial taxa varies according to diet. Vegetative cell walls are less digestible than nectar or invertebrate and vertebrate prey (McWhorter et al., 2009), and microbially derived enzymes thus play a key role underpinning digestion and nutrient uptake in herbivorous species. In many herbivorous mammals, foregut fermentation is a digestive method which allows the detoxification of secondary plant compounds and the breakdown of cellulose using fermentation in pregastric chambers (Grajal, 1995a,b; Lopez-Calleja & Bozinovic, 2000). However, the only bird species known to use this digestive system is the hoatzin (Opisthocomus hoazin) (Grajal, 1995b). In other herbivorous avian species, the microbiota involved in digestion occur primarily in the caecum (Soave & Brand, 1991; McWhorter et al., 2009; Hunt et al., 2019). In these species, some nutrients and short-chain fatty acids may be absorbed by the caecum (McWhorter et al., 2009), but primary absorption by the small intestine is missed. In such cases, coprophagy has been suggested to be a critical foraging behaviour that facilitates the uptake of lost or missing nutrients (Soave & Brand, 1991; Starks & Peter, 1991; Gallant, 2004; McWhorter et al., 2009). In some mammalian species, thiamine, K and B vitamins, and proteins are primarily excreted in faeces without absorption, but uptake can be increased via faeces reingestion (Soave & Brand, 1991). This level of detail is currently lacking from avian studies (González-Jáuregui, Esparza-Carlos & Mir, 2021) (Fig. 2), however, birds have been shown to be less efficient in metabolizing plant-based diets compared to mammals (Buchsbaum, Wilson & Valiela, 1986), suggesting coprophagy may play a significant role in nutrient uptake in birds, particularly those with an herbivorous diet. Interestingly, however, coprophagy has been described across a wide range of distantly related avian species from several different dietary niches (Fig. 1). For example, aquatic herbivores such as coot (Gruiformes: Rallidae) and ducks (Anseriformes: Anatidae), marine piscivores such as giant petrel (Macronectes giganteus) (Procellariiformes: Procellariidae) and Wilson's storm petrel (Oceanites oceanicus) (Procellariiformes: Oceanitidae), carrion-feeding Old World (Accipitriformes: Accipitridae) and New World vultures (Accipitriformes: Cathartidae), and insectivorous dunnock (Prunella modularis) (Passeriformes: Prunellidae), babax (Passeriformes: Leiotrichidae), and towhee (Passeriformes: Passerellidae) have all been reported as displaying coprophagic behaviour (Fig. 1). Most studies have presumed this behaviour is undertaken to access undigested or microbially digested food resources (Summers-Smith, 1983; Kraus & Stone, 1995; Negro et al., 2002; Gallant, 2004) (Fig. 2). Although some studies have suggested a role for coprophagy in seeding the microbiota of developing birds, to date there has been only one (Videvall et al., 2023) directly investigating the role of coprophagy in renewing or augmenting the gut microbiota of adult birds (Fig. 2). Yet the evidence detailed throughout this review suggests that shifts in microbiota, aided by coprophagy, could be particularly important for dynamic adaptation in adult birds.
