False alarms and information transmission in grouping animals
ABSTRACT
A key benefit of grouping in prey species is access to social information, including information about the presence of predators. Larger groups of prey animals respond both sooner and at greater distances from predators, increasing the likelihood that group members will successfully avoid capture. However, identifying predators in complex environments is a difficult task, and false alarms (alarm behaviours without genuine threat) appear surprisingly frequent across a range of taxa including insects, amphibians, fish, mammals, and birds. In some bird flocks, false alarms have been recorded to substantially outnumber true alarms. False alarms can be costly in terms of both the energetic costs of producing alarm behaviours as well as lost opportunity costs (e.g. abandoning a feeding patch which was in fact safe, losing sleep if an animal is resting/roosting, or losing mating opportunities). Models have shown that false alarms may be a substantial but underappreciated cost of group living, introducing an inherent risk to using social information and a vulnerability to the propagation of false information. This review will focus on false alarms, introducing a two-stage framework to categorise the different factors hypothesised to influence the propensity of animal groups to produce false alarms. A number of factors may affect false alarm rate, and this new framework splits these factors into two core processing stages: (i) individual perception and response; and (ii) group processing of predator information. In the first stage, individuals in the group monitor the environment for predator cues and respond. The factors highlighted in this stage influence the likelihood that an individual will misclassify stimuli and produce a false alarm (e.g. lower light levels can make predator identification more difficult and false alarms more common). In the second stage, alarm information from individuals is processed by the group. The factors highlighted in this stage influence the likelihood of alarm information being copied by group members and propagated through the group (e.g. some animals implement group processing mechanisms that regulate the spread of behavioural responses such as consensus decision making through the quorum response). This review follows the structure of this new framework, focussing on the causes of false alarms, factors that influence false alarm rate, the transmission of alarm information through animal groups, mechanisms to mitigate the spread of false alarms, and the consequences of false alarms.
I. INTRODUCTION
One of the key benefits of grouping is access to social information (Ward & Webster, 2016). Social information is gained by observing or interacting with another animal or its products (Heyes, 1994; Dall et al., 2005; Hoppit & Laland, 2008). For example, a deer watching another flee suddenly may be alerted to an incoming attack (Caro et al., 1995), a seabird seeing an aggregation of diving conspecifics could gain information about the presence of fish (Thiebault et al., 2014), or a fish releasing an alarm chemical could alert others in the area to the presence of a predator (Sosna et al., 2019). By using social information, an animal can gain biologically relevant information without incurring the costs of personal sampling, for example, greater mouse-tailed bats (Rhinopoma microphyllum) find food more quickly using social cues and, therefore, can forage more efficiently by spending less time personally searching for prey (Cvikel et al., 2015). By collating the knowledge of multiple individuals, social information allows animals access to a broader range of information than any one individual could gain from direct experience. This information can include resource distribution, quality, and quantity (Pitcher, Magurran & Winfield, 1982; Webster & Laland, 2015), and the detection of predators (Lima, 1995). When foraging as a group, the burden of predator vigilance is shared across members meaning that individuals can increase their foraging efficiency by decreasing their own vigilance effort (‘the-many-eyes-effect’; Lima, 1995). Information about predators can be transferred through groups using signals (evolved communications from a sender that alter the behaviour of a receiver) or by individuals monitoring the passive cues of conspecifics responding to threats (cues that have evolved to respond to predators, not to communicate with conspecifics, for example, fleeing; Ward & Webster, 2016). By responding to the behaviour of conspecifics as opposed to direct predator detection, not all members of a group need to identify a predator to respond (Treherne & Foster, 1981). While responding to social cues means that group members are more likely to respond to an approaching predator (Siegfried & Underhill, 1975; Lazarus, 1979; Treherne & Foster, 1981; Boland, 2003; Ward et al., 2011), by responding to conspecifics as opposed to direct detection, group members can become vulnerable to the propagation of false information and costly false alarms (Beauchamp & Ruxton, 2007).
A false alarm is defined here as the occurrence of alarm behaviour without a genuine threat (a false positive; Table 1). In animal groups, false alarm signals from a single individual can be transferred through the group leading to a costly misinformation cascade (Trail, 1987; Beauchamp, 2010). When responding to false predator information, animals incur both the energetic costs of producing an alarm response as well as lost opportunity costs such as lost foraging time (Beauchamp & Ruxton, 2007), loss of sleep/resting time (Beauchamp, 2010), or lost mating opportunities (Martín, López, & Cooper, 2003). False alarms constitute a surprisingly large proportion of all alarms, with high frequencies empirically documented in a range of birds (Table 2) and group-living mammals (Hoogland, 1981; Hare & Atkins, 2001; Blumstein, Verneyre & Daniel, 2004). Despite their widespread occurrence, the influence of false alarms remains relatively overlooked. False alarms are very rarely accounted for in models of group living and collective vigilance, however, more recent models have indicated that false alarms are likely a significant, often unrecognised, cost of group living (Beauchamp & Ruxton, 2007).
| Response | No response | |
|---|---|---|
| Predator present | Appropriate response | False negative |
Predator absent |
False positive | Appropriate response |
| Study species | Percentage of alarm behaviours that were false | False alarm rate per hour | Type of alarm behaviour quantified | Reference |
|---|---|---|---|---|
| Redshank (Tringa totanus) | >75% | 2 | Escape departures | Cresswell et al. (2000) |
| Guianan cock-of-the-rock (Rupicola rupicola) | 73.6% | 0.32 | Escape departures | Trail (1987) |
| Greylag goose (Anser anser) | >50% | 0.76 | Movement from land to water | Kahlert (2006) |
| Semipalmated sandpiper (Calidris pusilla) | >75% | 60 | Escape departures | Beauchamp (2010) |
| Willow tit (Poecile montanus) | 81% | (–) | Alarm calls | Haftorn (2000) |
| House sparrow (Passer domesticus) | (–) | 2 | Escape departures | Boujja-Miljour et al. (2017) |
(1) Brief overview: a two-stage framework
In groups of animals, false alarm behaviour is dependent on two factors: (i) the likelihood of each individual animal in the group producing a false alarm (the individual false alarm rate), and (ii) how alarm information from individuals is processed by the group (Fig. 1). The false alarm rate of an individual (see Section II) will depend on three primary factors: predator cue ambiguity, vulnerability to attack, and the cost of responding. When predator cues are harder to identify or are more ambiguous, false alarms are typically more common, for example, during rain (Hilton, Ruxton & Cresswell, 1999) or in lower light levels (Beauchamp, 2010). In addition, false alarms are likely more common when predation rates are higher and more successful, since false negatives (not responding to a true predator threat) would become more risky (Haftorn, 2000). Lastly, false alarms are typically less common when the cost of responding, in terms of the energetic cost of movement and lost opportunities, is higher (Beauchamp, 2010). These three factors will vary across an animal's lifetime meaning that individual propensity for alarm is constantly fluctuating.
