Quantitative meta-analysis reveals no association between mercury contamination and body condition in birds
[Corrections added on 1 March 2022, after first online publication: Affiliation for author Elena Golubovain has been corrected in this version.]
ABSTRACT
Mercury contamination is a major threat to the global environment, and is still increasing in some regions despite international regulations. The methylated form of mercury is hazardous to biota, yet its sublethal effects are difficult to detect in wildlife. Body condition can vary in response to stressors, but previous studies have shown mixed effects of mercury on body condition in wildlife. Using birds as study organisms, we provide the first quantitative synthesis of the effect of mercury on body condition in animals. In addition, we explored the influence of intrinsic, extrinsic and methodological factors potentially explaining cross-study heterogeneity in results. We considered experimental and correlative studies carried out in adult birds and chicks, and mercury exposure inferred from blood and feathers. Most experimental investigations (90%) showed a significant relationship between mercury concentrations and body condition. Experimental exposure to mercury disrupted nutrient (fat) metabolism, metabolic rates, and food intake, resulting in either positive or negative associations with body condition. Correlative studies also showed either positive or negative associations, of which only 14% were statistically significant. Therefore, the overall effect of mercury concentrations on body condition was null in both experimental (estimate ± SE = 0.262 ± 0.309, 20 effect sizes, five species) and correlative studies (−0.011 ± 0.020, 315 effect sizes, 145 species). The single and interactive effects of age class and tissue type were accounted for in meta-analytic models of the correlative data set, since chicks and adults, as well as blood and feathers, are known to behave differently in terms of mercury accumulation and health effects. Of the 15 moderators tested, only wintering status explained cross-study heterogeneity in the correlative data set: free-ranging wintering birds were more likely to show a negative association between mercury and body condition. However, wintering effect sizes were limited to passerines, further studies should thus confirm this trend in other taxa. Collectively, our results suggest that (i) effects of mercury on body condition are weak and mostly detectable under controlled conditions, and (ii) body condition indices are unreliable indicators of mercury sublethal effects in the wild. Food availability, feeding rates and other sources of variation that are challenging to quantify likely confound the association between mercury and body condition in natura. Future studies could explore the metabolic effects of mercury further using designs that allow for the estimation and/or manipulation of food intake in both wild and captive birds, especially in under-represented life-history stages such as migration and overwintering.
I INTRODUCTION
Chemical pollution is a major anthropogenic modification of the global environment, and a fundamental characteristic of the Anthropocene (Lewis & Maslin, 101). Humans are responsible for the synthesis and release of a plethora of chemical contaminants for agricultural uses [e.g. organochlorine and organophosphate pesticides (Jones & De Voogt, 85; Sánchez-Santed, Colomina & Herrero Hernández, 139)], and industrial or every-day life applications [e.g. metallic trace elements, perfluoroalkyl substances, chlorinated paraffins (Walker et al., 176; Sunderland et al., 162; Vorkamp et al., 174)]. Among these contaminants, and despite being a natural element, mercury (Hg) is particularly hazardous to humans and wildlife because it has no biological function and is highly toxic even at low concentrations (Walker et al., 176; UN Environment, 171). In its inorganic form, Hg has an atmospheric lifetime of 0.5–1 year and can be transported over vast spatial scales (Obrist et al., 117; UN Environment, 171). Once deposited on the Earth's surface, Hg can undergo complex, microbially mediated processes and be converted into methylmercury (MeHg), which is assimilated and accumulated by living organisms, and biomagnifies in food webs (Atwell, Hobson & Welch, 14; Evers et al., 48; Eagles-Smith et al., 41).
Anthropogenic Hg emissions have declined in North America and Europe over the past two decades (UN Environment, 171), but are still increasing in East Asia and in the Southern Hemisphere (Obrist et al., 117). In addition, concentrations measured in wildlife are still increasing in several regions of the Northern (Braune et al., 24; Wang et al., 177) and Southern Hemispheres (Mills et al., 114; Seco et al., 150). The Minamata Convention, an international treaty that came into force in 2017 (http://www.mercuryconvention.org), adopted a global strategy to reduce Hg emissions and protect human and environmental health. While these global restrictions may limit increases in future Hg emissions linked to economic growth, legacy Hg emissions will continue to affect the Hg cycle for decades to centuries (Eagles-Smith et al., 41). Monitoring Hg concentrations and effects in biota and the environment is thus a priority to oversee the effectiveness of the Minamata Convention (Evers et al., 48).
Adverse effects of Hg in humans and wildlife include neurological, endocrine, and immune disruption with consequences on development, neurocognitive function, and reproduction (Tchounwou et al., 169; Heinz et al., 73; Tan, Meiller & Mahaffey, 164; Tartu et al., 168; Goutte et al., 61; Eagles-Smith et al., 41; Evers, 47). Several intrinsic and extrinsic factors drive variation in Hg contamination and kinetics in wild organisms. Feeding ecology is a key explanatory factor of among- and within-species variation in tissue Hg concentrations (Anderson et al., 12; Carravieri et al., 29, 32; Polito et al., 124; Ma et al., 105) but other traits such as sex and age can also modulate this variation (Eagles-Smith et al., 40; Robinson, Lajeunesse & Forbes, 136; Jackson et al., 81; Chételat et al., 33). In addition, life-history traits such as breeding or migration strategies can influence diet, feeding rate, energy storage and expenditure, thus driving variation in Hg burdens (Seewagen, Cristol & Gerson, 153; Ackerman, Hartman & Herzog, 3; Adams et al., 6). All these intrinsic and extrinsic factors thus have the potential to modulate Hg toxicity. However, identifying sublethal effects of Hg in the field can be challenging, due to the potentially confounding influence of concurring environmental stressors (Marcogliese & Pietrock, 109; Marteinson, Marcogliese & Verreault, 110; Bårdsen, Hanssen & Bustnes, 15). Studies have shown species-specific sensitivity to Hg toxicity [e.g. embryotoxicity in birds (Heinz et al., 73); neuroreceptor inhibition in mammals (Basu et al., 17)], but the key phylogenetic and life-history traits or environmental factors that could explain these differences have yet to be identified clearly. Meta-analytical approaches quantifying the link between Hg and health endpoints in a large number of taxa could be effective in identifying the interactive factors that limit our capacity to detect significant effects of Hg on wildlife health.