(1) Dietary specialization
Specialization in diet leads to a dependence on specific, and often limited, resources, making specialists more vulnerable to environmental change (Morelli et al., 2021). A specialist species which feeds exclusively on a specific food source may be predicted to have a gut microbiome low in bacterial diversity, as a narrow specialist food range is less variable in its digestion pathways. For example, hummingbirds feed entirely on nectar and have been reported to harbour bacteria only from five or six phyla including Proteobacteria, Firmicutes, and Actinobacteria (Lee et al., 2019a; Herder et al., 2021). Kakapo (Strigops habroptilus), which feed on a limited diet of roots, bulbs, and leaves, also harbour low bacterial diversity (Waite, Deines & Taylor, 2012). Their microbiome comprises two main phyla, Gammaproteobacteria and Firmicutes, with Fusobacteria present only when feeding on rimu tree (Dacrydium cupressinum) fruits (Houston et al., 2007; Waite et al., 2012). However, this is not always the case. Although hoatzin have a primarily folivorous diet, their microbiome comprises a highly diverse bacterial community covering 40 phyla, with ~1400 different taxa (Godoy-Vitorino et al., 2010). In this species, Bacteroidetes are dominant in the crop whilst a higher proportion of Protobacteria and Firmicutes can be found in the caecum (Godoy-Vitorino et al., 2010, 2012). These differences in bacterial diversity may be more strongly correlated with diet items and not with feeding guild, as the hoatzin predominantly eat young leaves which are high in plant secondary metabolites and their foregut may be used to detoxify these compounds (Dearing, Foley & McLean, 2005). Small variations in a specialized diet as a result of environmental change could have a high impact within the gut. Thus, it could be predicted that dietary specialists consuming novel items may benefit from coprophagy to seed their gut microbiota with microbes that are adaptive for these new diets. Whilst the research is lacking in birds, faecal transplant/inoculation in clinical patients has been shown to alter the gut microbiota and allow for dietary expansion (Blyton et al., 2019) and has also influenced foraging behaviour and diet selection in mice (Trevelline & Kohl, 2022). Collectively, these findings suggest that coprophagy is likely to facilitate diet shifts, even for dietary specialists.
Assessing the role of coprophagy in facilitating diet switching, particularly in dietary specialist species, would aid in developing a mechanistic understanding of the potential for birds to adapt to novel diets which are increasingly common in the Anthropocene, and the risks this may entail. For instance, red knot (Calidris canutus canutus) offspring are becoming progressively smaller and shorter billed as a result of summers with early snowmelt, with these smaller individuals unable to consume highly nutritious bivalve prey and instead having to consume more seagrass rhizomes (van Gils, et al., 2016). Currently, shorter-billed individuals have reduced survival rates because they cannot gain the nutrients they need from their new diet (van Gils et al., 2016). Experimental studies in species that have undergone such massive diet shifts could yield critical insights into the nutritional, microbial, and synergistic benefits of auto-, allo- or heterospecific coprophagy, as well as potential risks in terms of exposure to novel pathogens (Fig. 4).
(2) Seasonal variation
Species that show flexibility in foraging behaviour and diet are known as generalists and may be comparatively responsive and adaptive to environmental change (Morelli et al., 2021). The microbiomes of generalists can be anticipated to be highly dynamic, particularly if feeding at different trophic levels at different times, as has been demonstrated for thick-billed murres (Uria lomvia) (Elliott, Gaston & Crump, 2010; Góngora et al., 2021). The microbiomes of generalist species are likely to be most dynamic at times of opportunistic foraging or seasonal diet shifts and may involve transient bacterial strains (Waite et al., 2012; Michel et al., 2018). For example, vampire finches (Geospiza septentrionalis) supplement their diet with blood during the dry season and their gut microbiota show an associated shift towards Fusobacteria, Tenericutes, and Deferribacterota, taxa which are typically attributed to carnivores and assist with blood digestion (Michel et al., 2018). Seasonal changes in gut microbiota have also been recorded for capercaillie (Tetrao urogallus), an omnivorous species that subsists on pine needles during winter when regular food sources are unavailable (Wienemann et al., 2011).