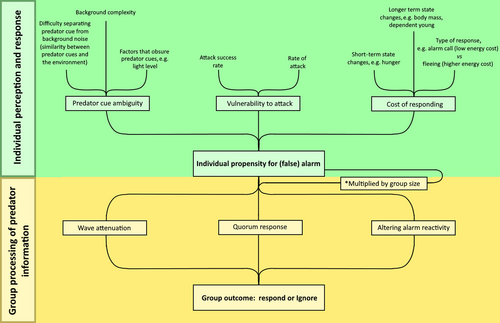
Theoretically, these factors combine to create a typical false alarm rate for individuals in the group, which is then multiplied by group size to give higher false alarm rates in larger groups (see Section III). In species with no group processing mechanisms, this would lead to a linear relationship between group size and the occurrence of false alarms within the group. While there is a predicted positive correlation between group size and false alarm rate, the relationship may not be directly proportional, as different group members may not have the same propensity for producing false alarms. For example, younger animals may be more prone to producing false alarms than adults (Cheney & Seyfarth, 1988), or different physical positions within a group may favour/impede predator vigilance. Generally speaking, however, false alarm rate is likely to increase with group size in animals without group processing mechanisms. However, many grouping species do employ mechanisms to restrict a cascade of false information (Hare & Atkins, 2001; Sumpter & Pratt, 2009; Sosna et al., 2019). The combination of both individual alarm rate and group processing mechanisms will determine how likely false alarms are to be generated and subsequently spread through the group.
Three information-dampening mechanisms are discussed in detail in this review (Fig. 1; see also Section III.3) (but note that there may be other dampening systems across the animal kingdom that are not considered herein): (i) altering alarm reactivity: some species of birds and mammals reduce their reactivity to the alarm behaviour of a previously unreliable conspecific (Cheney & Seyfarth, 1988; Lima, 1994; Hare & Atkins, 2001); (ii) the quorum response in which the likelihood that an animal copies a behaviour shows a non-linear relationship with the number of conspecifics performing that behaviour (Sumpter & Pratt, 2009); (iii) wave attenuation: some species of fish alter their group structure depending on the perceived level of predation risk, associating closely in high-risk environments and spreading out when predation risk is perceived to be lower (Sosna et al., 2019).
This review will follow the structure of Fig. 1, starting with an overview of false alarms at the individual level, with focus on the three key factors which determine individual false alarm rate (predator cue ambiguity, vulnerability to attack, and the cost of responding). It will then discuss the underlying mechanisms of information transfer within groups, how this can lead to a cascade of false information, and the group processing of predator information with reference to systems which impede the spread of false alarms (altering alarm reactivity, the quorum response, and wave attenuation).
(2) A note on deceptive alarm calling
Within the field of animal communication, there has been much interest in the role of deception. False alarms, while often produced mistakenly as a result of classification errors, are also used deceptively by some species. During a deceptive false alarm, the signaller will produce an alarm even though no threat is present, to gain an advantage over the receiver. For example, this behaviour is well documented in fork-tailed drongos (Dicrurus adsimilis), which both produce alarm calls of their own species and mimicked alarm calls of other species to steal food left behind by fleeing animals (Flower, 2011; Flower, Gribble & Ridley, 2014). The use of deceptive false alarms has been reported for only a relatively small number of animal species, but appears to be taxonomically widespread, with documented examples among insects (Regnier & Wilson, 1971), ungulates (Bro-Jørgensen & Pangle, 2010), primates (Wheeler, 2009), rodents (Tamura, 1995), and a variety of bird species (Munn, 1986; Møller, 1988; Flower, 2011). Deceptive false alarms can be used by animals to gain a range of advantages including food usurpation (Munn, 1986; Møller, 1988; Wheeler, 2009), mate retention (Tamura, 1995; Bro-Jørgensen & Pangle, 2010), or to confuse and disband aggressors (Regnier & Wilson, 1971). While an interesting aspect of animal communication, this review will focus on the more widespread occurrence of non-deceptive, ‘mistaken’ false alarms. The role of deceptive false alarms is likely to require a different framework, and will not be considered herein. Contexts where deceptive false alarms are a possibility can be distinguished from ‘mistaken’ false alarms by the possible benefits gained by the signaller; false alarms are only deceptive if the signaller stands to gain some form of advantage. In most contexts where false alarms occur, this is not the case. For example, a bird producing an escape flight from a profitable food patch, causing other birds also to flee, likely gains no benefits from the departure of the latter. A study on greylag geese (Anser anser) found that false alarms prevented geese from feeding for 19 min on average, resulting in the loss of valuable foraging opportunities (Kahlert, 2006).
II. MISCLASSIFICATION OF STIMULI AND FALSE ALARMS AT THE INDIVIDUAL LEVEL
(1) Classifying stimuli and signal detection theory
Identifying useful cues from a sea of distracting background information is a task faced by all animals. An animal must be able to identify a range of biologically relevant cues including predators, prey, food resources, mates, and conspecifics (Shettleworth, 2010). This task is made inherently difficult by predator cues that share characteristics with background information. For example, from the perspective of a fish, the movement of a swaying plant above the water may look very similar to that of a predatory bird. In fact, many predators and prey increase the difficulty of stimulus classification by using camouflage to increase their similarity to the background (Pembury-Smith & Ruxton, 2020). When responding to stimuli in the environment, an animal can make two types of recognition error: (i) a false negative where the target cue is rejected and classified as non-relevant (also known as a Type I error), and (ii) a false positive where non-target cues are misidentified and classified as relevant (also known as a Type II error; Bernal, Rand & Ryan, 2009; Table 1). False positives occur in a wide range of contexts, for example, red-winged blackbird (Agelaius phoeniceus) males consistently mistake the song of mimicking mockingbirds (Mimus polyglottos) for the song of conspecifics (Searcy & Brenowitz, 1988). In the context of predator detection, mistaking irrelevant background information for predator cues can give rise to costly false alarms. For example, redshanks (Tringa totanus) sometimes mistake non-raptorial corvids overhead for birds of prey, leading to false alarm flights and abandonment of feeding patches (Cresswell, Hilton & Ruxton, 2000).