Here, we performed a meta-analysis investigating the effect of Hg contamination on body condition using information extracted from 47 studies on 147 species of birds. Body condition indices are available in a large number of studies and species, and they can be calculated from morphometric measures that are used routinely in avian investigations. Albeit widely used, body condition indices may not be reliable indicators of health status (Fischer, Taborsky & Dieckmann, 52; Schultner et al., 146). Here, we consider body condition as an integrative measure of fat and lean mass (Peig & Green, 121; Labocha & Hayes, 98) that can be affected linearly or non-linearly by stressors (Pravosudov & Grubb, 125; Schultner et al., 146), for example via changes in behaviour (e.g. feeding performance) and/or physiology (e.g. disruption of nutrient metabolism and energy use). We chose birds as the study taxon because they have served widely as early sentinels of negative effects of Hg on wildlife and ecosystem health (Wolfe, Schwarzbach & Sulaiman, 188; Whitney & Cristol, 186). Birds are ubiquitous, being found in a large variety of terrestrial, freshwater and marine habitats from polar regions to the tropics. In addition, they belong to different dietary guilds, from herbivores to omnivores, are relatively accessible compared to other groups of vertebrates, and are thus extensively studied (Konishi et al., 92). Our aim was twofold: (i) to test for a systematic trend in the effect of Hg on body condition in birds; and (ii) to identify moderators of this relationship among intrinsic (e.g. species, age class, sex) and extrinsic factors (habitat type, dietary guild), as well as methodological aspects (tissue used to measure Hg concentrations, correlative or experimental approach). As Hg can affect bird behaviour [e.g. reduced ability to forage and/or compete for food (e.g. Evers et al., 49)], metabolic rates (Gerson, Cristol & Seewagen, 59), and physiological pathways involved in the stress response (Wada et al., 175; Franceschini et al., 56), which can have a range of contrasting consequences for physical condition, we expected either a positive or negative relationship between Hg concentrations and body condition. Given Hg biomagnification and naturally high bioavailability in aquatic environments (Atwell et al., 14; Fitzgerald, Lamborg & Hammerschmidt, 53), piscivorous species can be at greater risk of Hg exposure (Scheuhammer et al., 145), and are thought to have developed a better tolerance to toxicity over evolutionary timescales [e.g. through more efficient detoxification mechanisms (Robinson et al., 135; Manceau et al., 108)]. Piscivorous species might therefore show a smaller effect size of the Hg–body condition association.
II MATERIALS AND METHODS
(1) Search and inclusion criteria
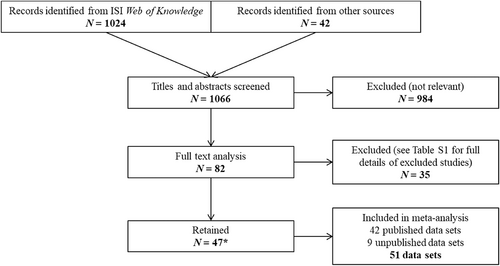
Our literature search was conducted in ISI Web of Science (latest search 22/01/2021) across all years, using the search terms “mercury”, “bird” and “body condition”. Since body condition is often calculated in studies of the effect of Hg on physiological and fitness endpoints without being the primary objective, we also searched the literature using the terms “mercury”, “bird” and one of the following key words: “effect”, “health”, “telomere”, “oxidative stress”, “hormone”, “corticosterone”, “testosterone”, “thyroid”, “immunity”, “parasite”, “DNA damage”, “energy”, “hatching/fledging/breeding success”, “hatch date”, “clutch size”, “foraging behaviour”, “survival”, “growth” and “body mass”. The reference lists of literature reviews found in this way were also checked to expand the database (Fig. 1). We contacted the authors of studies to obtain further details when (i) statistical information was missing, and/or (ii) the effect size was calculated across several species. Incomplete statistical information and a lack of a response by contacted authors meant that some relevant studies had to be discarded (see online Supporting Information, Table S1). The literature search resulted in the selection of 47 studies spanning publication years 2000 to 2020 (Table 1). These covered data mainly on passerines and seabirds with a few waders and raptors (Table 1). In addition, we included nine studies from the authors’ unpublished work in order to reduce the bias towards passerine birds. Overall, our meta-analysis included 147 species of birds, which were mainly passerines and seabirds. Inclusion criteria are detailed below.