Coprophagy may assist birds to adapt to seasonally variable food sources and could be particularly relevant when switching to diets with difficult-to-digest components whereby particular bacteria and enzymes may assist (Amato et al., 2015). Although there are no studies to date on birds, evidence from mammals suggests this is an area in need of investigation. For instance, in primates, the practice of coprophagy varies seasonally, in line with the seasonal availability of hard-to-digest food items (Krief, Jamart & Hladik, 2004; Sakamaki, 2009; Masi & Breuer, 2018) and/or times of food scarcity (Wall, 1983; Vogrin, 1997; Krief et al., 2004; Shimada, 2012; Masi & Breuer, 2018). Seasonal shifts in foraging behaviour involving coprophagy have been documented in birds, albeit in relation to changes in energy requirements rather than changes in the digestibility of available resources (Fig. 2). For instance, during the breeding season, giant petrels undertake extended incubation bouts necessitating periods of fasting of up to 15 days. At the end of their incubation bout, each parent starts their foraging trip by consuming seal faeces at haul-out sites (Corá, Finger & Kruger, 2020). This behaviour is thought to assist the birds, who are in a fasted state, to refuel quickly from an easily accessible and energetically rich food source before they undertake extended foraging trips across the Southern Ocean in search of more valuable prey (Casaux, Baroni & Carlini, 1997; Corá et al., 2020). However, it is also plausible that fasting may eliminate microbiota essential to digestion from the gut, as has been shown in other species (Dewar et al., 2014; Zarrinpar et al., 2014; Lee et al., 2019c), and that fasting giant petrels may be engaging in coprophagy to reinoculate their microbiota for a digestive state. Microbial re-seeding after fasting due to incubation has been surmised to occur in other species. During incubation and early chick rearing, adults of several species, including dunnocks (Lamb et al., 2017), spotted towhees (Pipilo maculatus) (McKay et al., 2009) and giant babax (Babax waddelli) (Gao et al., 2020) (Fig. 2), have been observed consuming nestling faecal sacs. This behaviour is suggested to be a means of not only retaining nutrients (Lamb et al., 2017) and microbes (Gao et al., 2020) but also keeping the nest hygienic (Azcárate-García et al., 2019), and removing cues for predators (Burrows, 2018). However, in the case of giant babax, nestling faecal sacs were found to have a high abundance of Firmicutes – a keystone taxon in the gut of many bird and mammalian species (Waite & Taylor, 2014; Grond et al., 2018). Coprophagic behaviour has therefore been suggested as a means by which parents could easily obtain these bacterial taxa (Gao et al., 2020). Indeed, the reduced feeding rate in parents and the increased physiological demands of raising young could both impact the gut microbiome, limiting some bacterial species, although further experimental studies are required to demonstrate this.
Coprophagy may also play a key role in the switch between fasting and fuelling metabolic states in long-distance migratory birds. For instance, shorebirds in an active migratory state have been found to have a gut microbiota that is similar across species yet distinct from the microbiota of those same species in the non-migratory phase of the annual cycle (Risely et al., 2018). Similarly, the gut microbiota of ruddy turnstones (Arenaria interpres) from the North Atlantic flyway has been shown to undergo dramatic changes in taxonomic composition and function, particularly in polyunsaturated fatty acid biosynthesis, over the course of refuelling during migratory stopover (Grond, Louyakis & Hird, 2023). How these migrants, arriving in a fasted state, can shift the composition of their microbiome so rapidly is not known, however direct sourcing of microbes from their foraging environment is unlikely (Risely et al., 2017). Recent observations in ruddy turnstones during stopover, albeit on the Central Asian flyway, have indicated that these birds consumed human faeces recently deposited on the beach, suggesting coprophagy may play a role in refuelling dynamics (Kasambe & Kasambe, 2020). Currently, our understanding of the underlying imperative for these behaviours, and the degree to which auto-, allo- and heterospecific coprophagy supplements metabolic, nutritional, and microbial needs following fasting remains speculative. Detailed investigations to tease apart the potential benefits of coprophagy in the context of different life stages, with varying resource demands and resource availability, are sorely needed (Fig. 4).