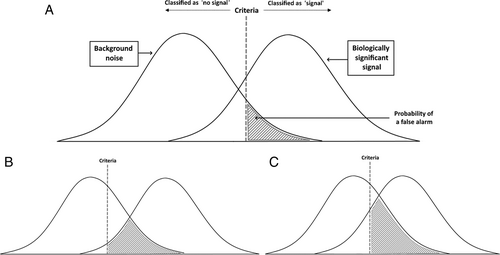
False alarms ultimately arise from the difficulty of identifying predator cues from a wealth of similar and distracting background information. False alarms are an inherent by-product of signal detection theory, a model that describes the process of identifying biologically important signals among irrelevant background noise (Wiley, 2006; Fig. 2). The overlapping distributions of this model illustrate the common scenario where background information shares qualities with the ‘useful’ signal the animal wishes to identify, for example, an approaching predator. Here the animal must set a criteria point above which it will classify input as a positive detection of the signal, and below which it will not. As shown in Fig. 2, the occurrence of false alarms is inevitable when the signal shares qualities with the background, and the probability of a false alarm will increase when the criteria for classifying a signal are lower or when background information and the signal are more similar (Wiley, 2006). An example of the use of a very low signal criteria threshold can be found in willow tits (Poecile montanus) which produce alarm calls in response to most large flying objects including aeroplanes and large non-raptorial bird species (Haftorn, 2000). With such a low alarm criterion, willow tits seem to be following a ‘better safe than sorry’ principle which is likely advantageous as their main predators use ambush attacks which they have little chance of escaping (Haftorn, 2000).
(2) False alarms in individuals
False alarms are recorded across a wide range of taxa including insects (Ings & Chittka, 2008; Hamel & Concroft, 2012), amphibians (Warkentin et al., 2019), fish (Fuiman, 1993; Godin & Morgan, 1985), mammals (Hoogland, 1981), and birds (Table 2). The misidentification of predator cues also spans a range of sensory modalities including auditory (Zhou, Radford & Magrath, 2019), physical (e.g. vibrational; Warkentin et al., 2019) and visual predator cues (Haftorn, 2000). For example, red-eyed treefrog (Agalychnis callidryas) tadpoles sometimes hatch prematurely in response to heavy rain vibrations, which may be mistaken for an approaching predator (Warkentin et al., 2019). Bumblebees (Bombus spp.) have been observed to produce false alarms (by rejecting a nectar-rich flower and fleeing) if they have previously encountered cryptic predatory crab spiders which visually mimic the flowers (Ings & Chittka, 2008).
When faced with ambiguous predator cues, the choice to respond is ultimately a trade-off between sensitivity and accuracy. Decreasing sensitivity to predator-like cues (the criteria for responding; Fig. 2) will lead to fewer false alarms, however, this will also increase the risk of not responding in a true predator attack. The cost–benefit ratio of this trade-off will vary depending on context, meaning the optimal propensity for alarm will change over time and across species. Three primary factors affect the optimal sensitivity to predator-like cues, and hence false alarm rate: (a) predator cue ambiguity; (b) the cost of responding; and (c) vulnerability to attack.
(a) Predator cue ambiguity
When predators are harder to identify, it is likely that false alarms will be more common (Hilton et al., 1999). The difficulty of identifying predator cues accurately is ultimately what causes false alarms. Identifying predators accurately will depend on three key factors: (i) the similarity between non-target background information and the target predator cues; (ii) background complexity; and (iii) factors which obscure predator cues.
(i) Similarity between background information and predator cues
As described in signal detection theory, predator and alarm cues are harder to identify when they share more attributes with background information (Wiley, 2006). For example, superb fairy-wrens (Malurus cyaneus) are less likely to flee in response to alarm call playbacks if the background noise overlaps in frequency with their alarm calls (Zhou et al., 2019). The more attributes a predator shares with the background, the higher the predicted rate of false alarms (Fig. 2C). This may lead to high false alarm rates in species whose predators are extremely cryptic, as is the case for the goldenrod crab spider (Misumena vatia). These spiders are ambush predators that are highly camouflaged on yellow flowers, from where they attack visiting insects. Ings & Chittka (2008) showed that bumblebees previously exposed to cryptic crab spiders showed a higher rate of false alarms compared to naive bees. The experienced bees rejected more foraging opportunities on ‘safe’ flowers that had no spiders present (Ings & Chittka, 2008).
(ii) Background complexity
Environments differ in their level of informational complexity. This concept is not exclusive to visual cues; ‘the cocktail party effect’ was originally coined to describe the difficulty of understanding speech in a loud social setting, and the task of focussing on a single voice while tuning out the others (Cherry, 1953). Similar examples of challenges in auditory classification are widespread across the animal kingdom, ranging from insect and frog choruses to the songbird dawn chorus (Hulse, 2002). When the acoustic signals of multiple conspecifics are heard concurrently, identifying each one individually becomes a challenge. The more stimuli there are to differentiate between, the harder it is for an animal to identify target stimuli, including predator information or alarm cues. Informational background noise also can mask and distract from real predator cues, making them more difficult to isolate and respond to. For example, both hermit crabs (Coenobita clypeatus) and eels (Anguilla anguilla) took longer to respond to predator cues and to produce appropriate antipredator responses in the presence of background anthropogenic noise. This suggested that the anthropogenic noise was masking and/or distracting from the predator cues, leading to less-reliable predator identification (Stahlman et al., 2011; Simpson, Purser & Radford, 2014). Another example is provided by great tits (Parus major), which were less likely to respond to conspecific alarm calls when there was a high level of background traffic noise (Templeton, Zollinger & Brumm, 2016).
Attwell et al. (2021) found that three-spined sticklebacks (Gasterosteus aculeatus) avoided areas with high visual noise (areas with higher surface waves and light bands) and were less successful at identifying projected model prey in those areas. It is plausible that sticklebacks would also be less successful in detecting predators under these conditions, although this was not tested directly. In another example, Römer & Holderied (2020) investigated the responses of sword-tailed crickets (Trigonidiinae) living in areas with high levels of background noise. Sword-tailed crickets are predated by bats and immediately cease flying and drop to the ground if they detect a predator. Römer & Holderied (2020) found that the crickets only responded to bat calls over a certain threshold amplitude and ignored the calls of bats that were lower in amplitude. They suggested that evolving a higher alarm threshold enabled the crickets to lower their false alarm rate by responding only to calls with a high likelihood of indicating an approaching predator, and represented an adaptation to an environment with very high levels of informational background complexity (Römer & Holderied, 2020). Generally, in informationally complex environments, false alarms are predicted to be more common, however, this prediction is yet to be empirically tested.
