Interaction between nanomaterials and the innate immune system across evolution
ABSTRACT
Interaction of engineered nanomaterials (ENMs) with the immune system mainly occurs with cells and molecules of innate immunity, which are present in interface tissues of living organisms. Immuno-nanotoxicological studies aim at understanding if and when such interaction is inconsequential or may cause irreparable damage. Since innate immunity is the first line of immune reactivity towards exogenous agents and is highly conserved throughout evolution, this review focuses on the major effector cells of innate immunity, the phagocytes, and their major sensing receptors, Toll-like receptors (TLRs), for assessing the modes of successful versus pathological interaction between ENMs and host defences. By comparing the phagocyte- and TLR-dependent responses to ENMs in plants, molluscs, annelids, crustaceans, echinoderms and mammals, we aim to highlight common recognition and elimination mechanisms and the general sufficiency of innate immunity for maintaining tissue integrity and homeostasis.
I. INTRODUCTION
The development of nanotechnologies and the consequent introduction of new man-made nano-entities into our environment has raised attention and concern about the possible detrimental effects of engineered nanomaterials (ENMs) on human and environmental health. Extensive evaluation has been implemented to understand the effects of the interaction of ENMs with biological systems (Oberdörster, Oberdörster & Oberdörster, 2005; Krug & Wick, 2011; Fadeel, 2012, 2019; Fadeel et al., 2018; Zhu, 2020; Cronin et al., 2020; Boraschi et al., 2020).
Living organisms are endowed with powerful protective mechanisms, developed throughout evolution for counteracting invading pathogens (e.g. viruses, bacteria) and for eliminating threatening exogenous and endogenous entities (mechanical stress, dust, allergens, anomalous, senescent and dead cells). We can therefore expect that those mechanisms may be involved in the recognition and response to ENMs. Notably, ENMs may resemble some infectious agents in terms of size and shape, but their interaction with living organisms is passive, resembling that with dust. Thus, to understand the possible detrimental effects of ENMs, it is important to examine the features of their interaction with such protective mechanisms and assess whether and how ENMs may escape recognition or circumvent protective responses.
The protective mechanisms activated upon contact with invading agents are mainly those of immunity. We can define them as innate immune mechanisms, although this definition is only necessary for vertebrates, which also have another type of immunity (adaptive immunity). Innate immunity is the only type present in over 95% of living organisms and in vertebrates is estimated to account for about 80–90% of protective efficacy (R. Zinkernagel, personal communication; Murphy, Weaver & Berg, 2022; Chumakov et al., 2021). Innate protection (physical, chemical and cellular barriers) prevents invasion by the majority of exogenous agents (about 97%), with the tissue-resident innate immune mechanisms subsequently tackling and eliminating (about 90%) those that successfully invade the body (Chumakov et al., 2021). Innate immunity shows several evolutionarily conserved molecular and cellular features that are present with little variation from plants to mammals. We focus here on two of these, the main defensive cells, i.e. phagocytes, and the main danger-sensing molecules, i.e. the pattern-recognition receptors (PRRs). Phagocytic cells are widespread across the tree of life, from unicellular organisms such as amoebae, with the capacity to engulf and digest exogenous material for nutrition, to specialised cells in multicellular animals that are involved in feeding and elimination of unwanted materials. Apart from plants, whose cells are not mobile, phagocytes are present in all multicellular eukaryotic organisms and can be found both as circulating cells and as tissue-resident sentinels, in particular in barrier tissues at the interface between the body and the external environment (Mowat, Scott & Bain, 2017). Thus, it can be assumed that interaction of ENMs with phagocytic cells is the first and primary interaction to occur. When a foreign agent comes into contact with phagocytes (or other cells in an organism), these cells have mechanisms for sensing and recognition, and for removal or destruction. PRRs are usually surface molecules, but can also be soluble, such as the complement components C1q and mannose-binding lectin (MBL) that perform this sensing function linked to an active downstream defensive/adaptation reaction. Notably, PRRs seem able to perceive different kinds of threats, including changes to environmental conditions (e.g. temperature, pH, salinity) that could hamper the organism's homeostasis, and can trigger appropriate adaptive responses. PRRs encompass a wide range of receptors that recognise sugars, nucleotides, glycoproteins, glycolipids and structural patterns typical of microorganisms (usually viruses and bacteria) although they are unable to recognise endogenous patterns unless damaged (Murphy et al., 2022). Toll-like receptors (TLRs) are the best-known PRRs and are present in all multicellular organisms (homologous sequences have also been identified in bacteria).
In this review, we examine the interaction between ENMs and innate immunity by focusing on phagocytic cells and TLRs to understand whether and when such interaction results in successful removal of ENMs and under which circumstances it leads to detrimental consequences. We examine immune interactions in plants, invertebrates (molluscs, annelids, crustaceans, echinoderms) and vertebrates (with a focus on humans).
Notably, ENM physico-chemical characteristics are expected to play a major role in determining the mechanisms of interaction with and uptake by phagocytes. However, after many years of research, no clear correlation between ENM characteristics and mechanisms of uptake has been identified that can allow us to predict the outcome of such interactions. The hope of ‘safe by design’ nanotechnological products is still unfulfilled. It is known that uptake depends on particle size, shape, stiffness and surface charge, among other characteristics, but the physiology of organisms remains the decisive parameter. The functional anatomy of the gut makes it a formidable barrier to ENMs. Some organisms, especially vertebrates, use mucus to keep adsorbed nanomaterial in the gut lumen and prevent it from interaction with epithelial cells. Invertebrates also have protective layers of cuticle or peritrophic membranes in the gut, which affect ENM uptake (Van der Zande et al., 2020). Phagocytosis occurs only when ENMs are able to cross such barriers and reach the cells lining the gut. In organisms in which phagocytosis is mainly a defence mechanism and preserves tissue integrity, phagocytosed particles are directed towards destruction or, if indigestible, towards ‘strenuous’ isolation and seclusion from the rest of the body, as shown for example by tattoos (Baranska et al., 2018). In addition, the health condition of the organism can affect the outcome of the interaction with ENMs, as well as the route of exposure and the type of affected cells.
II. IMMUNITY AND NANO-IMMUNE INTERACTIONS IN PLANTS
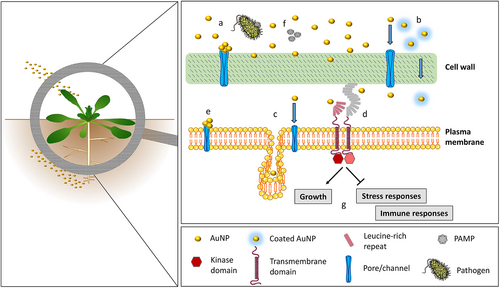
In plants, immunity does not rely on specialised tissues or organs but it is an autonomous process that can be activated in all cells. Innate immune receptors reside on the plasma membrane and intracellularly in the cytoplasm in all tissues and can recognise non-self structures on the surface (pattern-triggered immunity) and within the cell (effector-triggered immunity) (Zhou & Zhang, 2020; Ngou, Ding & Jones, 2022). Many cell surface receptors share similar features with TLRs, such as the extracellular leucine-rich domains. These receptors likely evolved independently in the plant and animal kingdoms and recognise pathogen-associated molecular patterns (PAMPs), but also other non-self and host-specific patterns that indicate hazards. The transmembrane domain transfers the signal into the plant cell and activates immune responses via intramolecular kinase domains in receptor kinases (complexes), which belong to the same phylogenetic family as the IL-1 receptor-associated kinases (IRAKs) in animals (Nürnberger et al., 2004). Intracellular nucleotide oligomerisation domain (NOD) like receptors (NLRs) display a Toll-IL-1 receptor (TIR) domain similar to the TLR intracellular domain and can recognise intracellular effector molecules introduced by potential pathogens (Han, 2019). ENMs come into contact with plants mostly extracellularly, but some studies also report transport within the plant and into the cells (see Section II.1). Thus, in principle EMNs could contact both types of receptors. Phytocytokines, endogenous peptides produced in response to stress, can amplify stress-adaptation reactions and alert neighbouring cells, similar to the role of animal cytokines (Gust, Pruitt & Nürnberger, 2017). Animal innate immune memory also has a plant counterpart in systemic acquired resistance (SAR). Primary local infections with a pathogen prime the plant not only at the infection site but also systemically, making the whole plant more resistant to a broad spectrum of subsequent infections, and suggesting that local ENM recognition could activate the defence repertoires within the entire plant (Vlot et al., 2021). As in animals, immune responses in plants include the production of reactive oxygen species (ROS) and changes to the global transcriptome and proteome, enabling effective defence against the vast majority of microorganisms in the environment (Yu et al., 2017).