Profound changes in gut microbiome composition have also been observed over the course of the moult period (Dewar et al., 2014; Lee et al., 2019c), including changes in alpha diversity in some species, and reductions in Firmicutes (Lee et al., 2019c), Proteobacteria and Bacteroidetes (Dewar et al., 2014) and increases in Fusobacteria (Dewar et al., 2014) in others. However, microbial dynamics during moult in free-living birds have, to date, only been reported from penguin species (family Spheniscidae), who have an intense moult during which they are confined to land for weeks and therefore abstain from eating (Lee et al., 2019c). Similarly, studies in poultry have been confounded with fasting, as moult has been experimentally induced through an extended period of food removal (Han et al., 2019). As a result, moult represents a critical, yet poorly studied, phase for the gut microbiota of birds. Although moult is characterized by the replacement of plumage, the energetic and nutritional costs incurred during this period vastly exceed the calorific and nutritional content of the feathers themselves (Murphy, 1996; Hoye & Buttemer, 2011), instead reflecting a period of systemic physiological rejuvenation (Murphy & Taruscio, 1995; Buttemer, Addison & Klasing, 2020). In birds, adults typically lose the ability to reconfigure the immune system and proceed to a state of immunosenescence (Janeway et al., 2004). Although the adaptive immune response is, by definition, adapting to novel pathogen's throughout an animal's life, preferential use of immunological memory stemming from a previous infection to a subsequent, slightly different, version of that pathogen hampers the immune system by preventing it from mounting potentially more effective responses during subsequent infections (Focosi et al., 2021). In birds, however, profound changes in immune function have been reported in adults during the moult period, including enlargements of key organs like the thymus (Brake, Morgan & Thaxton, 1981) and spleen (Silverin et al., 1999), increased abundance of basophils and monocytes (Nava, Veiga & Puerta, 2001; Buehler et al., 2008) and immunoglobulins (Pap et al., 2010), and suppressed inflammatory responses (Alodan & Mashaly, 1999; Martin, 2005; Buehler et al., 2008; Moreno-Rueda, 2010). Collectively, these findings indicate that peak moult is associated with decreased innate immune responses, paired with increased cell-mediated (T-cell) and antibody-mediated (B-cell) acquired immunity, long-lived macrophages and antigen-presenting cells important for initiating acquired immune responses (Alodan & Mashaly, 1999; Buehler et al., 2008). Historically, these shifts in immune strategy have been viewed as systemic adaptations to reduce the risk of innate immune responses competing with the increased energetic and nutritional demands of feather production (Buehler et al., 2008; Martin, Weil & Nelson, 2008). Yet, moult-related shifts in immune function have also been observed in near-featherless birds, such that these adjustments are indicative of a recalibration of entire immune repertoires and recognition systems, with adults during peak moult being immunologically comparable to a newly hatched chick (A. DeRogatis & K. Klasing, unpublished data) and potentially capable of undoing their ‘original antigenic sin’ (Focosi et al., 2021). Given the tight association between gut microbiota and immune repertoires and recognition systems (Broom & Kogut, 2018), moult may be a critical period for re-configuring avian microbiomes. Although there are currently no data on temporal changes in use of coprophagy in wild birds, poultry are known to increase coprophagy during moult. It is therefore plausible that coprophagy may interact with moult-associated recalibration of the immune system. Studies assessing the degree and type of coprophagy birds undertake before, during and after moult, both in captivity and in the wild, are needed to assess the plausibility of this process. Ideally, these studies would be paired with dynamic quantification of immune function and coincident microbiotas to understand the potential for coprophagy to shape immune function and microbiota–immune interactions.