(iii) Factors that obscure predator cues
In addition to background complexity increasing the difficulty of differentiation among signals, a variety of environmental factors can obscure predator cues, making them harder to identify. The factors in this category directly obscure predator cues, as opposed to distracting from them. For example, rain can make predator cues harder to identify by visually impeding detection of the predator, increasing background noise, and washing away predator scent. In redshanks, false alarms were more common during rain, and this was attributed to a higher likelihood of cue misclassification when vision is compromised (Hilton et al., 1999). Similarly, false alarms were found to be more common at lower light levels in flocks of staging semipalmated sandpipers (Calidris pusilla; Beauchamp, 2010). Any conditions where predator detection becomes more difficult are likely to increase the false alarm rate as well as the number of false negatives.
(b) Cost of responding
False alarms are likely to have both an energetic cost of producing the alarm behaviours and a subsequent opportunity cost, where time is lost that could have been spent on other activities such as foraging or roosting/sleeping (Beauchamp & Ruxton, 2007). This creates a trade-off between the anti-predator benefits of responding to a perceived predator cue and the loss of energy and opportunities if the cue has been misidentified. When faced with ambiguous predator cues, an animal must identify the optimal trade-off between false positives and false negatives. If an animal responds too frequently it wastes valuable resources, but if it responds too infrequently it becomes more vulnerable to predation (see Section II.2.c).
Thus, where the costs of responding are high, the false alarm rate is predicted to be lower (Beauchamp, 2010), and where alarm behaviours are more costly to produce, an animal is likely to be less sensitive to ambiguous cues (Fig. 2), both leading to decreased false alarm rates. Beauchamp (2010) presented empirical evidence for this effect in flocks of staging semipalmated sandpipers where individuals can double their body mass over 10–14 days. False alarms were less common later in staging, when body mass was higher and thus the energetic costs associated with alarm flights had increased. As referred to above, tadpoles of red-eyed treefrogs sometimes hatch prematurely in response to vibrational predator cues. This behaviour is more costly earlier in development when hatchling mortality is higher. False alarms in response to non-predator vibrations were found to be more common later in development when the cost of premature hatching was lower (Warkentin et al., 2019).
The cost of responding varies both within and among species depending on the type of alarm behaviour and other factors such as body condition. For example, in birds, producing an alarm call is likely a less energetically costly behaviour than an escape flight. This would lead to the prediction that false alarm calls will be more common than false alarm flights, however we are unaware of any empirical tests of this prediction. Among different species, the energetic costs of movement can vary substantially, even between animals that are a similar body mass. For example, Winter & Von Helversen (1998) found that bats expend 20–25% less energy in flight than bird species of a similar body mass. Differences in energy expenditure are also present across locomotory modes: the energetic costs of swimming are substantially lower than flying per unit distance travelled. In flight, energy is required to generate lift, whereas swimming animals can use buoyancy to maintain their position in the water column with minimal energy expenditure (Schmidt-Nielsen, 1972). Thus, the costs of responding to alarms are likely to vary substantially among different behaviours and different species, with the consequences of false alarms also likely to differ.
(c) Vulnerability to attack
False alarm rate is also likely impacted by the likelihood of being captured if an animal ignores a predator-like cue that represents a real threat (i.e. a false negative response; Table 1). Higher predation rates will therefore lead to higher levels of false alarms. This is exemplified by the willow tit (see Section II.1) which have very high false alarm call rates (81%) and demonstrate a very low criteria for alarm (Fig. 2B). In another example, flocks of house sparrows (Passer domesticus) respond more quickly to social alarm cues (rapid departures) when in larger groups (Boujja-Miljour, Leighton & Beauchamp, 2017). Boujja-Miljour et al. (2017) suggested that their predators may preferentially target larger flocks leading to higher vulnerability in larger groups, higher sensitivity to social cues, and hence to more false alarms. High vulnerability to predation means that the costs of an alarm response (see Section II.2.b) are offset by the risks associated with not responding to a true predator attack. This idea is exemplified in error management theory, a model based on signal detection theory (Johnson et al., 2013). Error management theory models the trade-offs in different decision-making scenarios where the costs of false negatives and false positives are asymmetric. In the context of predator detection, false alarms may be costly in terms of wasted energy or lost opportunities, but false negatives could result in immediate death. Therefore, in scenarios where predator attacks are invariably successful, the trade-off will be heavily weighted towards the avoidance of false negatives. In this situation, error management theory shows that biasing towards the least costly error (here, false alarms) may be the best option (Johnson et al., 2013).
Several factors could affect an animal's vulnerability to attack, for example, joining a group may reduce an individual animal's vulnerability to attack through the dilution effect. If a group of five animals is attacked, the probability of each individual being targeted is 1/5, however, in a group of 20 the probability is just 1/20. There are exceptions to this rule: some animals in a group may be more likely to be predated than others (Ward & Webster, 2016), for example, non-vigilant individuals (Roberts, 1996; Turner & Pitcher, 1986), ‘odd’ individuals (Ohguchi, 1981; Rutz, 2012), or those at the edges of the group [Krause, 1994; Ioannou et al., 2019; but see Lambert, Herbert-Read & Ioannou (2021) for moving groups]. While, generally, in a larger group individual vulnerability to attack is likely to decrease (groups can be subject to higher attack rates than lone individuals due to increased conspicuousness; Turner & Pitcher, 1986). Any effect that decreases the probability of predation is thus predicted to reduce the false alarm rate, as the costs associated with a false positive response would increase relative to those of a false negative response.
III. FALSE ALARMS IN GROUPING ANIMALS – GROUP PROCESSING OF ALARMS
(1) Social information and group information transfer
(a) Access to social information
A key benefit to group living is access to social information. Social information provides individuals in a group with access to a broader range of information than any one individual could gain from direct experience. This can include information about predators or the distribution, quality, and quantity of resources (Pitcher et al., 1982; Webster & Laland, 2015; Ward & Webster, 2016). When reacting to a predator or engaging with a resource, animals produce cues that can alert other group members (mostly passively, but sometimes intentionally; Evans, 1982; Haftorn, 2000; Freeberg & Lucas, 2002), for example, rapid escape flights and ‘wing clapping’ in pigeons (Columba livia) alert conspecifics to danger (Davis, 1975) and striking at food resources in predatory fish alerts conspecifics to food (Webster & Laland, 2015; Webster et al., 2019). By responding to cues from conspecifics that have successfully located resources, a group can search over a greater area than would be possible for a single individual (Pitcher et al., 1982), and the greater the chance that resources will be found. Larger groups can locate resources more rapidly, as was shown empirically in fish shoals (Pitcher et al., 1982) and bats (Cvikel et al., 2015).