(1) Uptake of ENMs
Plants do not have specialised phagocytic cells, instead particle uptake can result from interactions with all cells. The uptake of ENMs in plants is a controversial topic (Ali, Mehmood & Khan, 2021). In general, ENM uptake is prevented by physical barriers such as the cuticule, cell wall and the plasma membrane, which protect the plant from the entry of non-self-particles (Schwab et al., 2016). Entry of ENMs into the plant cell can potentially occur via pores, channels or by endocytosis (Ali et al., 2021). In aerial tissues, ENMs may be taken up through natural openings. In roots, they may enter the plant via cell wall pores and then travel apo- or symplastically. The cell wall pores are estimated to be 3–6 nm in diameter, thereby precluding the entry of larger molecules (Sabo-Attwood et al., 2012). In the case of gold nanoparticles (AuNPs), 4 and 12 nm particles are blocked within the cell wall and cannot be taken up by Arabidopsis thaliana roots (Ferrari, 2022). Nevertheless, in several plant species uptake was reported for several ENMs larger than the cell wall pore size, suggesting that cell wall alterations, flexibility or engagement of active uptake processes can allow particle passage (Ali et al., 2021). Small positively charged AuNPs were taken up more effectively than neutral or negatively charged larger particles (Spielman-Sun et al., 2019; Ali et al., 2021). Thus, depending on the species, ENM dose, size and surface charge, many types of nanomaterials have been shown to accumulate in roots (the majority) or shoots after soil or hydroponic exposure (Kranjc et al., 2018). These include Ag particles, CuO, TiO2, SiO2, NiO, La2O3, ZnO and several others (Kranjc & Drobne, 2019). Some remain intact after uptake and are accumulated, while others undergo transformation either before adsorption through the roots or after uptake as nanoparticles (NPs) (Ali et al., 2021). For example, in the soya bean Glycine max, CeO2 NPs remain in this form after translocation from the soil, whereas ZnO particles are transformed into Zn-citrate within the plant tissues (Hernandez-Viezcas et al., 2013). In maize Zea mays, they are taken up as Zn phosphate or Zn ions (Lv et al., 2015). In general, uptake is often reported for unstable NPs that can dissolve in the environment, suggesting that extracellular particle disintegration, uptake of ions and subsequent intracellular formation of new particles occurs rather than uptake of intact NPs (Taylor et al., 2014). Thus, uptake mechanisms remain poorly understood and therefore unpredictable, and generalisations cannot be made on the nature of ENMs taken up, the plant species in which this occurs, or the conditions under which internalisation takes place.
(2) AuNP effects on immune responses
Caution is needed to interpret the toxic effects of ENMs on plants because, in some cases, the bulk material can be intrinsically toxic and, in other cases, the coatings can be toxic (Barrena et al., 2009; Ferrari et al., 2021). AuNPs are an example of a nanomaterial that is generally considered safe and inert, i.e. unable to cause direct toxic effects, and are therefore useful for identifying potential effects of the nanoparticulate form on plant immunity. Different and sometimes controversial effects of AuNPs on plants have been described, with plant responses to AuNPs depending on specific conditions and specific interactions (Siddiqi & Husen, 2016; Zia-ur-Rehman et al., 2018; Sanzari, Leone & Ambrosone, 2019). Several studies show that there is no uptake of AuNPs (Taylor et al., 2014; Zhang et al., 2022; Ferrari, 2022). Any effects caused by AuNPs are therefore likely not caused by direct activation of receptors but are a consequence of other AuNP actions, e.g. clogging pores, or scavenging of environmental factors or PAMPs that induce defence responses (Barrena et al., 2009; Asli & Neumann, 2009; Feichtmeier, Walther & Leopold, 2015; Ferrari et al., 2021). In many studies no effects were detected using different assays and concentrations (Pacheco & Buzea, 2018). Negative results also may be underrepresented in the published literature because studies reporting no effect are often not published. At very high concentrations (≥100 mg/l) AuNPs have been reported to have detrimental effects on plants (Siegel et al., 2018), with smaller particles (diameter < 5 nm) showing higher toxicity. This size is in the cell wall pore size range, implying that AuNPs may have direct access to plant cell surfaces. By contrast, lower concentrations of AuNPs were reported to enhance seed germination and chlorophyll content and improve growth and productivity in several crops and model plants (Arora et al., 2012; Kumar et al., 2013; Mahakham et al., 2016; Ferrari et al., 2021; Sabo-Attwood et al., 2012).
A limited number of studies have investigated the effects of stable and well-characterised AuNPs on the plant immune system, with most focussed on oxidative burst phenomena. Kumar et al. (2013) reported that, in Arabidopsis thaliana, plant antioxidant potential under stressful conditions is enhanced by AuNP exposure through the activation of free radical scavenging activity. By contrast, other studies reported increased oxidative stress caused by AuNPs (Gunjan, Zaidi & Sandeep, 2014; Siddiqi & Husen, 2016). It was found that AuNPs at moderate concentrations can increase growth and reduce PAMP-induced ROS bursts in Arabidopsis thaliana (Ferrari et al., 2021), in agreement with similar results in Brassica plants (Arora et al., 2012). AuNPs alone did not induce ROS bursts or other defence responses, suggesting that AuNPs do not activate self-destructive immune reactions. At the transcriptome and proteome level, growth and metal response genes overall were upregulated, while immune and oxidative stress-responsive genes/proteins were downregulated (Ferrari et al., 2021). This indicates that the trade-off between growth and immunity could be shifted by AuNPs towards growth. Similar to the effects described for exposure to exogenous AuNPs, it was found that defence and oxidative stress-responsive genes are downregulated after Au ion treatment and intracellular formation of AuNPs (Tiwari et al., 2016).
ENM–plant interactions are clearly very diverse and, based on current knowledge, cannot be predicted or extrapolated. AuNPs and several other ENMs do not appear to exert detrimental effects on A. thaliana plants at environmentally relevant concentrations, but rather display antioxidant and growth-promoting activity. A general scheme of the interaction of AuNPs with plants and plant immune mechanisms is provided in Fig. 1.
III. PHAGOCYTES AND TLRS IN MOLLUSC IMMUNITY AND NANOPARTICLE–IMMUNE INTERACTIONS
Phylum Mollusca includes aquatic and terrestrial bivalves, cephalopods, and gastropods. Haemocytes, molluscan immune cells, are generally classified on a morphological basis as hyaline, semigranular or granular. Different molluscan taxa show differences in abundance and function of these subtypes, reflecting environmental and endogenous factors including infection by different microorganisms (protozoans, bacteria, viruses) (Dyachuk, 2016).
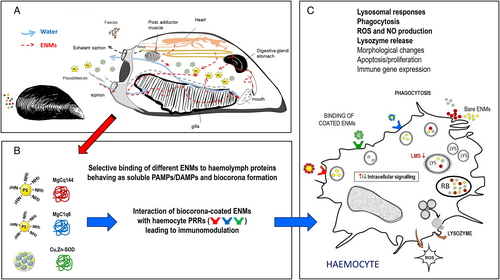
Bivalves are sessile suspension-feeders abundant in freshwater and marine ecosystems, where they are constantly exposed to abiotic and biotic stressors. Due to their feeding habit involving gill-mediated water filtration, bivalves have highly developed processes for the uptake of nano- and micro-scale particles (endo- and phagocytosis), integral to physiological functions such as intracellular digestion and cellular immunity. Bivalves have become a target group for investigations of ENM toxicity (Canesi et al., 2012) and to date represent the best studied group of invertebrates regarding NP–immune interactions (Canesi & Corsi, 2016).
(1) Immune effectors in bivalve molluscs
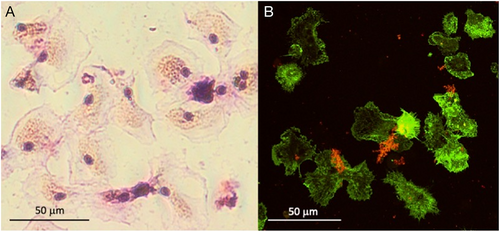
In bivalves, haemocytes are the main defence cells and act through a combination of phagocytosis and the production of humoral defence factors such as agglutinins (e.g. lectins), lysosomal enzymes (e.g. acid phosphatase, lysozyme), ROS and antimicrobial peptides (AMPs) (Canesi et al., 2002). In the mussel Mytilus galloprovincialis, different haemocyte subpopulations were identified by cytofluorimetric analysis of size, granularity, phagocytic activity, and ROS and nitric oxide (NO) production capacity (García-García et al., 2008). Representative images of M. galloprovincialis haemocytes are shown in Fig. 2.
Several cellular and soluble PRRs play a crucial role in activating the bivalve immune system. These include lectins, peptidoglycan recognition proteins, gram-negative binding proteins, TLRs, scavenger receptors, etc. (Canesi & Pruzzo, 2016; Gerdol et al., 2018). The best studied PRRs show high versatility and flexibility, although some degree of specificity could be identified (Canesi & Pruzzo, 2016). Compared with more basal phyla, many molluscan genomes exhibit a higher number of TLR-encoding genes; this expansion and divergence may contribute to molluscan immunity and their ability to adapt to stressors (Brennan & Gilmore, 2018). The recent application of single-cell RNA sequencing (scRNA-seq) techniques to bivalve haemocytes allowed identification, in the oyster Crassostrea hongkongensis, of 13 different subpopulations of immune cells, which have distinct expression patterns of genes encoding TLRs and other PRRs (Meng, Zhang & Wang, 2022).
Among the soluble factors, AMPs represent one of the main effectors in bivalve immunity. Several AMPs have been identified in different bivalve species. They are generally characterised by structural differences (peptide sequence, length, net charge, per cent hydrophobic residues), different activities and antimicrobial properties, differential tissue/cell expression, accessory functions, and often species-specific biological properties (reviewed in Zannella et al., 2017; Gerdol et al., 2018; Canesi et al., 2022).