(3) Opportunistic coprophagy
Birds are also known to engage in coprophagy opportunistically, in line with local weather conditions rather than predictable seasonal cycles. For instance, Eurasian coots (Fulica atra) common moorhen (Gallinula chloropus), tufted ducks (Aythya fuligula), and mallard (Anas platyrhynchos) were observed eating black-headed gull (Larus ridibundus) faeces from boulders and ice (Wall, 1983; Boot, 1987). Ducks (Anas platyrhynchos, A. crecca, A. formosa) were also observed consuming whooper swan (Cygnus cygnus) faeces under similar conditions (Shimada, 2012). In both cases, coprophagy was considered an opportunistic adaptation to the bird's regular food source being inaccessible due to ice covering the lakes where they feed (Wall, 1983; Boot, 1987; Shimada, 2012) (Fig. 2). This behaviour is often considered indicative of resource limitation (Wall, 1983; Boot, 1987; Krief et al., 2004; Shimada, 2012; Masi & Breuer, 2018), however coprophagy has been seen in dusky moorhens (Gallinula tenebrosa) at times of high resource availability (Starks & Peter, 1991). Importantly, the nutritional, energetic and microbial consequences of opportunistic coprophagy, particularly the consumption of faeces from other species, has yet to be examined (Fig. 2).
IV. POTENTIAL RISKS ASSOCIATED WITH COPROPHAGY
Although coprophagy has extensive potential to improve host health through access to nutrients and beneficial microbiota, there are several potential risks posed by this foraging behaviour (Fig. 3). These include potential ingestion of pathogens or antibiotic-resistant microbes, and exposure to compounds that might affect the microbiome (e.g. antimicrobials). The avian gastrointestinal tract is host to an array of bacterial species, most of which are beneficial and commensal (Benskin et al., 2009; Fuirst et al., 2018). However, several opportunistic gastrointestinal pathogens that can cause severe disease in birds are transmitted via the faecal–oral route, including bacteria belonging to the genera Enterococcus, Salmonella, Clostridium, Escherichia, Campylobacter and Staphylococcus (Benskin et al., 2009; Fuirst et al., 2018). These potentially pathogenic taxa could be transmitted through coprophagy, which has been extensively speculated in domestic poultry (Montrose, Shane & Harrington, 1985; Folz et al., 1986; Line, 2006; Line et al., 2008; Pan & Yu, 2014; Alpigiani et al., 2017; Craft et al., 2022). However, the role of coprophagy in the transmission of opportunistic pathogens has rarely been studied (von Waldburg-Zeil, van Staaveren & Harlander-Matauschek, 2019). To date, the only studies in birds have been in domestic species of poultry, particularly in mass-production environments (Hörnicke & Björnhag, 1980). Moreover, the effects of coprophagy have been inferred from increased incidence of infection after feed withdrawal (Byrd et al., 1998; Corrier et al., 1999), rather than through direct assessment of the effect of coprophagy. These studies have also been conducted on birds living at high stocking density with direct contact, where they might ingest contaminated feed and water (Shanker, Lee & Sorrell, 1990; Line et al., 2008).
In wild birds, the degree to which a species aggregates, particularly in mixed-species assemblages, may be particularly important for unintentional coprophagy (Kohl, 2012), and hence intra- and inter-species pathogen transmission (Kohl, 2012). Indeed, studies using animal social networks have shown similarities in gut microbiota between individuals of the same species groups (Downing, Griffin & Cornwallis, 2020) and mixed-species aggregations (Grond et al., 2018). To investigate the risk of pathogen transmission presented by coprophagy, experimental studies altering ability to engage in allo- and heterospecific-coprophagy and using whole-genome sequencing of pathogenic bacterial strains to give insight into the phylogenetic relationships among strains and accessory genes, including resistance and virulence genes (Ingle, Howden & Duchene, 2021; Djordjevic et al., 2023), are needed.