In addition to more rapid resource location, access to social information also allows larger groups to forage more efficiently due to an increase in feeding rate enabled by decreased individual vigilance: ‘the-many-eyes-effect’ (Lima, 1995). When two different activities are mutually exclusive, an animal must budget the time it dedicates to each behaviour. If remaining vigilant for predators is incompatible with feeding, the animal must alternate between scanning the environment for predators and foraging (Barbosa, 2002). However, when in a group, the burden of vigilance is shared across multiple members. As other group members are also vigilant for predators, each individual can allocate more time to feeding without decreasing the group's collective vigilance. In larger groups, at any particular moment there are more animals scanning for danger, therefore, it is more likely that one will identify an approaching predator, making larger groups more effective at detecting predators sooner and at a greater distance (Siegfried & Underhill, 1975; Lazarus, 1979; Treherne & Foster, 1981; Boland, 2003). Decreased individual vigilance in larger groups (the ‘group size effect’) has been reported widely in mammals and birds (Elgar & Catterall, 1981; Elgar, 1989; Lima & Dill, 1990; Beauchamp, 2008; Beauchamp et al., 2021).
(b) Information transmission and alarm signals
Animals can benefit from the ‘many eyes effect’ because they are able to recognise when a conspecific has detected a predator and this information is transferred through the group. Many animal species have intentional alarm signals (e.g. alarm calls) but in the absence of these, indirect cues such as cessation of feeding, adoption of scanning postures, orientation changes, or fleeing can be monitored to infer detection of a predator (Ward & Webster, 2016). For example, pigeons actively fleeing a predator as opposed to departing for other reasons use fewer preparatory motions before initiating flight and this is exploited by other group members to differentiate an alarm from a regular departure (Davis, 1975). By responding to cues from other group members, not all individuals need to observe a predator directly for a whole-group response to occur, as tested empirically in ocean skaters (Halobates robustus; Treherne & Foster, 1981). This information travels through groups from neighbour to neighbour in a wave of local communication.
(c) Local communication and self-organisation
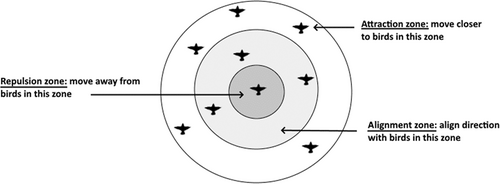
Most animal groups do not require a top-down decision-making process but instead are self-organised using local interactions (Sumpter, 2005; Ward & Webster, 2016). In self-organised systems, each group member bases its behaviour on that of its close neighbours (e.g. speed, orientation, or discrete behaviours such as a startle response) and information is transferred locally from one neighbour to the next in waves across the group (Rosenthal et al., 2015). Thus, interactions of local individuals within the group lead to the global properties of the system in emergent patterns including the group's shape, speed, and direction (Reynolds, 1987; Giardina, 2008; Rosenthal et al., 2015). Simulation models of these systems have been created. Reynolds (1987) modelled a three-dimensional (3D) landscape with dots (‘Boids’) acting as model birds. Reynolds gave each Boid three simple rules to follow: (i) maintain a close distance to your neighbour; (ii) do not move too close to your neighbour; (iii) follow your neighbour's travel direction. The model resulted in a cohesive group structure that responded to objects in the environment and behaved remarkably similarly to bird murmurations. Ultimately, the model showed that these cohesive structures could be produced by simple rules concerning only an individual's local neighbourhood (Reynolds, 1987). The assumptions of this model were tested empirically by Giardina (2008) in large European starling (Sturnus vulgaris) murmurations. The study recorded and mapped the locations and movement patterns of individual starlings in murmurations, and the flock was then digitised using points that followed the exact movement of the individual birds. Modelling using these data provided evidence for topological response zones where the birds maintained their position by following rules for repulsion and attraction zones and orientating with respect to their neighbours (Fig. 3). Giardina's (2008) analysis suggested that individual starlings responded to their nearest six or seven neighbours.
Similar studies of local interactions have shown how startle behaviour in fish is spread rapidly and effectively through local communication. Radakov (1973) first quantified these waves of information transfer in shoals of silversides (Atherinomorus sp.) by filming startled groups and monitoring the rate of behavioural cascades. The fright response began with a small number of individuals turning, this behaviour was copied by their neighbours, and so on until there was a whole-group response. The speed of this informational wave was 15 ms−1 which is faster than their own maximum swimming speed (1 ms−1) and, crucially, faster than the swimming speed of their predator (Radakov, 1973). Herbert-Read et al. (2011) analysed local interactions and escape waves in Pacific blue-eyes (Pseudomugil signifer) startled with an artificial predator. They found that changes in the direction and speed of a small percentage of individual fish that detect the predator initiate the beginning of an escape wave. The ‘front’ of this behavioural wave is a tightly packed band of individuals which causes the other fish to turn and move in the same direction. This front passes through the group in a wave, forming a group escape response (Herbert-Read et al., 2011).
(d) Reliability of social information
Although group information can be advantageous for both locating resources and responding to predators, with each animal basing its behaviour on that of other group members, vulnerability to the propagation of poor information is introduced. Behavioural cascades based on false information can spread through a group, with a whole-group response being generated by just a few misinformed individuals (Giraldeau, Valone & Templeton, 2002). For example, some redshank whole-group alarm flights were generated from a few individuals in the group mistaking a non-raptorial corvid for a bird of prey (Cresswell et al., 2000). In some circumstances, even when social information conflicts with individual experience, individuals follow the behaviour of the majority of the group members. For example, sticklebacks follow their group to a foraging site even if they have previously experienced that area to be low in resources (Webster & Hart, 2006). For this reason, blind copying of group members with no individual validation of socially acquired information could be maladaptive. In some species, the risks of misinformation lead to avoidance of the use of social information entirely unless personal information gathering is not possible. For example, in European starlings social information about resource distribution was only used if personal information gathering was difficult or costly (Templeton & Giraldeau, 1996).
When considering the use of socially acquired information on predators (as opposed to individual detection), it is important to note that alarms may not be universally ‘true’ or ‘false’ since what constitutes a threat is not universal across all individuals. For example, juvenile willow tits are vulnerable to corvids, while adults are not (Haftorn, 2000), therefore, a juvenile willow tit alarm calling in response to a corvid is a true alarm, but from the perspective of an adult it is false. The threats facing one individual may be genuine, making the alarm true, but the same stimulus may be irrelevant to another individual, creating a false alarm from the perspective of the receiver. In degus (Octodon degus), adults are less likely to respond to alarm calls from juveniles, likely because predator identification by juveniles is less likely to be reliable, and they likely face different threats (Nakano et al., 2013). This difference in threat perception may apply particularly to animals that form mixed-species groups. Ruddy turnstones (Arenaria interpres) use alarm information from both conspecifics and other wader species such as oystercatchers (Haematopus ostralegus) (Metcalfe, 1984). When turnstones are associating with conspecifics, they rely on social predator information more often than when they associate with oystercatchers (Metcalfe, 1984). It is possible that this is, in part, due to these two different species having different perceptions of what constitutes a threat. Oystercatchers are three times larger in body mass and are attacked less often by smaller birds of prey compared to the smaller wading turnstones (Whitfield, 1985). Oystercatchers, therefore, may not produce alarms in response to the same predators as turnstones, resulting in less shared vigilance. Even between members of the same species, state effects likely influence how an animal perceives risk. For example, an animal that is injured or hungry may approach threat differently, with trials in 15-spined sticklebacks (Spinachia spinachia) showing that starving individuals take more risks when presented with predator cues than do satiated individuals (Croy & Hughes, 1991). Whether an animal is the producer or receiver of an alarm thus may alter the validity of that alarm, and the trade-offs associated with the use of socially derived predator information, and false alarms may be altered by this variation in the perception of threat.