| Reference* | Year | Location | Species | ES | Tissue | Age class | Condition type |
|---|---|---|---|---|---|---|---|
| Correlative studies | |||||||
| Ackerman et al. (3) | 2012, 2013 | Central Valley of California | 40 passerine species | 78 | Blood & Feather | Adult | Body condition |
| Adams et al. (6) | 2013–2017 | Multiple sites in New York State, USA | 54 passerine species | 54 | Blood | Adult | Body condition |
| Adams et al. (7) | 2009–2012 | Bill Baggs Cape Florida State Park | 8 passerine species | 12 | Blood | Adult | Body condition |
| Amélineau et al. (11) | 2004–2015 | East Greenland | Alle alle | 1 | Feather | Adult | Body condition |
| Unpublished O.G., G.Y.* | 2018, 2019 | North-East Greenland | Pagophila eburnea | 2 | Blood | Adult | Body condition |
| Unpublished E.G.* | 2017, 2018 | Talan Island, Sea of Okhotsk, Russia | 2 seabird species | 2 | Feather | Adult | Body condition |
| Unpublished H.S.* | 2015–2018 | Bjornoya Island, Barents Sea, Norway | 4 seabird species | 7 | Blood & Feather | Adult | Body condition |
| Unpublished A.P.W., A.K.* | 2015, 2016 | Saint Lawrence Island, Bering Sea | 6 seabird species | 11 | Blood & Feather | Adult | Body condition |
| Carravieri et al. (31) | 2011–2014 | Dronning Maud Land, Antarctica | Thalassoica antarctica | 2 | Blood | Adult | Body mass |
| Carravieri et al. (26) | 2018 | Isle of May, Firth of Forth, Scotland | Phalacrocorax aristotelis | 2 | Blood | Adult | Body condition |
| Eckbo et al. (42) | 2015 | Svalbard, Norwegian Arctic | Cepphus grylle mandtii | 1 | Blood | Adult | Body condition |
| Unpublished J.F.* | 2019 | Tromelin & Ile dy Lys, Indian Ocean | 3 seabird species | 6 | Blood | Adult | Body condition |
| Gurney et al. (62) | 2002, 2003 | Redberry Lake, Canada | Melanitta fusca | 1 | Blood | Adult | Body mass |
| Hargreaves et al. (65, 66) | 2009 | Southampton Island, Nunavut | 6 wader species | 7 | Blood | Adult | Body condition |
| Unpublished K.L.* | 2017 | Karrak Lake, Nunavut | 2 wader species | 2 | Blood | Adult | Body mass |
| Kojadinovic et al. (90) | 2004 | Multiple sites, Western Indian Ocean | Onychoprion fuscatus | 1 | Feather | Adult | Body mass |
| Provencher et al. (126) | 2013, 2014 | Mittivik Is, Northern Hudson Bay, Canada | Somateria mollissima | 1 | Blood | Adult | Body condition |
| Rowse et al. (137) | 2011, 2012 | Upper Scioto River, Ohio, USA | Empidonax virescens | 1 | Blood | Adult | Body condition |
| Scheuhammer et al. (144) | 2008 | Manitoba & Saskatchewan, Canada | Gavia immer | 4 | Blood & Feather | Adult | Body mass |
| Seewagen (151) | 2008, 2009 | New York, USA | Parkesia noveboracensis | 1 | Blood | Adult | Body mass |
| Unpublished C.L.S.* | 2007, 2008 | New York, USA | Hylocichla mustelina | 1 | Blood | Adult | Body mass |
| Soldatini et al. (157) | 2017 | Natividad Island, Mexico | Puffinus opisthomelas | 1 | Blood | Adult | Body condition |
| Tartu et al. (168) | 2008, 2011 | Svalbard, Norwegian Arctic | Rissa tridactyla | 2 | Blood | Adult | Body condition |
| Tartu et al. (167) | 2008 | Terre Adéie, Antarctica | Pagodroma nivea | 2 | Blood | Adult | Body condition |
| Tartu et al. (165) | 2010 | Terre Adélie, Antarctica | Pagodroma nivea | 2 | Blood | Adult | Body condition |
| Tartu et al. (166) | 2012, 2013 | Svalbard, Norwegian Arctic | Rissa tridactyla | 2 | Blood | Adult | Body condition |
| Albertos et al. (10) | 2005–2020 | Alicante, Spain | 2 seabird species | 2 | Feather | Adult | Body mass |
| Ackerman et al. (4) | 2006–2010 | South San Francisco Bay, USA | Rallus longirostris obsoletus | 2 | Blood & Feather | Adult | Body condition |
| Wayland et al. (179, 180, 183, 181) | 1997, 1998 | East Bay Migratory Bird Sanctuary, Nunavut | Somateria mollissima | 1 | Blood | Adult | Body condition |
| Lerma et al. (100) | 2011, 2012 | Bahia Santa Maria, Sinaloa, Mexico | Sula nebouxii | 3 | Blood | Adult & Chick | Body condition |
| Unpublished P.B., A.C., O.C., Y.C.* | 2012 | French Austral Territories, Southern Ocean | 15 seabird species | 52 | Blood & Feather | Adult & Chick | Body condition |
| Sebastiano et al. (149) | 2013 | Grand Connetable, French Guyana | 6 seabird species | 11 | Blood | Adult & Chick | Body condition |
| Weech et al. (184) | 2000–2002 | lakes, British Columbia | Haliaeetus leucocephalus | 2 | Blood | Adult & Chick | Body mass |
| Clarkson et al. (34) | 2009, 2010 | Virginia and New York, USA | 1 seabird, 1 wader species | 2 | Feather | Chick | Body mass |
| Costantini et al. (35) | 2016–2018 | Linosa, Italy | Calonectris diomedea | 2 | Blood | Chick | Body mass |
| Carravieri et al. (28) | 2012 | French Austral Territories, Southern Ocean | 13 seabird species | 13 | Blood | Chick | Body condition |
| Unpublished P.B., J.F.* | 2015–2017 | French Coast, English Channel | 4 seabird species | 8 | Blood & Feather | Chick | Body condition |
| Carravieri et al. (30) | 2012 | French Austral Territories, Southern Ocean | 2 seabird species | 3 | Blood | Chick | Body condition |
| Herring et al. (75) | 2006, 2007 | Everglades, Florida | 2 wader species | 2 | Blood | Chick | Body condition |
| Kojadinovic et al. (91) | 2002–2004 | Reunion Island, Western Indian Ocean | 2 seabird species | 2 | Feather | Chick | Body condition |
| Ortiz-Santaliestra et al. (118) | 2010, 2011 | Castilla and Leon, Andalusia, Spain | Aquila fasciata | 4 | Blood | Chick | Body condition |