(2) Immune interaction with ENMs
A few studies have focused on the effects of ENMs on bivalve haemocyte function and expression of immune-related genes, including PRRs (Canesi, Auguste & Bebiano, 2019). Large variability was observed depending on the type of ENM used, bivalve species, and exposure conditions. In M. galloprovincialis exposed to TiO2 NPs (4 days, 1–100 μg/l), a concentration-dependent decrease in lysosomal membrane stability and phagocytic activity was observed, together with an increase in extracellular ROS and NO production – indicating cellular stress and frustrated phagocytosis – and a general upregulation of messenger RNA (mRNA) levels for different AMPs and lysozyme (Barmo et al., 2013). In Tegillarca granosa, prolonged exposure to higher concentrations of TiO2 NPs (30 days, 10–100 mg/l) significantly reduced total haemocyte number and phagocytosis. Expression of genes encoding PRRs (TLR1, TLR2, TLR4, TLR5, TLR6, RIG1) and downstream immune-related molecules (IRAK4, TRAF2, TRAF3, TRAF6, NFkb1, TRIM58) was significantly downregulated (Shi et al., 2017).
Most information on interactions between bivalve haemocytes and different types of ENMs has been obtained from in vitro studies with freshly isolated or primary cell cultures. In M. galloprovincialis haemocytes after short-term exposure to nano-carbon black (NCB) (Canesi et al., 2008), concentration-dependent uptake of NCB was associated with a rapid increase in extracellular ROS production, lysozyme and NO release, and induction of pre-apoptotic processes. The effects were mediated by rapid activation of mitogen-activated protein kinases (MAPKs), which play a key role in immune and inflammatory responses. The results demonstrated that in mussel haemocytes, as in mammalian cells, exposure to ENMs can induce inflammatory processes, and indicate that bivalve immunocytes could represent a suitable model for investigating the effects and modes of action of ENMs in marine invertebrates (Canesi et al., 2008). Several immunotoxic or immunomodulatory effects were observed upon exposure to different types of metal and carbon-based ENMs and nano-oxides in haemocytes from M. galloprovincialis and other bivalve species (clams, oysters); however, the effects were dependent on particle type and exposure conditions (reviewed in Canesi et al., 2019). A summary of the effects of ENMs on bivalve haemocytes is reported in Table 1.
| Species | ENM | Effect | References |
|---|---|---|---|
| In vitro | |||
Marine mussel M. galloprovincialis |
50 nm PS-NH2 |
|
Auguste et al. (2021a) |
Marine mussel M. galloprovincialis |
100 nm PS-NH2 |
|
Auguste et al. (2021a) |
Marine mussel M. galloprovincialis |
PS-NPs |
|
Sendra et al. (2020) |
Marine mussel M. galloprovincialis |
n-CeO2 |
|
Canesi et al. (2017) |
Marine mussel M. galloprovincialis |
50 nm PS-NH2 |
|
Canesi et al. (2016, 2017) |
Marine mussel M. galloprovincialis |
NCB NPs |
|
Canesi et al. (2008) |
Hong Kong oyster Crassostrea hongkongensis |
37 nm Ag NPs |
|
Luo & Wang (2022) |
| In vivo | |||
Marine mussel M. galloprovincialis |
50 nm PS-NH2 |
|
Auguste et al. (2021a) |
Marine mussel M. galloprovincialis |
100 nm PS-NH2 |
|
Auguste et al. (2021a) |
Marine mussel M. galloprovincialis |
50 nm PS-NH2 |
1st exposure
2nd exposure
|
Auguste et al. (2020) |
| Marine blood clam Tegillarca granosa | TiO2 NPs |
|
Shi et al. (2017) |
Marine mussel M. galloprovincialis |
TiO2 NPs |
|
(Barmo et al., 2013) |
- Abbreviations: LMS, lysosomal membrane stability; MAPK, mitogen-activated protein kinase; MgC1q6, M. galloprovincialis putative C1q domain containing protein 6; MgC1q44, M. galloprovincialis putative C1q domain containing protein 44; n-CeO2, cerium nano-oxide; NCB, nano-carbon black; NO, nitric oxide; NP, nanoparticle; PRR, pattern recognition receptor; PS-NH2, amino-modified polystyrene; PS-NP, polystyrene nanoparticle; ROS, reactive oxygen species; SOD, superoxide dismutase; THC, total haemocyte count.
(3) Immune interaction with nanoplastics
In the last few years, increasing concern regarding the potential toxicity of nanoplastics (NPLs), mainly resulting from plastic degradation in oceans (Gigault et al., 2018) has led to renewed interest in NP–immune interactions in bivalve haemocytes. In the absence of reliable ‘natural’ NPLs, studies have relied on the availability of different types of nanopolymers for use as proxies of NPLs, in particular polystyrene nanoparticles (PS-NPs) of different sizes and surface characteristics (e.g. plain or labelled with different fluorochromes, surface modified with cationic and anionic groups). In vitro, NPLs can be internalised in the lysosomal compartment of haemocytes and induce a wide range of responses, from lysosomal reactions to decreased phagocytosis and increased production of toxic radicals and apoptosis [see Auguste et al. (2021a,b) and references therein]. Again, the effects were dependent on particle characteristics (size, surface charge) and experimental conditions (exposure media, concentration, exposure time). Interestingly, distinct responses were observed in different haemocyte subpopulations, with large granular haemocytes (fully mature phagocytes), being most involved (Sendra et al., 2020). None of these studies investigated the role of PRRs in mediating particle recognition and effects.
As in other invertebrate groups, in addition to cells, molluscan immune defences also rely on many soluble haemolymph factors, which can interact with non-self material often by targeting common (rather than specific) structural motifs (Gianazza et al., 2021). Studies carried out with surface-modified NPLs reveal the role of individual haemolymph proteins in particle recognition. Results in mammals show that soluble serum components form a biomolecular corona on the ENM surface, which is responsible for the particle interaction with target cells. In molluscs, the influence of haemolymph serum (HS) on the effects of positively charged NPLs (50 nm amino-modified polystyrene, PS-NH2) was investigated using M. galloprovincialis haemocytes. In the presence of HS, PS-NH2 caused increased cellular damage and ROS production, mediated by dysregulation of p38 MAPK signalling (Canesi et al., 2016). The putative C1q domain-containing protein 6 (MgC1q6) was identified as the only component of the 50 nm PS-NH2 hard biocorona. MgC1q6 has a high affinity for cations, explaining why it can adhere to the positive surface charge of this type of NPL, and acts as an opsonin in immune recognition (Pezzati et al., 2015). The preferential association of PS-NH2 with this opsonin thus is likely responsible for the observed effects. In vivo, repeated exposure to NPLs induced upregulation of MgC1q6 (but not TLR) expression, associated with increased bactericidal activity (Auguste et al., 2020). By contrast, larger PS-NH2 (100 nm, PS100-S) showed a peculiar behaviour in HS (absence of agglomeration and inversion of the zeta potential – the electrokinetic potential in a colloidal system) and weaker effects on lysosomal destabilisation and phagocytosis, suggesting that interactions with HS components can reduce potential adverse effects. The main HS protein that forms a stable association with PS100-S is MgC1q44, an MgC1q complement component unrelated to MgC1q6 (Auguste et al., 2021a). Similarly, decreased ROS production by haemocytes exposed to cerium nano-oxide (n-CeO2) was associated with the formation of a biocorona mainly involving Cu,Zn-superoxide dismutase (SOD) (Canesi et al., 2017). This evidence indicates that the formation of a biomolecular corona is ENM selective and involves distinct soluble serum factors, acting as PRRs for particle recognition, thereby mediating the interactions with immune cells and resulting in different functional outcomes (Canesi et al., 2017). These data underline the importance of nano-bio interactions that confer a recognisable biological identity to ENMs in physiological exposure media. This is important when testing the potential environmental immunotoxicity of ENMs in model organisms (Canesi et al., 2017), but also to reveal unexpected serum components than can act as PRRs for non-self particles.
The above results represent the most extensive data available on bivalve immune responses to NPL exposure. It should be noted, however, that an experimental approach using commercial spherical NPLs, necessitated by the absence of standardised environmental NPL samples for toxicity testing, has obvious limitations as underlined by Auguste et al. (2021a,b). Studies are underway for testing the effects of non-homogeneous NPLs, such as those obtained from weathering or fragmentation of larger plastic debris, fibres, and from different polymers. Recent data indicate that both in vitro and in vivo exposure to high concentrations of polyester microfibres (length 50–100 μm) arising from textile fragmentation/abrasion significantly affects haemocyte functions (adhesion, tissue infiltration) and immune responses (lysozyme release, NO and ROS production) in M. galloprovincialis (Auguste et al., 2022).
A schematic depiction of the interaction of ENMs (including NPLs) with the bivalve immune system, and its functional consequences, is presented in Fig. 3.
IV. PHAGOCYTES AND TLRS IN ANNELID IMMUNITY AND NANOPARTICLE–IMMUNE INTERACTIONS
The annelids include marine worms (Polychaeta), earthworms (Oligochaeta), and leeches (Hirudinea). They rely on highly evolved mechanisms of innate immunity, especially in wound repair, clotting and coagulation, phagocytosis or cytotoxic killing, encapsulation reactions, and the action of antimicrobial factors. Since earthworms live in close contact with the soil, their coelomic cavity (which is in direct contact with the external environment through dorsal pores) is not aseptic and contains diverse microorganisms. Like earthworms, marine worms and freshwater leeches are also in direct contact with dispersed ENMs (Cuvillier-Hot, Boiddin-Wichlacz & Tasiemski, 2014; Bodó et al., 2020a).