(2) False alarms in grouping animals
The use of social information leads to an inevitable vulnerability to false information, giving rise to potentially costly false alarms. When a member in a group misidentifies a stimulus and produces a false alarm, it can create a wave of behavioural false responses which spreads through the group. The propagation of false alarms in groups has been documented widely in flocks of birds (Trail, 1987; Haftorn, 2000; Cresswell et al., 2000; Kahlert, 2006) and group-living mammals (Hoogland, 1981; Blumstein et al., 2004) with false alarms making up a surprisingly large percentage of all alarms (Table 2). In many cases, the exact cause of a false alarm is unclear, with studies in birds often documenting alarms occurring with no obvious cause (Trail, 1987; Cresswell et al., 2000; Boujja-Miljour et al., 2017). In groups of animals, the propagation of false alarms is dependent on the mechanisms of information transfer and processing implemented in that species.
(3) Group processing of alarm information
By pooling information and interacting with conspecifics, groups of animals exhibit an emergent ability to process information from their environment (Reynolds, 1987; Giardina, 2008; Rosenthal et al., 2015). In biology, an emergent system describes the gain of properties/abilities in a collective biological system where the system's individual parts do not have the same properties on their own (Parrish, Viscido & Grünbaum, 2002), for example, a brain can complete complex information processing whereas a single neuron cannot. In the same way, groups of animals are capable of processing and reacting to information in ways that would not be possible for a single individual (Parrish et al., 2002). Different animal species implement different mechanisms of information processing, thereby reacting differently to alarm behaviours produced by individuals in their group. Many species employ mechanisms that impede the spread of false information, including: (i) altering alarm reactivity; (ii) the quorum response; and (iii) wave attenuation.
(a) Altering alarm reactivity
In some animal species, it is known that individuals can alter their reactivity/sensitivity to conspecific alarm signals. In these species, reactivity to conspecific alarm cues is a malleable trait, with individuals reducing their responsiveness to signallers whose alarm calls have been previously unreliable. This has been tested empirically in Richardson's ground squirrels (Spermophilus richardsonii; Hare & Atkins, 2001), yellow-bellied marmots (Marmota flaviventris; Blumstein, Verneyre & Daniel, 2004), Western Australian magpies (Cracticus tibicen dorsalis; Silvestri, Morgan & Ridley, 2019), and vervet monkeys (Chlorocebus pygerythrus; Cheney & Seyfarth, 1988). In these studies, animals were more responsive to conspecific alarm calls from individual conspecifics whose calls were previously correlated with the presentation of a model predator (compared to individuals whose alarm calls were not). In house sparrows, where social alarm information is often unreliable (Table 2), individuals self-validate alarm signals from individual conspecifics by increasing reaction times, likely to scan for danger before copying alarm behaviours (Boujja-Miljour et al., 2017). Similarly, yellow-bellied marmots were more likely to respond to alarm calls when multiple calls were played concurrently, likely because the alarm is more likely to be accurate if it has been verified by multiple animals (Blumstein et al., 2004). In an alternative mechanism to adjusting sensitivity based on reliability, in mixed-species reef fish shoals, modelling suggested that fish dynamically alter their sensitivity to socially transmitted cues depending on group density to supress the transmission of false alarms (false alarms can be more common at higher densities; Fahimipour et al., 2022).
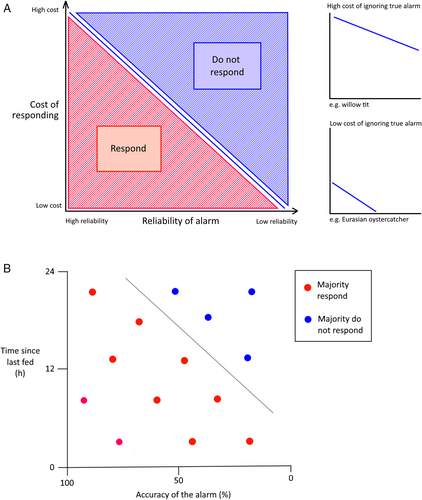
Optimal reactivity to a conspecific alarm should theoretically vary between different species depending on their specific social and ecological niche. An animal's optimal reactivity level to alarm will change according to the risks and benefits associated with responding. This could range from using a ‘better-safe-than-sorry’ principle where animals respond to most alarms (e.g. in willow tits; Haftorn, 2000), to contexts of low predator threat where the response to any alarms could be muted. Theoretically, the decision to respond to an alarm should be based on its likely reliability, the cost of responding, and the cost of a false negative (the risk of predation if a true alarm is ignored) (Fig. 4). If alarms are typically reliable and the cost of responding is low, an individual should respond to most alarms. However, if an alarm is likely to be false and there is a high cost of responding, it could be more beneficial to ignore the alarm. The gradient and position of the optimal response line should vary depending on the costs of ignoring a true alarm. If this cost is high, then an individual may need to respond to most alarms – the ‘better-safe-than-sorry-principle’. However, if the costs of ignoring an alarm are low, it may be beneficial to ignore most alarms, even if there is a reasonable likelihood of the alarm being true. Whether these dynamics play out in real animal systems could be investigated in trials that experimentally alter these factors (Fig. 4B).