| Santos et al. (140) | 2012, 2013 | Belgium | Larus fuscus | 1 | Feather | Chick | Body condition |
| Santos et al. (141) | 2015 | Ostend, Belgium | Larus fuscus | 1 | Feather | Chick | Body mass |
| Experimental studies | |||||||
| Seewagen et al. (153); Gerson et al. (59) | 2015 | Laboratory | Taeniopygia guttata | 4 | Blood | Adult | Body mass |
| Kobiela et al. (89) | 2014 | Laboratory | Taeniopygia guttata | 2 | Blood | Adult | Body condition |
| Seewagen et al. (155) | 2016–2017 | Long Point, Lake Erie, Canada | Setophaga coronata | 1 | Blood | Adult | Body condition |
| Ma et al. (106) | 2014–2017 | Long Point, Lake Erie, Canada | Setophaga coronata | 4 | Blood | Adult | Body mass |
| Kenow et al. (87) | 1999, 2000 | Laboratory | Gavia immer | 1 | Blood | Chick | Body condition |
| Fallacara et al. (50) | 2005 | Laboratory | Falco sparverius | 2 | Blood | Chick | Body condition |
| Spalding et al. (159) | 1996 | Everglades, Florida | Ardea alba | 2 | Blood | Chick | Body condition |
| Yu et al. (189) | 2015 | Laboratory | Taeniopygia guttata | 4 | Blood | Chick | Body condition |
- ES, number of effect sizes.
- * Authors involved in unpublished work, in alphabetical order: Paco Bustamante (P.B.), Alice Carravieri (A.C.), Olivier Chastel (O.C.), Yves Cherel (Y.C.), Jérôme Fort (J.F.), Olivier Gilg (O.G.), Elena Golubova (E.G.), Alexander Kitaysky (A.K.), Katelyn Luff (K.L.), Chad L. Seewagen (C.L.S.), Hallvard Strøm (H.S.), Alexis P. Will (A.P.W.), Glenn Yannic (G.Y.).
(a) Body condition indices
where Mi and Li are the body mass and the body length measure of individual i, respectively; the exponent bSMA is estimated by the standardised major axis (SMA) regression of log body mass on log body length; L0 is an arbitrary value of body length, and Ṁi is the predicted body mass for individual i when the body length is standardised to L0. We calculated L0 as the arithmetic mean of the body length variable chosen for each study. As different measures of body length (e.g. tarsus, bill, and wing length) can scale differently with body mass depending on species, we used the body size measure selected by the authors to calculate L0 and thus SMI. When no preference was communicated, and several body length measures were available, we used tarsus or bill length rather than wing length, which is difficult to measure reproducibly and can be a poor indicator of structural body size (Jenni & Winkler, 83). The choice between tarsus or bill length was made by taking the measure that correlated better with body mass on a log basis, as this is likely to be the best one explaining the fraction of mass associated with structural size (Peig & Green, 121).
(b) Hg chemical form
The form of Hg in avian diets may vary depending on prey types. High-trophic-level prey, such as fish and squid, mainly contain Hg as MeHg, whereas invertebrates may have higher proportions of inorganic Hg (Bloom, 20; Mason, Laporte & Andres, 111; Bustamante et al., 25; Seco et al., 150). All included studies measured total Hg, which is an accurate proxy of MeHg in tissues such as blood and feathers (e.g. Bond & Diamond, 22; Renedo et al., 132).
(c) Exposure pathway and measure
We included experimental studies where adults and chicks were exposed to Hg via dietary exposure as MeHg, as well as MeHg egg injections. We considered studies where Hg contamination was inferred from concentrations [in parts per million (ppm) or μg per unit wet or dry mass) measured in whole blood, red blood cells, and feathers, in adult birds and chicks. We excluded effect sizes obtained from other tissues given their heterogeneity and small number (Table S1). In experimental studies all Hg concentrations were reported on a wet mass basis. By contrast, the majority of correlative studies reported Hg concentrations on a dry mass basis. In order to provide homogenous Hg concentration estimates, correlative studies presenting results on a wet mass basis were converted to dry mass. The latter calculation was based on a moisture content of 65% in red blood cells (P. Bustamante & O. Chastel, unpublished data), and 77% in whole blood [mean of moisture values measured in Eagles-Smith et al. (39), Ackerman, Hartman & Herzog (2) and Ackerman et al. (3)]. Blood is a better representative of Hg body burden than are feathers in birds, and should be preferred in toxicity risk estimations (Fuchsman et al., 57; Chételat et al., 33). In addition, Hg temporal integration into feathers can be highly variable depending on species, feather type, moult strategy and moult stage at sampling (Carravieri et al., 27; Albert et al., 9; Peterson et al., 122). However, in some species, feather Hg concentrations correlate well with concentrations in internal tissues, including blood, and can thus also be useful representatives of Hg body burdens (Ackerman et al., 4, 3; Fort et al., 55). Therefore, we decided to include studies reporting associations between feather Hg concentrations and body condition in our meta-analysis, and test tissue type (blood or feather) as a moderator. Body feathers are preferentially sampled for ethical and practical reasons, and, to avoid further heterogeneity in the data set, we excluded the few effect sizes obtained from other feather types (Table S1).