In addition to exposing birds to a wider range of pathogens, coprophagy could represent a mechanism by which these infections can be treated. Although some ingested bacterial strains are only transient and not retained in the gut, many are retained and can affect the resident bacterial community, both directly and indirectly (Derrien & van Hylckama Vlieg, 2015). In addition to becoming a member of the microbiome, ingested bacteria can stimulate the growth of resident bacteria (Derrien & van Hylckama Vlieg, 2015) or have a negative impact on them through competitive exclusion (Derrien & van Hylckama Vlieg, 2015). Recently, faecal transplants have been used to treat infections and metabolic disease and to re-establish a healthy microbiome after medical treatment of clinical patients (West et al., 2019). Whilst there have been limited studies in birds, there is evidence to suggest that faecal transplant may improve gut microbiomes and could be particularly beneficial for (re)introduction of animals into the wild after periods in captivity and/or following medical treatment (Eason & Moorhouse, 2006; West et al., 2019; Li et al., 2022). For example, faecal transplant has been successfully used in critically endangered kakapo to establish normal gastrointestinal microbiota (Eason & Moorhouse, 2006). Investigation into self-medication through coprophagy is a promising line for future research, particularly in terms of the conservation of threatened species and management of synanthropic species (Eason & Moorhouse, 2006; Waite, Deines & Taylor, 2013; Guo et al., 2020).
(1) Anthropogenic influences
Many generalist avian species are capable of exploiting human-dominated habitats, such as areas rich in refuse or agricultural remains (Smith & Carlile, 1993; Mennechez & Clergeau, 2006; Dolejska et al., 2015; Thabethe & Downs, 2018), and some of these species are also intentionally fed (Feng & Liang, 2020). Association with humans has been linked to lower diversity gut microbiota in some species (Knutie, Chaves & Gotanda, 2019), and could be a result of birds feeding on refuse or being provisioned and becoming specialized on a very narrow range of food items such as bread (Murray et al., 2018; Thabethe & Downs, 2018). Species such as gulls (family Laridae) and ibis (family Threskiornithidae) foraging at a variety of natural sites have greater microbial diversity compared to those foraging at sites of significant human impact (Merkeviciene et al., 2017; Fuirst et al., 2018; Murray et al., 2020). Long-term shifts towards increased synanthropic foraging behaviours are therefore expected to have profound consequences for wildlife microbiome composition and function (Fig. 4).
Synanthropic birds, through their use of waste depots, sewage treatment plants, and human refuse, are exposed to chemicals and heavy metals that can profoundly alter their microbiota (Poole, 2017). Although some antimicrobial compounds occur naturally in the environment, the increased use of anthropogenically produced analogues in agriculture, veterinary medicine and human medicine combined with suboptimal waste disposal and treatment has resulted in active antimicrobial compounds inadvertently being released into some environments through contaminated landfill leachate, wastewater, and effluents (Christou et al., 2017). Many of these antimicrobials also persist in the environment in active forms, resulting in an increased number and diversity of these compounds in the environment, particularly in areas dominated by anthropogenic activities (Arnold, Williams & Bennett, 2016). Exposure to antimicrobial compounds can eliminate a considerable proportion of the microbiota, opening ecological niches within the gut for bacteria that carry antimicrobial resistance genes to survive the effects of antimicrobial compounds and confer the ability to invade and/or proliferate (Munita & Arias, 2016). Experimental administration of antibiotics in birds has been shown to alter their gut microbiota, impacting digestion, metabolic and immune functions (Kohl et al., 2018; Zhou et al., 2020b; Li et al., 2022). Such profound changes in microbiota, together with reductions in host immune function by bacteria carrying antibiotic resistance genes (Jiang et al., 2019), can lead to a state of dysbiosis (Schjørring & Krogfelt, 2011). As demonstrated in mammals, coprophagy may be a method for birds to overcome antimicrobial-induced dysbiosis (Soave & Brand, 1991). However, coprophagy may also increase exposure to antimicrobials, particularly those that have high environmental persistence and/or bioaccumulate, including heavy metals (Fig. 3).