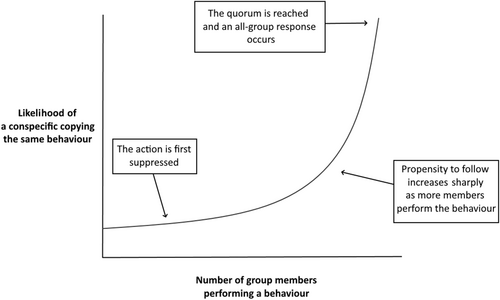
(b) The quorum response
A second mechanism that may act to limit the spread of false information is the quorum response (Sumpter & Pratt, 2009). In a quorum response, the likelihood that a group member copies a behaviour shows a non-linear relationship with the number of other group members that are performing that behaviour (Sumpter & Pratt, 2009; Fig. 5). When a behaviour is performed by one group member (e.g. an escape flight), the behavioural response of the group is first suppressed to lower the likelihood of a whole-group response to a small number of misinformed individuals. As more group members perform the behaviour, it becomes more likely that they are responding to true information, therefore, the propensity of other members to copy the behaviour rises sharply after a certain threshold. Once this threshold is met, a quorum has been reached, and a whole-group response is initiated. The initial suppression of the group response thus impedes a cascade of poor information as several individuals must validate the behaviour before other group members begin to copy it (Sumpter & Pratt, 2009). The quorum response has been applied to a variety of contexts including foraging and group predator detection, with empirical data available for insects (Pratt, 2005; Seeley & Visscher, 2004), fish (Ward et al., 2008), birds (Cresswell et al., 2000; Collins & Sumpter, 2007), and mammals (Sueur, Deneubourg & Petit, 2011). Cresswell et al. (2000) found that foraging redshanks will only initiate an immediate escape response if a certain threshold of members departs simultaneously. Even in a real attack, when just a single redshank departed there was a longer delay before other group members responded, suggesting that they were first assessing the reason for departure before copying (Cresswell et al., 2000). By allowing time to assess the reason for single departures, redshanks can avoid missed feeding opportunities caused by false alarm flights. While quorum responses are effective in suppressing the impacts of poor information, they still cannot fully eliminate the risk of cascading false information (Laland & Williams, 1998; Dall et al., 2005).
(c) Wave attenuation
The propagation of behavioural cascades through animal groups can vary in terms of the magnitude and distance travelled by the cascade. Radakov (1973) described and measured this effect in fish, showing that some behavioural cascades propagate throughout groups of fish while others attenuate rapidly. The distance travelled by a behavioural cascade was correlated with the level of risk, for example, in higher risk scenarios such as a barracuda attack, fright responses propagated further through the group (Radakov, 1973). Further work by Rosenthal et al. (2015) showed that most individual startle behaviours do not cascade through the whole group but instead dampen rapidly, only affecting a portion of the group. Following these studies, many analyses have attempted to decipher the mechanisms behind why some waves propagate fully through a group while others attenuate. Sosna et al. (2019) investigated the underlying mechanisms by exposing golden shiners (Notemigonus crysoleucas) to the chemical alarm cue Schreckstoff. After exposure, the average nearest-neighbour distance decreased substantially, and the group clustered together. Golden shiners school using visual cues, and this change in structure significantly altered the number of neighbours in their visual field and subsequently how they responded to the behaviour of their nearest neighbours. Sosna et al. (2019) suggested that these changes in the physical group structure indicated a structural encoding of risk; in riskier environments the fish arrange themselves in a structure that allows behavioural fright cascades to propagate more easily through the group. This mechanism allows groups to alter their alarm reactivity depending on the perceived risk of their environment, lowering reactivity when alarm information is more likely to be false, and increasing reactivity when predators are more likely to be present (Sosna et al., 2019).
(4) Group size and false alarm rate
Different studies have shown conflicting relationships between group size and false alarm rate. Theoretically, if there are more members in a group, then it should be more likely that at least one individual will misclassify harmless stimuli as a threat, therefore, false alarm rate should increase with group size (Beauchamp & Ruxton, 2007). This relationship has been documented in roosting semipalmated sandpipers (Beauchamp, 2010), lekking Guianan cock-of-the-rock (Rupicola rupicola; Trail, 1987), and feeding greylag geese (Kahlert, 2006), however, increases in false alarm rate are often confounded by larger groups experiencing higher rates of attack. By contrast, in studies of redshanks and finches (Fringilla coelebs and F. montifringilla) no relationship between group size and false alarm rate was found (Lindström, 1989; Cresswell et al., 2000). In fact, Cresswell et al. (2000) predicted that false alarm rate should decrease with group size as individuals in larger groups are more likely to assess the cause of an alarm before initiating a response. With such conflicting evidence, it is clear that the relationship between group size and false alarm rate is not universal.
We suggest that the relationship between group size and false alarm rate will be determined largely by how individual alarm information is processed and transferred by the group. As discussed in Section III.3, different species may employ very different mechanisms of group information processing. In species with very little post-processing of alarm information (most alarm behaviours are copied), an increase in false alarm rate with group size would be expected. However, in species with more complex processing mechanisms which dampen the spread of behavioural cascades, there may be little change in false alarm rate with group size (Fig. 6).
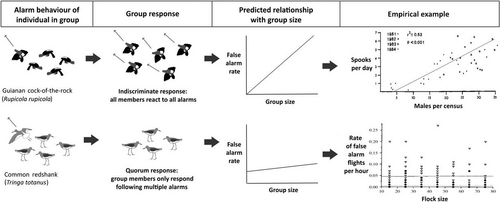
Incorporating both individual alarm rate and mode of information processing could therefore explain conflicting results on the effect of group size on false alarm rate. False alarm rate increases with group size in Guianan cock-of-the-rock leks (Trail, 1987) and staging flocks of semi-palmated sandpipers (Beauchamp, 2010), but not in flocks of redshank (Cresswell et al., 2000). In both species an increase in group size likely results in an increase in the number of individual false alarms, however, in the group processing stage redshanks employ a quorum response (Cresswell et al., 2000), which dampens false information transfer and group-level false alarms are rare. Compared to redshanks, the Guianan cock-of-the-rock is relatively solitary and likely lacks mechanisms for group information processing. For semipalmated sandpipers (which have a high false alarm rate; Table 2), Beauchamp (2010) noted that all birds departed from the roost in response to a false alarm, but returned within 30 s. Such an indiscriminate response to alarm cues suggests that semipalmated sandpipers do not use information transmission dampening systems. Beauchamp (2010) also found that, as in Guianan cock-of-the-rock flocks, false alarm rate increased with group size in semipalmated sandpipers. Numerous other factors could contribute to the high false alarm rate of these sandpipers, including low costs associated with false alarms (birds returned within 30 s and were roosting, not feeding), relatively high vulnerability to predators, and very high group sizes (a maximum observed group size of 15,000).