(1) Immunocytes and pattern-recognition receptors in annelids
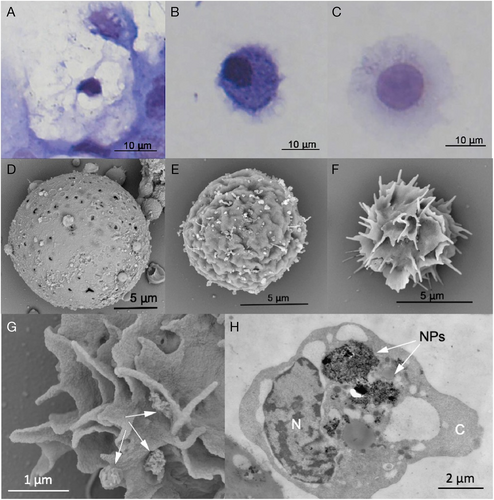
The body cavity of an earthworm is filled with a protein-rich coelomic fluid containing several types of immune cells (coelomocytes) that differ in morphology and function. There are three main coelomocyte types: eleocytes have mainly nutritive functions and produce antimicrobial factors, and hyaline and granular amoebocytes represent effector immunocytes, responsible for phagocytosis and encapsulation (Engelmann et al., 2016a) (Fig. 4). Amoebocytes engulf material including microbial components, foreign cells, inert particles, and ENMs. Similar to vertebrates, phagocytosis by coelomocytes can be modulated by humoral components, opsonins, which coat the particles and thus promote their phagocytosis (Bilej et al., 2010). Similar cell types and immune defence principles have been described for leeches and marine worms, where three subtypes of granulocytes (G1, G2 and G3) were described (Lefebvre et al., 2008; Cuvillier-Hot et al., 2014).
In the phylum Annelida, only a few PRRs have been described, including TLRs, peptidoglycan recognition proteins (PGRPs), coelomic cytolytic factor (CCF), NLRs, and scavenger receptors (Bilej et al., 2010; Engelmann et al., 2016b). The first earthworm TLR with an immune function was described in Eisenia andrei (EaTLR) (Skanta et al., 2013). Its significant intra-species variability suggests the possibility of a vast number of Toll-like gene paralogues in the E. andrei genome, for TLRs with tissue-specific expression profiles. Another E. andrei TLR-like protein (multiple cysteine cluster type of TLR, mccEaTLR), with a potential role in earthworm early development, was described by Prochazkova et al. (2019). In marine worms, 105 TLR homologues in Capitella capitata and 16 TLR homologues in Helobdella robusta genomes were found by in silico analysis of whole genomes (Davidson et al., 2008). Of the four Hm-TLRs in the leech Hirudo medicinalis, Hm-TLR1 is associated with the leech central nervous system (Tasiemski & Salzet, 2017).
(2) Effects of ENMs on annelid innate immunity
A limited number of studies have investigated the effects of ENMs on phagocytic cells or their recognition by TLRs in annelids. Besides phagocytic activity, the production of ROS during phagocytosis has been assessed following ENM exposure. The involvement of TLRs in EMN recognition has mainly been studied in terms of changes to their expression after exposure to the particles.
Most studies on the effects of ENMs on annelid immunity examine the role of silver nanoparticles (AgNPs), especially using in vitro studies. Exposure of Eisenia earthworms to AgNPs led to the accumulation of NPs in cells (mainly amoebocytes), oxidative and mitochondrial stress, apoptosis, and altered expression of TLR and other genes involved in stress and intracellular signalling (Hayashi et al., 2012; Bodó et al., 2020b). Similarly, transcriptional profiling of cells exposed to nanosilver NM-300K revealed upregulation of TLR and signalling-related genes (Hayashi et al., 2016). Increased phagocytic activity of earthworm coelomocytes was detected after exposure to CuO NPs and nanoscale zero-valent iron (nZVI), concomitant with higher levels of lipid peroxidation (Semerad et al., 2020; Pacheco et al., 2021a). Coelomocytes of Lumbricus rubellus treated with fullerene C60 NPs showed decreased gene expression of pattern recognition factor CCF, suggesting immunosuppression (van der Ploeg et al., 2014). By contrast, except for altered TLR expression when cells were exposed to AuNPs, neither AuNPs nor TiO2 NPs induced cytotoxicity or altered immune properties (Bodó et al., 2020b; Pacheco et al., 2021b). In vitro treatment of leech cells with multiwalled carbon nanotubes (MWCNTs) induced a strong inflammatory response, activation of macrophages, encapsulation of the foreign bodies, and increased apoptotic rate together with elevated ROS production (Girardello et al., 2017, 2015a) (Table 2).
| Species | ENM | Effect | References |
|---|---|---|---|
| In vitro | |||
Earthworm Eisenia andrei |
TiO2 NPs |
|
Pacheco et al. (2021b) |
Earthworm E. andrei |
CuO NPs |
|
Pacheco et al. (2021a) |
Earthworm E. andrei |
nZVI |
|
Semerad et al. (2020) |
Earthworm Lumbricus rubellus |
Fullerene C60 NPs |
|
van der Ploeg et al. (2014) |
Earthworm E. fetida |
AgNPs |
|
Hayashi et al. (2012) |
Earthworm E. andrei/fetida |
AgNPs |
|
Bodó et al. (2020b) |
Earthworm E. fetida |
nanosilver NM-300 K |
|
Hayashi et al. (2016) |
Earthworm E. andrei/fetida |
AuNPs |
|
Bodó et al. (2020b) |
Earthworm E. fetida |
ZnO NPs |
|
Gupta et al. (2014) |
Leech Hirudo medicinalis |
MWCNTs |
|
Girardello et al. (2015a) |
Leech H. verbana |
MWCNTs |
|
Girardello et al. (2017) |
| In vivo | |||
Earthworm L. rubellus |
AgNPs |
|
Roelofs et al. (2020) |
Earthworm L. terrestris |
AgNPs |
|
Lapied et al. (2010) |
Earthworm L. rubellus |
AgNPs |
|
Diez-Ortiz et al. (2015) |
Earthworm E. fetida |
AgNPs |
|
Novo et al. (2015) |
Earthworm E. fetida |
AgNPs |
|
Garcia-Velasco et al. (2016) |
| Earthworm Aporrectodea caliginosa | AgNPs |
|
Saleeb et al. (2020) |
Earthworm E. fetida |
AgNPs |
|
Gomes et al. (2015) |
Earthworm E. fetida |
AgNPs |
|
Courtois et al. (2020) |
Earthworm E. andrei |
AgNPs |
|
Pacheco et al. (2022) |
Earthworm Metaphire posthuma |
CuO NPs |
|
Gautam et al. (2018) |
Earthworm E. fetida |
CuO NPs |
|
Swart et al. (2020) |
Earthworm L. rubellus |
Fullerene C60 NPs |
|
van der Ploeg et al. (2013) |
Leech H. medicinalis |
MWCNTs |
|
Girardello et al. (2015a,b) |
| Marine worm Nereis diversicolor | MWCNTs |
|
De Marchi et al. (2018) |
Marine worm N. diversicolor |
CuO NPs |
|
Thit et al. (2015) |
- Abbreviations: CCF, coelomic cytolytic factor; MWCNTs, multi-walled carbon nanotubes; NO, nitric oxide; NP, nanoparticle; nZVI, nanoscale zero-valent iron; PO, phenoloxidase; ROS, reactive oxygen species.
The majority of in vivo studies assessing the effects of ENMs on annelids focus on the toxic effect of AgNPs. Two transcriptomic studies revealed the upregulation of genes involved in phagocytosis and autophagy pathways as well as in endocytosis (Novo et al., 2015; Roelofs et al., 2020). This is consistent with endocytosis being one of the possible routes by which ENMs can enter the tissues. Accumulation of AgNPs in tissues was detected in three earthworm species (Diez-Ortiz et al., 2015; Garcia-Velasco et al., 2016; Saleeb et al., 2020). The greatest concentrations of AgNPs were found in the gut wall, liver-like chloragogenous tissue, and the excretory organ nephridia, suggesting potential uptake, detoxification, and excretion via this pathway. Furthermore, AgNPs induced oxidative stress, lipid peroxidation, apoptosis, and mass loss in earthworms (Lapied et al., 2010; Gomes et al., 2015; Saleeb et al., 2020; Pacheco et al., 2022). While CuO NPs did not induce changes in immune gene expression in the earthworm E. fetida (Swart et al., 2020), a decrease in phagocytic activity and NO generation was observed in the earthworm Metaphira posthuma (Gautam et al., 2018). Analysis of leech tissues after exposure to MWCNTs revealed internalised nanomaterial, a strong inflammatory response with inflammatory cytokine expression, particle uptake by phagocytes, and encapsulation (Girardello et al., 2015b). Few papers describe the effect of ENMs on marine worms. One study reported that exposure to CuO NPs provokes a substantial accumulation of Cu in tissues (Thit, Banta & Selck, 2015). Other work reported increased antioxidant enzyme activity and elevated lipid peroxidation as a consequence of MWCNT exposure (De Marchi et al., 2018) (Table 2).