(2) Antimicrobial resistance emergence and spread
Increased use of antimicrobial compounds has also altered the selective pressure on microbial populations (Martinez, 2009; Djordjevic, Stokes & Chowdhury, 2013; Smalla et al., 2018; Ben et al., 2019; Oniciuc et al., 2019), increasing the prevalence of organisms resistant to antimicrobial compounds in the environment (Borges et al., 2017). Synanthropic birds are increasingly found to have been exposed to microbial communities rich in antibiotic resistance genes (Borges et al., 2017; Poole, 2017; Marcelino et al., 2019; Wang et al., 2019; Murray et al., 2020; Wyrsch et al., 2022). Coprophagy may further enhance the transmission of antibiotic resistance genes from humans to wildlife (through foraging on human faeces; Kasambe & Kasambe, 2020), and between wildlife, through several pathways. Firstly, the faeces from humans or animals (either domestic or wild after being treated in captivity) that have recently been treated with antimicrobials are expected to contain antimicrobials, which will directly select for resistant microbes in the gut of a bird engaging in coprophagy. Secondly, these faeces will also contain bacteria enriched in antimicrobial resistance genes (Baros Jorquera et al., 2021). Birds engaging in heterospecific coprophagy, consuming faeces from medicated populations, may therefore be at greater risk of direct inoculation with bacterial strains carrying resistance genes to clinically important antimicrobials. Subsequent coprophagy between wild birds (either allo- or heterospecific) may then lead to widespread transmission through a population that would not otherwise occur in birds not utilizing this behaviour. Finally, resistance genes can be transmitted from bacteria ingested from food to gastrointestinal microbiota via horizontal gene transfer (Lester et al., 2006; Feld et al., 2008; Pinto et al., 2015). High numbers of resistant strains in the gut, as may be the case when a bird consumes large quantities of faecal material from a bird carrying resistant bacteria, increases cell-to-cell contact and therefore further increases the opportunity of resistance gene transfer from ingested bacteria to bacteria in a bird's microbiota (Schjørring & Krogfelt, 2011).
In wild birds, whole-genome and metagenomic sequencing has demonstrated that antibiotic resistance genes are predominantly associated with phyla Bacteroidetes, Firmicutes and Proteobacteria (Wang et al., 2019; Cao et al., 2020; Feng et al., 2021). Whilst many bacteria belonging to such phyla are harmless, some can colonize and become pathogenic, causing intestinal or extraintestinal disease, and may also be zoonotic (Woolhouse, Haydon & Antia, 2005; Santos et al., 2020; Wyrsch et al., 2022). Horizontal gene transfer may result in these bacteria acquiring and spreading antimicrobial resistance genes along with virulence genes, which would make infection exceedingly difficult to treat. Although there is evidence that antibiotic resistance genes are found in excreta, particularly in birds with synanthropic associations (Silva et al., 2009; Hernandez et al., 2013; Mukerji, et al., 2019; Wyrsch et al., 2022), to date there have been no studies on the transmission of antibiotic-resistant bacteria or onward transmission of antibiotic resistance genes through coprophagy. Studies quantifying the connection between diet, coprophagy and resistance gene burden and transmission in different land-use contexts would shed valuable light on these risks. Whether there are traits that give some species a propensity for higher resistance-gene carriage should also be explored.
V. FUTURE PERSPECTIVES
The ongoing development of next-generation sequencing has greatly accelerated the number of studies characterizing the avian microbiome (Waite & Taylor, 2014; Cao et al., 2020; Aruwa et al., 2021). Yet, the intrinsic and extrinsic factors driving dynamics in these microbial communities have yet to be explored in detail. In particular, the impact of the use of coprophagy on these dynamics, both in terms of benefits and risks to host health and nutrition warrants targeted investigation across a range of species and environmental contexts (Fig. 4). Given the fundamental role of gut microbiota for the maintenance of organismal health and performance, and the demonstrated potential for auto-, allo- and heterospecific coprophagy to impact gut microbiota, there is a pressing need to understand better when, where and why birds engage in coprophagy. In parallel, we need to understand the mechanistic impact of coprophagy on host health and performance, in order to understand the degree to which this foraging behaviour can assist wild birds in adapting to predictable and unpredictable change processes as well as the risks it might pose to host health and pathogen transmission. In this review, we have outlined several key life stages where there is considerable potential for coprophagy to modulate gut microbiota and have a profound influence on host health and fitness. Greater use of, and benefit from, coprophagy may be expected during ontogeny, moult, long-distance migration, and extreme weather events (Fig. 4). Coprophagy may also assist animals in adapting to longer-term changes, including adapting to novel environments as a result of anthropogenic land-use change. However, anthropogenic environments are predicted also to pose the highest risks as a result of coprophagy, particularly in terms of exposure to microbiota-altering antimicrobials as well as pathogenic and antimicrobial-resistant microbes.