(d) Other criteria
Studies comparing body condition indices between populations at polluted sites where pollution was not clearly Hg-related were also excluded, as well as studies on dead, emaciated individuals (Table S1), where body condition could be biased. Effect sizes obtained from less than four individual birds were discarded.
(2) Moderators included and categorisation
Our meta-analysis included a maximum of 15 moderators: (i) bird type (passerine, raptor, seabird, wader); (ii) age class (chick, adult); (iii) whether the effect size had been corrected for sex; (iv) whether the effect size had been corrected for other factors that were specific to the study (e.g. individual random factor, sampling date, season); (v) dietary guild (carnivore, herbivore, invertivore, omnivore); (vi) habitat type (freshwater, marine, terrestrial); (vii) geographical zone (polar/subpolar, temperate, tropical/subtropical); whether the sampled population was (viii) wintering (yes/no), (ix) migrating (yes/no) or (x) breeding (yes/no); (xi) tissue type used for Hg quantification (blood, feather); (xii) body condition index (body mass, size-corrected body mass); (xiii) species-specific basal metabolic rate (BMR); (xiv) Hg concentration; (xv) ratio of species-specific maximum body mass to average body mass (hereafter BM ratio, which reflects the maximum body condition of the species; Vincze et al., 173). All moderators, their modalities, the number of effect sizes, and the justification for including them to study the link between Hg concentration and body condition are reported in Table 2. Dietary guild was assigned based on results in the sampled population of each study when available, or from the Wilman et al. (187) database. Species-specific BMR values were extracted from Ellis & Gabrielsen (46), Møller (115), Londoño et al. (103) and McKechnie, Noakes & Smit (112), but were not available for all species. Body mass information to calculate the BM ratio was extracted from the Dunning (38) database, or from additional references (Glahn & McCoy, 60; Shirihai et al., 156; Kooyman et al., 93; Helseth, Stervander & Waldenström, 74; Tobón & Osorno, 170; Kojadinovic et al., 90; Overton et al., 119; Hancock, Kushlan & Kahl, 1992; Rising, 133; García, Moreno-Opo & Tintó, 58; Maccarone & Brzorad, 107; studies included in the meta-analysis). For each study, we extracted the moderators, as well as sample and effect sizes as detailed below.
| Moderator | Modalities (number of effect sizes)a | Expected influence on the Hg–body condition association |
|---|---|---|
| Bird typeb | Passerine (145; 15), Raptor (6; 2), Seabird (150; 1), Wader (14; 2) | Physiological or life-history related differences in sensitivity to Hg |
| Age class | Adult (256; 11), Chick (59; 9) | Age-related sensitivity to Hg |
| Accounted for sex | Yes (18; 5), No (297; 15) | Sex-related sensitivity to Hg |
| Accounted for other variables | Yes (6; 11), No (309; 9) | Accounting for other confounding factors may modulate effect sizes |
| Dietary guild | Carnivore (114; 5), Herbivore (25; 10), Invertivore (143; 5), Omnivore (33; 0) | Potential exposure to different Hg chemical forms |
| Habitat typeb | Freshwater (9; 1), Marine (153; 0), Terrestrial (153; 19) | Potential exposure to different Hg chemical forms |
| Zoneb | Polar/subpolar (105; 0), Temperate (156; 18), Tropical/subtropical (54; 2) | Different energetic demands may change susceptibility to Hg |
| Winteringb | Yes (16; 0), No (299; 20) | Physiological status, energetic demands and behaviour of different life-history stages may change susceptibility to Hg |
| Migrating | Yes (41; 5), No (274; 15) | |
| Breedingb | Yes (197; 0), No (118; 20) | |
| Tissueb | Blood (213; 20), Feather (102; 0) | Tissue-specific temporal integration of Hg |
| Body condition indexb | Body mass (19; 17), Size-corrected body mass (296; 3) | Index-associated influence on the Hg–body condition relationship |
| Covariate | Unit (number of effect sizes) | |
| Hg concentration | μg/g (315; 20) (dw and ww for correlative and experimental studies, respectively) | Concentration-dependent susceptibility to Hg |
| BMR | kJ/day (132; 17) | Energetic needs may influence the susceptibility to Hg |
| BM ratio | No unit (315; 20) | Potential effect of fat load on body condition |
- BM, body mass; BMR, basal metabolic rate; dw, dry mass; ww, wet mass.
- a The first and second number are for correlative and experimental studies, respectively.
- b Moderators not included in meta-analytic models of experimental studies given the limited sample size.
(3) Effect size extraction or calculation
Given that most studies were correlative, we chose Pearson's r as the effect size. Based on the information given in correlative studies, we calculated effect sizes using t-values, F-values, P-values, means and standard deviations, or correlation coefficients, following formulae given in Koricheva, Gurevitch & Mengersen (95). For studies reporting the comparison of experimental groups, we calculated standardised mean differences (Cohen's d), which were then transformed to Pearson's r, following Koricheva et al. (95). To adhere to normality assumptions, Pearson's r were then converted to Fisher's Zr following the equation in Lipsey & Wilson (102). Sampling variances associated Z-scores were calculated as (n–3)−1 following Koricheva et al. (95). Positive effect sizes indicate a positive effect of Hg on body condition, while negative effect sizes denote a decrease in body condition with increasing Hg concentrations.