(3) Role of biomolecules adsorbed onto the ENM surface
Annelid coelomic fluid contains a large number of proteins, which form a biomolecular corona around NPs. The secretory protein lysenin was found to be an important component of the biocorona around nanosized silver particles in earthworms (Hayashi et al., 2013), thus its opsonisation properties participate in ENM interaction with cells. Particles covered by a biocorona may be eliminated by encapsulation and subsequently expelled via dorsal pores in the epidermis. Earthworms treated with nanosilver NM-300K showed suppressed lysenin expression and thus a reduction in its potential incorporation into the biocorona, thereby reducing lysenin-assisted ENM uptake (Hayashi et al., 2016). It remains unclear whether the coating of ENMs with endogenous biomolecules is a way to facilitate ENM clearance or whether it results in concealment or in a do not-eat-me signal. Although some studies point to ENMs affecting TLRs, the exact mechanism is unknown. Since many ENMs are known to absorb bacterial lipopolysaccharide (LPS) readily onto their surface, the observed changes in TLR expression or activation may be due to interaction with LPS or other PRRs adsorbed on the particle surface. Particularly in annelids, whose body cavities are not aseptic, activation of TLR signalling is potentially more likely due to microbial molecules adsorbed on the ENM surface than to the ENMs themselves. Moreover, in earthworms, TLR expression changes during regeneration, which can be part of a response to ENM-caused tissue damage (Bodó et al., 2021). A schematic depiction of the interaction of ENMs with annelid phagocytes and TLRs, and its functional consequences, is presented in Fig. 5.
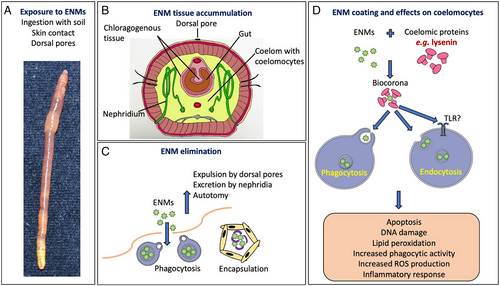
V. PHAGOCYTES AND TLRS IN CRUSTACEAN IMMUNITY AND NANOPARTICLE–IMMUNE INTERACTIONS
Crustaceans are a large arthropod group comprising marine, freshwater and terrestrial species. As in other invertebrates, the crustacean innate immune response encompasses diverse humoral and cellular activities dependent on the activation of specific signal transduction pathways and is generally initiated by the recognition of PAMPs or endogenous damage-associated molecular patterns (DAMPs) by PRRs. Immune responses are also triggered by internal/endogenous and/or external/environmental stress signals (Lorenzon, 2005). For example, hyperglycaemia is a typical response of many aquatic animals, including crustaceans, to physical and chemical environmental changes. Exposure to environmental stresses (e.g. hypoxia, salinity changes, thermal stress, emersion, heavy metal pollutants, ENMs) results in increases in hyperglycaemic hormone levels, as is also the case for viral diseases or parasitic dinoflagellate infections. In infections, homeostasis is maintained by a complex neuroendocrine–immune regulatory network (Lorenzon, 2005).
(1) Crustacean haemocytes and pattern-recognition receptors
The central players in the immune response of crustaceans are haemocytes (Fig. 6), which are responsible for phagocytosis, encapsulation, and lysis of foreign particles and cells, and the production and release of different humoral components, such as coagulation factors, protease inhibitors, antimicrobial substances, lectins and others (Iwanaga & Lee, 2005; Griffith, Sokol & Luster, 2014). Phagocytosis by haemocytes is a crucial defence mechanism against infectious agents such as bacteria and viruses, and a scavenging mechanism to remove necrotic debris (reviewed in Liu et al., 2020; Westman, Grinstein & Marques, 2020). Different receptors, such as lectins, scavenger receptors, immunoglobulin-related proteins, and fibrinogen-related proteins, have been reported to be involved in phagocytosis (Liu et al., 2020). Soluble factors (opsonins) bind to pathogens or damaged cells, marking them for phagocytosis. A phagocytic receptor present only in arthropods is the Down Syndrome cell adhesion molecule (Dscam), which in its soluble isoforms binds with bacteria in an opsonisation-like manner, while membrane-bound Dscam promotes phagocytosis in haemocytes (Li et al., 2018b). Dscams are highly variable immunoglobulin-related PRRs, they can be either constitutive or inducible during acute-phase reactions (Jin et al., 2013; Tran et al., 2020), and also have a role in promoting phagocytosis (Liu et al., 2020). It has been suggested that membrane-bound and soluble Dscams can directly bind to pathogens to promote their elimination. Exposure to tyre particles for 4 days, but not 2 weeks, caused downregulation of the Dscam gene in the hepatopancreas of Porcellio scaber (Dolar et al., 2022). This finding suggests that Dscams may have an important role in enabling arthropods to cope with external challenges and preserve homeostasis. Among factors enhancing phagocytosis, AMPs can bind to bacteria and promote their phagocytosis, in addition to their known direct antimicrobial functions (Liu et al., 2015; Matos & Rosa, 2021). The expression of some AMP gene families is induced in response to tissue injury, suggesting a possible role in tissue repair.
In crustaceans, the main uptake mechanism for particles is direct uptake from the water through the gills by pinocytosis or phagocytosis (Ribeiro et al., 2017). Traditionally, phagocytosis is assessed directly as a phagocytic index, i.e. the percentage of haemocytes containing endocytosed bacteria or latex beads in in vitro tests, or indirectly by measuring the production of ROS. Injected particles accumulate in the gills and can be eliminated together with haemocyte aggregates (Ikerd, Burnett & Burnett, 2015). When circulating haemocytes detect the presence of pathogens, foreign particles, or endogenous anomalous entities, they rapidly aggregate, entrapping the pathogens/particles. These aggregates are then trapped in the gill microvasculature where they are melanised and eliminated during the next moult (Martin et al., 2000). While most studies pertain to bacteria and infectious events, there is little information on phagocytosis/activation as a consequence of exposure to pollutants, and the available evidence is mostly from terrestrial isopods in ecotoxicological studies. Mayall et al. (2021) reported an increase in NO concentration as an indirect indication of phagocytic activity resulting from CeO2 NP exposure in the terrestrial crustacean P. scaber, explaining this as a (micro)damage-related response. The observed response of P. scaber to CeO2 NPs was significantly different from that in AuNP-fed animals, both in terms of NO production and increased total haemocyte counts, suggesting that AuNPs are biologically inert compared to CeO2 NPs (Mayall et al., 2021). A summary of the available information on the interaction between ENMs and crustacean haemocytes is reported in Table 3.
| Species | ENM | Effect | References |
|---|---|---|---|
| In vivo | |||
American lobster Homarus americanus Spiny lobster Panulirus interruptus Crayfish Procambarus clarkia Ridgeback prawn Sicyonia ingentis |
India ink (Higgins) particles injected |
|
Martin et al. (2000) |
| Crayfish Cherax quadricarinatus | Fluorescent polystyrene beads 5 μm |
|
Duan et al. (2014) |
| Crayfish C. quadricarinatus | Fluorescent polystyrene beads |
|
Li et al. (2018a) |
| Amphipod Gammarus fossarum | AgNPs and AuNPs 20–80 nm |
|
Mehennaoui et al. (2018) |
| Terrestrial isopod Porcellio scaber | AuNPs in diet (14 days) |
|
Mayall et al. (2021) |
| Terrestrial isopod P. scaber | CeO2 NPs in diet (14 days) |
|
Mayall et al. (2021) |
| Terrestrial isopod P. scaber | AuNPs and CeO2 NPs, injected |
|
Mayall et al. (2021) |
| Terrestrial isopod P. scaber | AuNPs and CeO2 NPs, injected with LPS |
|
(D. Drobne et al., unpublished data) |
| Terrestrial isopod P. scaber | Microplastics, ingestion |
|
(D. Drobne et al., unpublished data) |
| White-leg shrimp Litopenaeus vannamei | Exposure to microplastics |
|
Wang et al. (2021) |
| Chinese mitten crab Eriocheir sinensis (juvenile) | Exposure to microplastics |
|
Liu et al. (2019) |
- Abbreviations: ACP, acid phosphatase; AKP, alkaline phosphatase; CIT, citrate; GC, granular cell; HC, haemocyanin; IMD, immunodeficiency gene; NOS2, inducible nitric oxide synthase gene; GC, granular cell; LPS, lipopolysaccharide; LSZ, lysozyme; NO, nitric oxide; NP, nanoparticle; PEG, polyethylene glycol; PO, phenoloxidase; SGC, semigranular cell; SOD, superoxide dismutase.
Sensing of possible dangers by crustacean haemocytes mainly occurs through PRRs, among which the best described are TLRs (Wang & Wang, 2013). Eighteen TLR genes have been identified and studied extensively in several crustacean species. These are present on haemocytes and many other cells (Lai & Aboobaker, 2017; Habib & Zhang, 2020). As in insects, crustacean TLRs bind to Spätzle, an endogenous ligand generated by proteolysis of a pro-protein that is usually activated in response to an infectious agent. TLR engagement by Spätzle leads to the activation of an intracellular signalling cascade that initiates the expression of several immune genes, including those encoding anti-LPS factors (ALFs), crustins, penaeidins, histins, arasins, and hyastatin (Habib & Zhang, 2020; Sánchez-Paz & Muhlia-Almazán, 2020). Components of the Toll pathway in malacostracan crustaceans (crabs, lobsters and shrimps) include a chain of interacting proteins: in addition to the TLRs themselves, these include the endogenous ligand Spätzle, the intracellular signalling molecules myeloid differentiation factor 88 (MyD88), Tube, Pelle, Dorsal, Cactus and the Toll-interacting protein (TOLLIP) (Arancibia et al., 2007; Lai & Aboobaker, 2017).