(5) Consequences of false alarms
(a) Modelling the costs of false alarms
Despite the surprisingly high proportion of false alarms recorded in some animal species (Table 2), the consequences of false alarms are not well understood. It is presumed that false alarms are costly in terms of the energetic cost of producing alarm behaviours and subsequent loss of opportunities (Beauchamp & Ruxton, 2007), with this cost of false alarms dependent on the current activity of the animal. For example, foraging animals may lose valuable feeding time (Kahlert, 2006), roosting animals may lose sleep (Beauchamp, 2010), and courting animals may lose mating opportunities (Martín et al., 2003). However, very few empirical studies have quantified these costs. In greylag geese, it was estimated that each false alarm prevented feeding for 19 min, creating a relatively substantial reduction in total foraging time given that false alarms occurred multiple times per day (Kahlert, 2006). Beauchamp & Ruxton (2007) modelled the consequences of false alarms on behaviour using a genetic algorithm model to investigate how animals should adapt their behaviour to optimise vigilance. Their model showed that the increased likelihood of misclassifications that arises with increasing group size would lead to an increase in false alarm rates, and that this added a non-negligible cost to foraging in larger groups. To compensate for this additional cost, individual vigilance of group members should decrease as group size increases. They also found that it would be beneficial to rely less on collective detection in larger groups (Beauchamp & Ruxton, 2007).
Most traditional models of group vigilance assume that alarms are perfectly accurate (Pulliam, 1973; Pulliam, Pyke & Caraco, 1982; McNamara & Houston, 1992), however, it is now clear that this is not true. While many factors contribute to the costs of living in large groups, such as competition for resources, false alarms are likely an additional cost of group living that may significantly impact fitness trade-offs.
(b) The fire-drill hypothesis
The surprisingly high rate of false alarms observed across animals poses a question: why do false alarms occur so often, when they are likely to be costly? As explained in Section II.2.b, it has been argued that false alarms inevitably involve a trade-off between the benefits of socially acquired predator information and energetic/lost opportunity costs. However, a recent paper (Root-Bernstein, 2021) argued that false alarm flights in birds can be explained by the so-called ‘fire-drill hypothesis’. In this hypothesis, false alarm flights are not maladaptive because they allow birds to practice the mechanical actions of emergency evasive flight that may increase their chances of survival in a real predator attack. Since the body mass of a bird can fluctuate dramatically (e.g. Beauchamp, 2010), Root-Bernstein (2021) argues that false alarm flights allow birds to practice and adjust their take-off manoeuvres to compensate for differences in body mass over time. This could explain the seemingly contradictory high rates of false alarm flights observed in birds by providing a significant associated benefit. This new hypothesis provides a clear alternative to the classical explanation that false alarms are unavoidable when identifying predators using ambiguous cues (as described by signal detection theory; Fig. 2) and thus occur as an inevitable cost associated with the use of using social information. We agree with Root-Bernstein (2021) that there is a lack of theoretical modelling to support this hypothetical trade-off. However, the costs associated with false alarms are evidenced by the widespread use of mechanisms to impede the cascade of false information (see Section III.3). If false alarms are beneficial, it would be difficult to explain the evolution of these mechanisms. Root-Bernstein (2021, p. 31) also argued that false alarm flights cannot be maladaptive as evolution cannot select against conditional outcomes (‘the bird is alive given the real presence of a predator at time x’) but rather acts only on absolute outcomes (‘the bird is alive at time y’). However, this line of reasoning considers only a single flight, neglecting the costs of repeated false alarm flights over a longer term. With false alarms occurring multiple times over a given period, a bird that responds to all alarms (true or false) will survive predation attempts for longer than a bird that does not respond. However, a bird that filters out and responds only to true alarms will both avoid predation and expend less energy on unnecessary departures. The latter strategy would likely lead to a higher probability of survival. There will therefore be an inevitable trade-off between the responses to false alarms and the cost of these responses. These two conflicting arguments await detailed modelling before we can resolve their differences.
IV. FUTURE DIRECTIONS
A key area limiting our current understanding of false alarm behaviour in groups is the lack of a comprehensive model of the hypothetical trade-off between the costs of false alarms and the benefits of socially acquired predator information. To the best of our knowledge, no study has reported such a model, and there have been no attempts to quantify the costs of false alarms. Appropriate modelling will allow us to determine the presence of trade-offs and to test alternative ideas such as the fire-drill hypothesis.
Studies on false alarms in groups of animals appear to be dominated by observational trials in bird species. While these studies have provided valuable insights, focusing on a single taxon reduces the generality of any conclusions drawn, and a lack of control over the conditions in observational studies means that confounding variables cannot be excluded. Studies analysing the factors that influence alarm rate are typically correlative, with their results often having multiple interpretations: for example, an increase in false alarm rate with body mass in semipalmated sandpipers (Beauchamp, 2010) was proposed to be due to an increase in the cost of producing alarm behaviours, but other factors that vary over time, such as temperature differences, could potentially be involved. Identifying the exact cause of an alarm behaviour in the field is very difficult, especially in complex environments where views are often obstructed. There are thus inevitable sources of error when studying false alarms in the field: observers may fail to identify the true threat perceived by the focal animal. More studies including trials in controlled environments and with different animal groups will be a valuable addition. Most experimental work under controlled conditions has been conducted in shoals of fish, leading to a divide in the literature: observational work conducted on birds, and experimental work conducted on fish shoals. Future laboratory studies could expand experimental trials to include a range of established model organisms that show characteristic fright responses such as rodents (e.g. rats Rattus norvegicus), captive birds (e.g. zebra finches Taeniopygia guttata), and insects (e.g. field crickets Gryllinae and water striders Gerridae).
Exact measures of the rate of false alarms are rare (with the exception of some flocking bird species; Table 2), however, they may also be common in other groups, for example, in mammals (Hoogland, 1981; Hare & Atkins, 2001; Blumstein et al., 2004). Detailed information on false alarm rate across a range of animal groups is required to understand their potential impacts across different taxa. Large multi-species comparisons would be valuable to gain insight into how different ecologies impact the rates and use of false alarms, and the selection processes which led to the evolution of information dampening systems such as the quorum response. Phylogenetic analyses could also highlight how these kinds of responses evolve. These comparisons will provide insight into the processes underlying false alarms and information transmission in grouping animals.
V. CONCLUSIONS
- (1)
False predator detections and false alarms appear to be common across the animal kingdom and are likely an underappreciated but significant cost of group living. In groups, false alarms can give rise to maladaptive cascades where group members incur both the energetic costs of producing alarm behaviour and lost opportunity costs.
- (2)
The propensity for individuals to produce false alarms (and hence false alarm rate) increases when predator cues are harder to identify/more ambiguous, when animals are more vulnerable to predators, and when the cost of producing alarm behaviour is lower.
- (3)
Many group-living animal species regulate responses through group processing mechanisms which mitigate the spread of false information, decreasing the likelihood of whole-group false alarms. These mechanisms include altering alarm reactivity depending on previous reliability, the quorum response, and wave attenuation through the modification of group structure.
- (4)
In species without group processing mechanisms to dampen the spread of false alarms, false alarm rate often increases with group size. This creates increasing costs with increasing group size, likely affecting optimal group size.