(4) Meta-analytic technique
In a preliminary step, we conducted a meta-analysis on the full data set including 335 effect sizes and a study type moderator (correlative or experimental) (results not shown). However, experimental studies appeared to be highly influential in the effect estimates, because of extreme values and high leverage. Therefore, we carried out separate meta-analyses for correlative and experimental data sets.
(a) Potential bias in effect size reporting
To test for the presence of potential bias in the results of our meta-analysis, we first explored funnel plots produced between effect sizes and their corresponding measure of precision (here standard error, SE) for signs of asymmetry. Second, we performed Egger's regression tests (Egger et al., 43) using overall intercept-only models with the regtest function (metafor package, v. 2.4-0; Viechtbauer, 172). Egger's test evaluates the relationship between effect sizes and measurements of study precision (Egger et al., 43), and can reveal different types of bias, such as reporting bias or poor methodological quality (Sterne et al., 160). For instance, studies reporting a significant effect may be more likely to be published than studies reporting no effect (Koricheva et al., 95; but see Koricheva, 94). Here, most effect sizes for correlative studies, and all effect sizes for experimental studies, were extracted from published resources (Fig. 1). Our meta-analysis also included unpublished resources for the correlative data set. We thus tested (i) potential bias in published and unpublished studies separately, as well as combined; (ii) whether the publication status (used as a moderator) of the effect size had an effect on the meta-analytical results.
(b) Random structure and overall effect of Hg on body condition
All meta-analytic models were performed using the rma.mv function in the metafor package (v. 2.4-0; Viechtbauer, 172) in R (v. 4.0.5; R Core Team, 128). We constructed multilevel meta-analytic linear mixed effect models, which facilitates the control of multiple sources of non-independence. Our data were affected by multiple types of non-independence (Noble et al., 116), including non-independence of effect sizes, multiple effect sizes originating from the same studies, non-independence of observations of the same species, as well as non-independence of species (shared ancestry). We aimed to control for these dependencies by testing the effect of three random variables: individual effect size identity (individual effect size ID, unique per data row, necessary to estimate residual heterogeneity; Noble et al., 116), study identity (study ID), and species identity (species ID). In the correlative data set, we also tested the influence of the phylogenetic variance–covariance matrix representing the phylogenetic history of the species. For the latter, we used a rooted ultrametric consensus tree that was inferred from the SumTrees Python library (Sukumaran & Holder, 161), based on 1000 random trees obtained from birdtree.org (Jetz et al., 84), using the Hackett backbone tree (Hackett et al., 63). Phylogeny was not accounted for in the experimental data set, as including phylogenetic random effects with less than 15 species can lead to unreliable estimates (Bolker et al., 21). To select the appropriate random structure, we constructed intercept-only meta-regression with all combinations of the three random variables, as well as the phylogenetic signal for correlative studies, using the maximum-likelihood (ML) method. We then compared the models using the Akaike Information Criterion (AIC) and chose the random structure of the model with the lowest AIC value, and lowest number of variables when AIC values were similar between models (parsimony criteria) (Table S3). Using the random structure of the best selected model, we tested the overall effect size of Hg contamination on body condition using restricted maximum likelihood approximation (REML). We also calculated the heterogeneity statistic (I2total) based on an intercept-only meta-analytic model (built using the rma function in metafor), without any random effects. I2 represents the percentage heterogeneity in the effect sizes (0–100%) due to true heterogeneity rather than random sampling variance (Higgins & Thompson, 76; Higgins et al., 77). I2 was moderate to high (defined as I2 > 50%; Higgins et al., 77; see Section III), therefore we ran moderator analyses to explore which life-history, ecological or physiological parameters could explain the high heterogeneity observed in the effect of Hg contamination on body condition in birds.
(c) Moderator analysis
In order to study the influence of different moderators on the association between Hg contamination and body condition, we constructed multifactorial meta-analytic models using the MuMIn package (Bartón, 16). Chicks and adults, as well as blood and feathers, are known to behave differently in terms of Hg accumulation and health effects (e.g. Whitney & Cristol, 186), and could show different associations between Hg and body condition. Therefore, their single and interactive effects were always accounted for in models of the correlative data set, as follows: Z score ~ age class + tissue type + age class*tissue type + Moderator (Table S4). Models were constructed with the selected random structure (see Sections II.4b and III), fitted using ML, and compared to the base model: Z score ~ age class + tissue type + age class*tissue type. In preliminary steps the age class ‘juveniles’ was considered, but data were too scarce to enable inclusion in the final meta-analysis. The available data on body condition and Hg concentrations in blood or feathers of juveniles were pooled with those of adults. We considered that a moderator had a significant effect on the association between Hg and body condition when its addition to the base model decreased the AIC corrected for small sample size (AICc) by at least 2. Data on BMR were available only for a subset of observations. To test the effect of BMR on the association between Hg and body condition we thus constructed a separate set of models (Table S4). Using these models, we obtained parameter estimates for each predictor after refitting models with REML.
The experimental data set was too small for multifactorial statistical analysis (N = 20 effect sizes). Therefore, each moderator was entered as a single predictor to an intercept-only multilevel meta-analytic model (fitted by REML) with the selected random structure (Table S5). Using these models, we obtained parameter estimates for each predictor and factor level. The overall significance of each predictor was assessed using an omnibus test (Viechtbauer, 172).