(2) Crustacean immune reaction to environmental stressors
While the role of TLRs in pathogen recognition and reaction is established, much less is known about their role in responses to other types of stress. A low salinity shock can trigger an innate immune response by activating the TLR and MAPK signalling pathways (Zhao et al., 2015). In the Indian spiny lobster, Panulirus homarus, environmental changes (salinity, dissolved oxygen levels, and ammonia–N concentration) affect immune responses, measured as changes in total haemocyte counts, phenoloxidase (PO) activity, and ROS production (Verghese, Radhakrishnan & Padhi, 2007). In the crab Carcinus aestuarii, extreme temperatures (both high and low) influence immune parameters (mainly haemocyte proliferation, haemolymph protein concentration, and PO activity), most likely as mechanisms to cope with temperature changes (Matozzo, Gallo & Marin, 2011). Such observations are not restricted to crustaceans: similar findings have been reported for other invertebrate and vertebrate species and underline the broader role of TLRs in sensing environmental changes and coordinating subsequent adaptive/protective responses.
Crustaceans can exhibit immune responses to other environmental stressors, including ENMs or micro- and nanoplastics (Table 3). In the terrestrial isopod P. scaber, immune stimulation in response to ingested or injected ENMs was assessed as increased or decreased expression of several immune-related genes (Table 3) (Dolar et al., 2022). Whether and how particles are perceived as a potential threat by the crustacean immune system, and in particular by interaction with innate receptors such as TLRs according to Matzinger's danger model (Matzinger, 2002), may depend on the type of biomolecular corona on the particle surface. If the corona is composed of conformationally altered self-molecules, it can be recognised by the immune system as potentially dangerous altered/damaged self. Some proteins/molecules can undergo conformational changes upon adsorption onto particles (Li et al., 2013). Thus, crustacean TLRs seem to be involved in recognition and defensive response to various stimuli and conditions other than infective agents, suggesting a wider function in safeguarding the body's integrity. In this context, evaluating immune reactions to particles may enhance our understanding of the fundamental mechanisms of crustacean immune defences. A schematic depiction of the interaction of ENMs (including NPLs) with the crustacean immune system, and its functional consequences, is presented in Fig. 7.
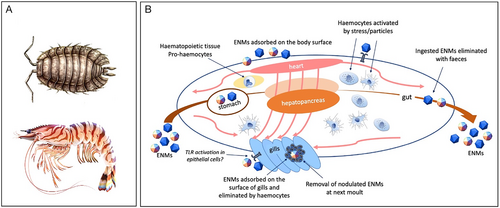
VI. PHAGOCYTES AND TLR IN ECHINODERM IMMUNITY AND NANOPARTICLE–IMMUNE INTERACTIONS
Several immune-related traits are functionally and evolutionarily conserved among deuterostomes, of which echinoderms and chordates are the major extant members. These include danger-sensitive innate immune cells (e.g. phagocytic cells), stimulation of TLR orthologues, and activation of the inflammasome upon immune challenge.
Echinoderms include five extant clades: Crinoidea (sea lilies and feather stars), Asteroidea (sea stars), Ophiuroidea (brittle stars and basket stars), Echinoidea (sea urchins and sand dollars), and Holothuroidea (sea cucumbers). The Echinoidea and Holothuroidea are the most highly evolved classes.
(1) Phagocytes in echinoderms
Three major types of freely circulating immune cells [phagocytes (Fig. 8), amoebocytes, and vibratile cells] are relatively conserved among sea urchin and sea star species, but less so among species of sea cucumbers. They are present in all coelomic spaces, including the perivisceral coelomic cavities, the open water-vascular system, and in organs and tissues. Although some cell types are better defined, less is known about the functions of the rarer types, including amoebocytes and vibratile cells. These cells are highly mobile and increase in number following an insult. Amoebocytes (red and white) are large motile cells with cytoplasmic granules rich in mucopolysaccharides and proteins. These cells are associated with the first phase of pathogen immobilisation (e.g. antibacterial and cytotoxic activity, encapsulation). Red amoebocytes in echinoderms produce the anti-bacterial agent echinochrome. White amoebocytes display cytotoxic activity against mammalian cells in vitro, which increases in the presence of phagocytes. Vibratile cells are fast-moving flagellated cells involved in clot formation, with cytoplasmic granules that contain clotting proteins.
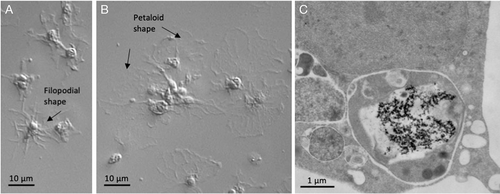
In all echinoderms, phagocytic cells are the main actors in defensive reactions and in sea urchins and sea stars are often the most abundant free-circulating cells present in the coelomic fluid (Smith et al., 2018; Andrade et al., 2021; Barela Hudgell, Grayer & Smith, 2022). Phagocytes include a few subsets of cells based on their size and cytoskeletal structure, with additional distinctions based on function, including the production of complement components (e.g. SpC3), opsonisation, phagocytosis, encapsulation, bacterial growth inhibition, cytotoxicity, and stimulus-dependent expression of immune-related genes (e.g. genes encoding SpTransformer proteins) (Smith et al., 2018; Clow et al., 2000; Chou, Liu & Smith, 2018). Depending on the conditions, these cells can change from a petaloid/bladder shape (mainly involved in phagocytosis) to a filopodial shape (mainly involved in clotting formation) via reorganisation of cytoskeletal microfilaments (Fig. 8). Phagocytes produce several types of AMPs, including strongylocins (cysteine-rich), centrocins, β-thymosin, and paracentrin. Other humoral defensive factors are lectins, lysozymes, clotting proteins, lysins, and the prophenoloxidase (proPO) system (Smith et al., 2018).
(2) TLRs in echinoderms
In Echinoidea, closely related but diversified variants of TLRs may respond to evolving pathogens with a higher dynamism than the PAMP-based systems of vertebrates and insects (Buckley & Rast, 2012). The sequence diversity and complexity of the Echinoidea TLR multigene families appears to be the result of an expansion specific to the echinoderm lineage; while a small number of TLRs may function similarly to those of vertebrates, the large number of TLRs likely implies novel immune-recognition functions. Depending on the species, the estimated size of the TLR gene family ranges from 68 in Lytechinus variegatus to 276 in Mesocentrotus franciscanus (syn. Strongylocentrotus franciscanus) (Smith et al., 2018), with the level of complexity related to the average lifespan. For example, M. franciscanus (Pacific Ocean) can live for over 100 years (Ebert, 2008), while L. variegatus (Atlantic) has an estimated maximal lifespan of only 3–10 years (Russell et al., 2012), which is a relatively short life expectancy among sea urchin species. Evidence for TLR expansion has not been detected in the genomes of other echinoderms, including the sea cucumber Apostichopus japonicas for which only two TLR genes were identified (AjTLR3 and AjToll) (Sun et al., 2013).
Genes involved in pathogen-specialised TLR signalling pathways are highly conserved and include the presence of TIR domain-containing adaptors (e.g. MyD88, SARM1), signalling mediators (e.g. IRAK1/4, TRAF6, M3K7, TAB1, TAB2), and cytokines and transcription factors activated by infection (e.g. NF-κB, TNFs, IL-17, IRFs) (Tassia, Whelen & Halanych, 2017). The evolution of gene family expansion to generate TLR diversity and other immune receptors is not limited to sea urchins, but is also found in other invertebrate deuterostomes, including the lancelet Branchiostoma floridae (cephalochordate) (Tassia et al., 2017), although it remains to be elucidated how TLR expansion is involved in pathogen recognition. Molecular phylogenetic analysis shows that most sea urchin or lancelet TLR candidates are paralogues, suggesting that these organisms independently underwent TLR-related gene family expansion (Satake & Sekiguchi, 2012).
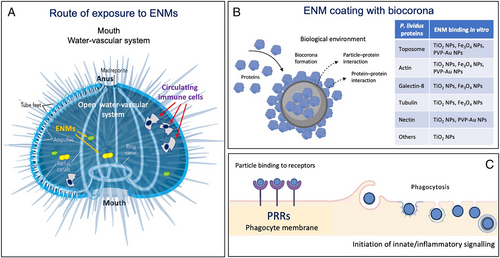
(3) Interaction between ENMs and the echinoderm immune system
Echinoderms, particularly sea urchins, represent a suitable model in developmental biology for evaluating environmental risks posed by ENMs (Fairbairn et al., 2011; Genevière et al., 2020; Pikula et al., 2020; Kukla et al., 2022). Only one species, the sea urchin Paracentrotus lividus, has been extensively used for environmental nano-immunotoxicological studies, addressing the issue of ‘nanosafety’ (Pinsino & Di Bernardo, 2022). P. lividus is a common sea urchin on European coasts (Mediterranean Sea and Eastern Atlantic Ocean), with an estimated lifespan of 15 years (Tomšic ́ et al., 2010).