Using coprophagy-prevention experiments and germ-free models to compare microbiota structure and dynamics during each of these periods, and assessing how this relates to physiology and health, would be highly beneficial. Coprophagy could be an underappreciated mechanism for obtaining limited or high-demand resources, and for augmenting a bird's gut microbiota to adapt to changing environmental conditions. In some species, for example ruddy turnstone (Kasambe & Kasambe, 2020) and Australasian darter (Anhinga novaehollandiae) (Yong, 2018) coprophagy has only recently been documented and could therefore represent a novel adaptive behaviour. Whether coprophagy in birds is predominantly a mechanism for supplementation of a metabolic or microbial need could be assessed using metagenomics to characterize the gut microbial community, potential microbial target species or strains, and insights into microbial function (e.g. Grond et al., 2023), from energy gains to pathogen exclusion and immune programming. Whole-genome sequencing approaches would provide complementary insights into the role of coprophagy in the transmission of pathogens and antimicrobial resistance genes (Ingle et al., 2021). Finally, it is unknown if coprophagic behaviour is a learned behaviour, or a heritable trait that has evolved ancestrally, or convergently. As more studies on coprophagy in wild birds become available, meta-analysis could provide additional insights into the evolutionary origins of this behaviour.
VI. CONCLUSIONS
- (1)
The avian gut microbiome regulates a broad range of biological functions yet is dynamic across a range of timescales. The gut microbiota may be fundamental in an individual's ability to adapt to new environments, due to its bacterial composition being dynamic both during development and after maturity.
- (2)
Understanding the drivers of avian gut microbiota dynamics is critically important to determining the capacity of avian hosts to adapt to new and changing environments but has received little attention to date.
- (3)
Although coprophagy in birds has been assumed to confer nutritional and energetic benefits, this foraging behaviour may present a unique and powerful mechanism for rapid seeding and augmenting the gut microbiota throughout a bird's life.
- (4)
Conversely, this behaviour has the potential to expose birds to antimicrobial compounds and pathogenic microbes and may contribute to the emergence and spread of antimicrobial resistance in wildlife and the environments they inhabit.
- (5)
Based on mechanistic predictions developed through this review, there is a pressing need for detailed investigation into the influence of auto-, allo-, and heterospecific coprophagy on avian gut microbiomes and their impact on host health and performance across several life stages and environmental contexts.
ACKNOWLEDGEMENTS
The authors declare that they have no competing interests. A. D. was supported by the University of South Australia's Science, Technology, Engineering, and Mathematics (STEM) RTP scholarship, and B. J. H. was supported by a DECRA Fellowship from the Australian Research Council (DE200100884). The authors confirm that the funding bodies had no direct influence on the review design, data collection, analysis/interpretation, or writing. We express our gratitude to all individuals who assisted in facilitating this review, as well as the Editorial Office and reviewers for their valuable and constructive feedback. In memoriam of Judith Dawson 07/12/1956 to 25/10/2022.
AUTHOR CONTRIBUTIONS
A. D.: conceptualization, data curation, writing of original draft, review & editing; B. D.: supervision, funding, conceptualization, data curation, writing of original draft, review & editing; S. P. D.: supervision, review & editing; E. D.: supervision, funding, conceptualization, review & editing; B. J. H.: supervision, conceptualization, data curation, writing of original draft, review & editing.