III RESULTS
(1) Bias in effect size reporting
For the correlative data set, Egger's tests indicated a tendency for biased reporting towards negative effect sizes among published studies (Egger's test: N = 224, z = −1.932, P = 0.053), and a significant bias towards positive effect sizes among unpublished studies (Egger's test N = 91, z = 2.148, P = 0.032; see Table S6 for funnel plots). By contrast, we found no statistical evidence of bias in the correlative database combining published and unpublished effect sizes (Egger's test: N = 315, z = −0.707, P = 0.480). In addition, publication status had no effect on the Hg–body condition association (Table S6). Therefore, all further meta-analytic models were only run on the full correlative data set combining published and unpublished effect sizes.
We detected a tendency for biased reporting towards negative effect sizes in experimental studies (Fig. 2; Egger's test: N = 20, z = −1.894, P = 0.058), but this bias was non-significant.

(2) Correlative studies
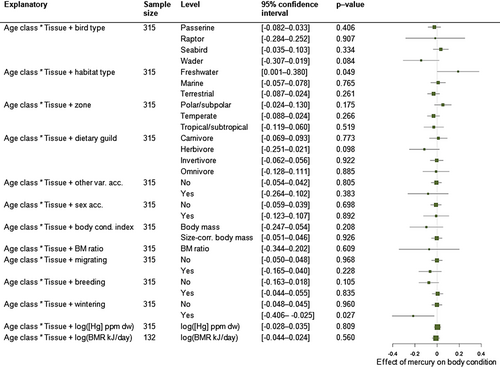
We used 315 population-specific effect sizes from 145 species to test the relationship between Hg concentration and body condition in correlative studies. These were carried out in wild populations, including mainly adult passerines and seabirds, from terrestrial and marine environments, in temperate and polar/subpolar regions (Table 2). The median number of individuals inspected per effect size was 16 (range: 4–1051). Comparison of intercept-only multilevel meta-analytic models indicated a significant increase in fit with the inclusion of individual effect size ID and species ID (Table S3), which were retained in the random structure of all subsequent models. Phylogenetic signal appeared to have little influence on the overall effect size and did not affect model fit. The overall effect of Hg contamination on body condition was non-significant (estimate ± SE = −0.011 ± 0.020, confidence interval, CI [−0.049; 0.027], while accounting for individual effect size ID and species ID). Effect size heterogeneity was moderate (I2 = 55%; Cochran's Q test = 668, df = 314, P < 0.0001), indicating suitability for moderator analyses. Multifactorial moderator analyses revealed that wintering status had an influence on effect size estimates (AICc 2.6 points lower than the base model, Table S4): wintering birds were more likely to show a negative effect of Hg on body condition (Fig. 3). Other moderators had no clear effects on the Hg–body condition association (Table S4).
(3) Experimental studies
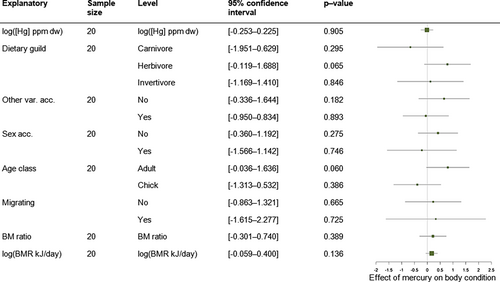
Experimental studies encompassed 20 effect sizes from five species (Ardea alba, Falco sparverius, Gavia immer, Setophaga coronata, Taeniopygia guttata). The median number of individuals inspected per effect size was 24 (range: 5–49). Only individual effect size ID was retained in the random structure of intercept-only multilevel meta-analytic models (Table S3). Hg contamination was not related to body condition (0.262 ± 0.309, [−0.344; 0.867], while accounting for individual effect size ID), and effect size heterogeneity was very high (I2 = 99%; Cochran's Q test = 887, df = 19, P < 0.0001). In the experimental data set, no moderator predicted the Hg–body condition relationship (Table S5, Fig. 4, P > 0.100 in all Omnibus tests).
IV DISCUSSION
Our extensive meta-analysis showed no overall effect of Hg contamination on body condition across 147 free-living or captive bird species. However, free-ranging wintering passerines were more likely to present a negative association between Hg concentration and body condition, but further studies are necessary to confirm this trend in other taxa. We found that waders and raptors, birds in freshwater habitats and tropical/subtropical regions, and especially migrating and overwintering birds, are under-represented in the literature on Hg contamination and body condition. Experimental studies were more likely to detect a significant effect of Hg concentrations on body condition, with 90% of estimated effect sizes being significantly positive or negative. Conversely, only 14% of correlative effect sizes for the effect of Hg contamination on body condition were significantly positive or negative. Although recent experimental studies exposed birds to environmentally realistic Hg doses, these were often in the upper range of levels encountered in the wild (Kobiela, Cristol & Swaddle, 89; Yu et al., 189; Ma et al., 106). Therefore, the difference in sensitivity to effects between correlative and experimental studies could stem from a threshold dose that can be reached under controlled conditions, but is unlikely in the wild.