The open water-vascular system allows water-dispersed ENMs to come into contact with the coelomic fluid and interact with various extracellular proteins to form a complex biocorona on the particle surface. Biocorona-coated ENMs then come into contact with cells – mainly phagocytes which represent more than 80% of the total freely circulating cell population (Alijagic et al., 2019, 2021a,b). In vivo studies on P. lividus immune cells show selective changes in specific pathways when exposed to a range of ENMs, a similar situation to that in humans (Falugi et al., 2012; Pinsino et al., 2015). For example, in vivo exposure of P. lividus to TiO2 NPs for 24 h activates receptor-mediated phagocytosis involving the TLR4 and p38 MAPK signalling pathways coupled to an anti-inflammatory response (Pinsino et al., 2015). Phagocytes exposed in vitro to the same NPs downregulate the expression of genes encoding TLR signalling-related proteins such as nuclear factor kappa B (NF-κB), c-Jun N-terminal kinase (JNK), p38 MAPK, caspase 8, and FAS-associated death domain protein (FADD), and increase their antioxidant metabolic activity (pentose phosphate pathway, cysteine–methionine and glycine–serine metabolism) that promotes immunological tolerance (Alijagic et al., 2020). By contrast, AuNPs coated with polyvinylpyrrolidone (PVP-AuNPs) and sea urchin extracellular molecules can elicit an immune response through transient involvement of the TLR4/p42/44 MAPK (ERK) signalling pathway, accompanied by metabolic rebalancing, to control/downregulate the response (Alijagic et al., 2021a). Upon exposure to PVP-AuNPs, phagocytes take up particles, and a rare cell subset shows increased expression of CD45 (which in mammals is a pan-leukocyte antigen transmembrane tyrosine phosphatase) and CD14 (which in mammals is an LPS-binding protein expressed on monocytes and macrophages) (Alijagic et al., 2021b). These cells may reflect an intermediate state of maturity and may be involved in several aspects of TLR-dependent immune activation. The particle-uptake mechanism is unknown, but may involve scavenger receptors and other PRRs and, as known for mammalian macrophages, a non-specific passive uptake mechanism, depending on the particle size (Geiser et al., 2008). The biocorona on the ENM surface represents the interface that is perceived by cells, and particles coated with P. lividus extracellular proteins are readily phagocytosed by both petaloid and filopodial phagocytic cells (Pinsino et al., 2015; Alijagic et al., 2020). A summary of studies describing the interaction between ENMs and the echinoderm immune system is reported in Table 4, and a schematic depiction is presented in Fig. 9.
| Species | ENM | Effect | References |
|---|---|---|---|
| In vitro | |||
Sea urchin Paracentrotus lividus |
TiO2 NPs |
|
Alijagic et al. (2019, 2020) |
Sea urchin P. lividus |
PVP-coated AuNPs |
|
Alijagic et al. (2021a) |
Sea urchin P. lividus |
PVP-coated AuNPs |
|
Alijagic et al. (2021b) |
Sea urchin P. lividus |
Fe3O4 NPs |
|
(A. Pinsino, unpublished data) |
| In vivo | |||
Sea urchin P. lividus |
TiO2 NPs |
|
Pinsino et al. (2015) |
Sea urchin P. lividus |
Fe3O4 NPs SnO2 NPs CeO2 NPs |
|
Falugi et al. (2012) |
- Abbreviations: CD14, cluster of differentiation 14; CD45, cluster of differentiation 45; ER, endoplasmic reticulum; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor κB; NP, nanoparticle; PVP, polyvinylpyrrolidone; TLR, Toll-like receptor.
VII. PHAGOCYTES AND TLRS IN MAMMALIAN IMMUNITY AND NANOPARTICLE–IMMUNE INTERACTIONS
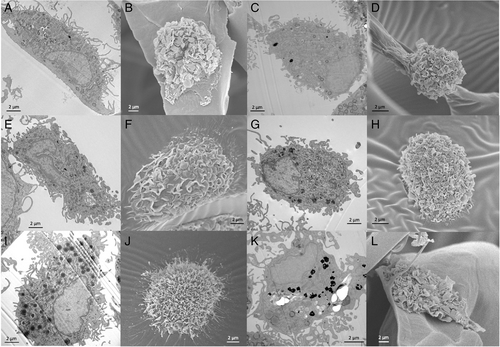
In vertebrates, the defensive role of phagocytes is similar to that in invertebrates, i.e. recognition and elimination of exogenous particles (scavenging), elimination of senescent or dead cells (surveillance), and initiation of an active immune/inflammatory response against exogenous agents such as pathogenic microorganisms. In mammals, including humans, there are two main types of phagocytic cells, mononuclear phagocytes (mainly blood monocytes and tissue-resident macrophages) (Fig. 10) and polymorphonuclear (PMN) phagocytes (mainly blood neutrophils). Monocytes and neutrophils exit the bloodstream to enter tissues where defensive reactions are ongoing, and are the main innate immune effector cells. PMN phagocytes represent the first defensive wave, with a very short lifespan and high phagocytic and degranulation capacity, followed by monocytes which may survive the immune reaction and remain in the tissue post infection. Other human leukocytes involved in inflammatory/innate immune reactions also resemble invertebrate defensive cells; these include circulating eosinophils and basophils and tissue mast cells, which are poorly phagocytic compared to macrophages and PMN phagocytes and have defensive roles mainly by releasing granules loaded with bioactive mediators.
(1) Pattern-recognition receptors in mammalian innate immunity
Phagocytes and other innate immune cells in vertebrates can sense, recognise and react to exogenous agents using a range of PRRs that selectively bind structural molecular patterns shared by different microbes and other particles. Vertebrate PRRs have much in common with those of invertebrates and include a large number of lectins, scavenger receptors, cytoplasmic NLRs and RIG-like receptors (RLRs), and TLRs (Murphy et al., 2022; Brennan & Gilmore, 2018). In mammals, 13 TLR paralogue proteins have been described (of which 10 functional proteins are expressed in humans) that selectively recognise groups of molecular patterns characteristic of different microbes and/or anomalous endogenous molecules. Different types of professional phagocytes display different arrays of functional TLRs; for instance, monocytes/macrophages are able to express all of them at levels that depend on the activation conditions, whereas PMN leukocytes do not express TLR3 (Hayashi, Means & Luster, 2003). TLR expression levels also vary between mononuclear phagocytes from different anatomical sites (e.g. monocytes and alveolar macrophages), with TLR2 expressed more abundantly in monocytes and TLR9 in alveolar macrophages (Juarez et al., 2010). In addition, there is compartmentalisation of TLR proteins within mononuclear phagocytes, with some TLRs present in the plasma membrane (TLR1, 2, 4, 5, 6, 10), others in endosomal membranes (TLR3, 4, 7, 8, 9), Golgi (TLR4), and endoplasmic reticulum (TLR3, 7, 8, 9) (Barton & Kagan, 2009). The presence of TLRs is not limited to professional phagocytes. The ‘amateur’ phagocytes, such as epithelial cells, endothelial cells and astrocytes, also express some TLRs and other PRRs, and use them for cell/particle recognition and subsequent elimination; their role mostly being the elimination of dead cells (efferocytosis) in synergy with professional phagocytes (Sihombing et al., 2021). Mammary epithelial cells can phagocytose apoptotic cells using PRRs (Monks et al., 2005). Intestinal enterocytes can use TLRs for the uptake of bacteria and their transcytosis (Neal et al., 2006), a process that contributes to the maintenance of tolerance for commensal bacteria (Rakoff-Nahoum et al., 2004), but that could also be the basis of systemic sepsis (Strober, Fuss & Blumberg, 2002). It is notable that colonocytes express TLR2, 4 and 5 only on the basolateral membrane, so that recognition and activation can only occur if bacteria pass the epithelial cell layer (i.e. in an invasive infection), whereas there is no recognition/activation upon contact of commensal bacteria with the apical cell membrane in the gut lumen. Conversely, enterocytes in the ileum also express TLR2 and TLR4 apically, while TLR5 is only detected basolaterally (reviewed in Yu & Gao, 2015). Different gut epithelial cells (crypt epithelial cells, Paneth cells, follicle-associated epithelial cells) differentially compartmentalise TLR proteins, with altered expression and compartmentalisation occurring in disease conditions (e.g. cancer, ulcerative colitis, Crohn's disease) (Yu & Gao, 2015). Thus, TLR expression and compartmentalisation depend both on cell types and microenvironmental conditions and allow modulation of the activation of cellular functions, adapting their responses to microenvironmental cues.
The modes of TLR recognition and signalling are almost identical to those described for Drosophila Toll and all invertebrate TLRs. Signalling involves the presence of the highly conserved intracellular domain TIR that, in vertebrates, is present in another class of innate receptors, the interleukin-1 receptor (IL-1R) family. Molecules of the IL-1R family show binding specificity for endogenous cytokines of the IL-1 superfamily, which then activate an innate inflammatory response in target cells (including monocytes and macrophages) (Boraschi et al., 2018). Thus, the development of adaptive immunity in vertebrates is accompanied by the development of a new class of innate TIR-bearing receptors, which are likely involved in the crosstalk between innate and adaptive immunity (Boraschi, 2022).
(2) Innate immune interaction with ENMs
Similar to invertebrates, the interaction of ENMs with immune cells in vertebrates and, in particular, in mammals occurs primarily with phagocytes (Fig. 10) and other innate immune cells, which are abundantly present in barrier tissues (Boraschi et al., 2017). Blood phagocytes are recruited to tissues following entry of a foreign object. These blood cells and tissue-resident phagocytes are the main cells that encounter ENMs and, when they recognise them as exogenous agents, engage in their elimination. ENMs in contact with biological systems readily become coated with biomolecules (e.g. serum proteins including complement and fibronectin, mucus components) that may function as opsonins and facilitate uptake by phagocytes through dedicated receptors (Barbero et al., 2017; Moghimi & Simberg, 2017). While the majority of ENMs contacting phagocytes are taken up and degraded intracellularly or secluded into vesicles (Krug & Wick, 2011; Baranska et al., 2018), high aspect ratio ENMs that cannot be phagocytosed because of their size can undergo extracellular degradation. This is the case for carbon-based ENMs such as single-walled carbon nanotubes (SWCNTs) and graphene oxide (GO), which can be trapped by neutrophil extracellular traps (NETs) and degraded by neutrophil and eosinophil myeloperoxidases and NADPH oxidase (Kagan et al., 2010; Andón et al., 2013; Farrera et al., 2014; Mukherjee et al., 2015). The interaction between ENMs and mammalian macrophages has been studied mostly in vitro, mainly employing transformed cell lines of human or mouse origin (Boraschi & Italiani, 2016; Boraschi et al., 2021; Andón & Bondarenko, 2021). In many cases, depending on the type and surface characteristics of ENMs, and on their size, shape, dose, and time of exposure, cytotoxic effects can be detected (inhibition of proliferation, cell death) or inflammatory activation, oxidative stress, and gene regulation can be observed (production of ROS and NO, inflammatory cytokines and chemokines, and enzymes) (Kodali et al., 2013; Fadeel, 2012, 2019). Several caveats, however, limit the relevance of this large body of results, which should therefore be considered with caution. First is the extensive use of transformed cell lines instead of primary monocytes/macrophages. Although some studies have been performed on primary cells (mouse bone marrow-derived macrophages and human blood monocytes or monocyte-derived macrophages), the intrinsic donor-to-donor variability of responses with primary cells has led most studies to use more reproducible models such as those offered by continuous cell lines. The gain in reproducibility is, however, hampered by the loss in representativeness, since transformed, mostly tumoral cell lines do not fully reflect the functional and metabolic features of primary cells and may provide misleading information on the effects of interaction with ENMs (Boraschi et al., 2021). Secondly, the exquisite sensitivity of human monocytes/macrophages to activation by bacterial agents such as LPS calls for caution in attributing to ENMs inflammatory effects that may be due to undetected contamination with LPS (Li & Boraschi, 2016; Li et al., 2017). Likewise, the presence of a biomolecular corona on the ENM surface should always be considered since naked ENMs are unlikely to come into contact with immune cells. Eventually, and most importantly, the overall features of innate/inflammatory responses should be considered. ENMs that can kill macrophages in vitro or activate an inflammatory response are not necessarily immunotoxic or able to cause damage in vivo. Activation of an inflammatory response underlies the fact that macrophages can recognise ENMs as potentially dangerous and mount a defensive response. That macrophages die during an inflammatory reaction is the rule (for most macrophages and all PMN leukocytes), and, in most cases, the reaction is successful, and the dangerous agent eliminated without significant damage to the body (Ghezzi et al., 2017). Thus, the overall outcome of the ENM–immune interaction cannot be judged reliably by examining the effects of a single ENM type on a single cell type in culture and cannot be assessed by examining a single time point after exposure since a successful innate immune reaction evolves over time and proceeds from activation to resolution and an eventual return to baseline. Anomalous immune reactions may only be captured by examining the entire course of the defensive response and by examining the balance between inflammatory and anti-inflammatory factors and mechanisms activated in response to ENMs (Li et al., 2016, 2017; Italiani et al., 2020; Swartzwelter et al., 2020). Table 5 summarises the effects that should be considered for a realistic assessment of the functional outcomes of ENM–immune interactions in the mammalian/human context.
| ENM effect on cells | Type/extent of effect | Effect at the organism level |
|---|---|---|
| No effect |
|
No overall effect, ENMs are eliminated silently (respiration, urine, faeces) |
| Inflammatory activation |
|
No overall effect, limited damage repaired without consequences |
| Inflammatory activation |
|
Long-term tissue damage, which may include both tissue destruction and neo-tissue formation (fibrosis, granulomas) |
| Death |
|
No overall effect, limited damage repaired without consequences |
| Death |
|
Long-term tissue damage possible |
Some studies have proposed that ENMs may interact directly with TLRs or other PRRs, thereby activating innate/inflammatory responses of macrophages (Bastús et al., 2009; Wolf-Grosse et al., 2018; Mukherjee et al., 2018), although conclusive proof is still not available. The fact that ENMs are often ‘contaminated’ with TLR ligands such as LPS suggests caution in the interpretation of results. On the other hand, the regularly patterned structure of engineered ENMs, similar to microbial patterns, implies that PRRs may indeed be able to recognise them. While the involvement of TLR activation is still questionable, it is known that ENMs can activate IL-1R family receptors indirectly by promoting the release of IL-1 superfamily ligands. The available information mostly concerns IL-1β, the prototypical inflammatory cytokine of this superfamily. Several types of ENMs can induce IL-1β production, in particular by providing the intracellular signals necessary for the cleavage/maturation of the inactive pro-form of this cytokine, based on inflammasome activation (Martinon et al., 2006; Dostert et al., 2008; Hornung et al., 2008; Yazdi et al., 2010; Morishige et al., 2010; Baron et al., 2015), while in other cases they can interfere with the same mechanisms, resulting in decreased IL-1β production (Wu et al., 2013). Whether ENMs may also provide the first signal required for IL-1β production, i.e. gene upregulation, is not clearly established, again because the undetected presence of bioactive agents such as LPS (which is an excellent inducer of IL1B gene expression) may lead to misinterpretation of results. In fact, LPS-free ENMs do not seem able to induce IL1B gene expression and consequent IL-1β production and are also unable to induce the production of the major IL-1β inhibitor, the IL-1 receptor antagonist IL-1Ra (Li et al., 2016, 2017; Della Camera et al., 2021; Verde et al., 2021). Conversely, they seem able to modulate the generation of innate memory in monocytes/macrophages, with cells primed with ENMs (alone or together with other agents) producing different amounts of IL-1β and IL-1Ra compared to unprimed cells (Italiani & Boraschi, 2017; Lebre et al., 2020; Swartzwelter et al., 2020; Della Camera et al., 2021). A schematic representation of the interaction between human mononuclear phagocytes and ENMs is provided in Fig. 11.

VIII. CONCLUSIONS
- (1)
Nanoparticles interact with the host immune defensive mechanisms in a conceptually similar way to interactions that occur with microorganisms or other non-self/damaged-self materials. Since ENMs and other particles are not alive, the task for the innate immune system may be easier. Interactions, depending on the size, shape and surface properties of the particles, are not different from those with inert microbial/organic particulate agents, which will depend on the same properties. A biocorona that determines the interface between particles and the immune system also can form on the surface of microorganisms (opsonisation) and likewise determines their interaction with the immune system.
- (2)
Immunity has developed and adapted to recognise and react to microorganisms and other biotic and abiotic materials (ashes, dust, etc.), we thus can expect it to deal successfully with ENMs as well.
- (3)
In the animal kingdom, phagocytic cells seem to have a major role in interactions with ENMs and can efficiently recognise and remove them without causing irreparable damage. The role of TLRs in ENM recognition is unclear, although the patterned structure of ENMs may facilitate their interaction with PRRs. Unlike plants, which do not have specialised immune cells, most animals appear to activate phagocyte-related defensive mechanisms, with some common to all (e.g. phagocytosis, production of ROS) and others restricted to particular clades (e.g. production of IL-1 in vertebrates).
- (4)
Observations on the capacity of innate immune mechanisms to recognise and eliminate ENMs across metazoans suggest that innate immunity can successfully deal with ENMs, without causing permanent damage to the organism (Boraschi et al., 2020; Pinsino et al., 2020; Swartzwelter et al., 2021). In plants, interaction with ENMs appears to shift the growth/immunity balance towards enhanced growth. In animals, exposure to ENMs can trigger an inflammatory reaction in phagocytes, which may cause transient cell death and tissue damage responses. Since inflammation is a physiological defensive response with a limited duration and is concluded by resolution, tissue repair, and a return to homeostasis, this cannot be considered a toxic effect.
- (5)
Pathological consequences of ENM–immune interactions seem to be rare and due to either persistence of the triggering stimulus or anomalous development of the immune reaction, similar to many other infectious and immune-related diseases, such as autoimmunity or degenerative diseases. The effects of ENMs, similar to those of pathogens and other challenges, depend strongly on the type of host organism and its conditions. Thus, ENMs that are safe for humans may be toxic for marine crustaceans; and particles that are safe in young healthy individuals may cause chronic inflammation and damage in old or diseased individuals. The integrity and efficiency of body barriers and the performance of the immune system are key determinants in the ‘toxicity’ of ENMs (Boraschi, 2014), besides the material intrinsic characteristics. Thus, ‘safe-by-design’ ENMs are unlikely to exist, and the outcome of the ENM–organism interaction needs to be assessed case by case, in a strategy of ‘personalised nanosafety’.
ACKNOWLEDGEMENTS
This review is the result of a fruitful collaboration fostered by the H2020 project PANDORA, and we acknowledge the enthusiastic work and discussion with all colleagues involved in that project. We also wish to thank the PANDORA project officer Giuliana Donini for her continuous support for our studies. We thank the Cell Imaging facilities of IBBC-CNR and SZN for the TEM and SEM images of human monocytes and macrophages, and the EM Facility of CAS (Prague, Czech Republic; supported by project LO1509 of the Ministry of Education, Youth and Sports of the Czech Republic) for the TEM image of earthworm coelomocytes. D.D. acknowledges the support of the Slovenian Research Agency (P1-0207 and J1-9162). We are grateful to Tom Secrest (Secrest Editing s.r.o., Prague, Czech Republic) for editing services. We dedicate this work to our friend and colleague Valeria Matranga. We miss her insight and original approach to research, and know that her courage and passion for science will remain a valuable heritage for future generations.