(1) Overview of experimental and correlative studies
Accumulation of energy stores can be part of the response to stressors, whereby perceived risk or unpredictable access to food can cause birds to store energy as a buffer against unpredictable environmental changes [Schultner et al. (146) and references therein]. Experimental exposure to environmentally realistic Hg levels caused an increase in energy (fat) stores and body mass in zebra finch Taeniopygia guttata (Gerson et al., 59). Feeding rate or ingested food were not quantified, but birds were offered food ad libitum. Hence, their increase in energy stores was likely linked to an increase in food intake, as shown in mallard ducks Anas platyrhynchos exposed to Hg and fed ad libitum (Heinz, 71). Alternatively, Hg could increase energy storage by disrupting the metabolism of carbohydrates or lipids [Seewagen (152) and references therein]. However, in another experimental study, Hg-treated Taeniopygia guttata individuals waited longer to commence foraging and showed a significant decrease in their body mass, after exposure to predation risk (Kobiela et al., 89). Other experimental studies showed no effect of Hg on body condition despite reduced appetite, motivation to forage, and possibly low foraging efficiency (Bouton et al., 23; Spalding et al., 159; Adams & Frederick, 5). Overall, experimental studies point to disruption of feeding behaviour, and/or nutrient and energy metabolism, with positive, negative or no consequences on body condition. This suggests that metabolic effects of Hg may be weak, and thus become (statistically) detectable only at high exposure levels, and/or under specific conditions that could not be identified by the meta-analysis. Experimental data sets were only eight in number and suffered from a slight publication bias. Therefore, we cannot exclude that further experimental work in a larger sample of individuals and species could reveal a different picture.
Previous investigations and literature reviews highlighted substantial heterogeneity in the strength and direction of the effect of Hg concentrations on body condition in wild birds: studies reported significantly negative (e.g. Ackerman et al., 4, 3; Fort et al., 54; Adams et al., 6), positive (e.g. Kalisińska et al., 86), or no associations (e.g. Heath & Frederick, 67; Herring et al., 75; Tartu et al., 165). This heterogeneity could stem from the small statistical power of several ecotoxicological field investigations. Meta-analytical approaches can overcome this drawback and provide higher precision in the estimation of effect sizes (Koricheva et al., 95). However, our meta-analysis confirmed the lack of a clear pattern. The correlative data set included a large number of effect sizes, with a balanced distribution of modalities for most moderators, and a lack of bias, thus suggesting that the output of this meta-analysis including both published and unpublished data is robust. Interestingly, we detected a (publication) bias towards studies that show a negative effect of Hg on body condition, while positive effects were more likely to remain unpublished (Table S6).
Among the 15 tested moderators in correlative studies, only wintering status was identified as a driving factor of cross-study heterogeneity in results. Wintering birds were more likely to show a negative effect of Hg concentrations on body condition, suggesting a more detectable, negative effect when food is scarce and/or energetic demand for coping with unfavourable weather conditions is high. This effect was driven by two studies on several passerine species (Ackerman et al., 4, 3) and needs confirmation from other species. As discussed above for metabolic and behavioural effects in experimental settings, food intake and predation risk could be critical in driving effects of Hg concentration on body condition. Feeding rates, food availability and predation risk are challenging to measure in the wild, and could thus be key factors potentially confounding the Hg–body condition association in natura. In conclusion, there is a need for further studies that measure Hg–body condition associations while accounting for food intake and concurrent stressors (e.g. predation risk), especially at challenging life-cycle stages such as chick-rearing, migration, overwintering and moult.
(2) Potential confounding factors and directions for future studies
Results from our meta-analysis indicate that body condition indices are not sensitive endpoints of Hg sublethal effects in birds. In accordance with conclusions from Fuchsman et al. (57) and Evers (47), reproductive endpoints should be preferred to estimate Hg toxicity risk. Effects of Hg on reproductive success also have the advantage of being comparable between laboratory-based and field studies in similar taxa (Evers, 47). The results of our meta-analysis also refute our prediction of smaller effect sizes of the Hg–body condition association in piscivorous species, because of their naturally high Hg exposure over evolutionary timescales (Scheuhammer et al., 145; Evers, 47). The lack of sensitivity of body condition indices to Hg effects could stem from several non-exclusive factors, some of which are inherently linked to the concept of ‘body condition’. Body condition indices have been used as indicators of fat reserves, although not always explicitly so (reviewed in Labocha & Hayes, 98). However, body mass variation can be driven largely by lean mass, not only fat mass, especially in migrating birds (Piersma, Gudmundsson & Lilliendahl, 123; Seewagen & Guglielmo, 154). As such, body condition indices may be poor indicators of energy stores in species with intrinsically low percentage of body lipids (Jacobs et al., 82), and be no more informative than body mass alone (Labocha & Hayes, 98). Here, we found no effect of body condition index type on the Hg–body condition association (Tables S2 and S4). Previous studies have shown that it is complicated to draw generalisations on which body condition index best represents body condition, but that different indices are often correlated (Labocha et al., 99; Kraft et al., 96). We can speculate that if Hg had a clear impact on body condition in birds, effects would be detected irrespective of the index used, but further studies are needed to address this point specifically. In addition, body condition indices can vary substantially with season, sex and other factors, complicating comparisons among studies (Labocha & Hayes, 98; Labocha et al., 99). For instance, effects of Hg on body condition have been shown to depend on time of day in migrating passerines (Adams et al., 7), as body mass can fluctuate strongly across the day in small birds. To investigate further the potential role of energy storage and use on the relationship between Hg concentration and body condition, we tested the effect of BM ratio and BMR as moderators. The BM ratio is an indicator of maximum body condition and energy reserves (Vincze et al., 173), while BMR represents the energy needed for basal body maintenance [in a resting, post-absorptive phase, under thermoneutral conditions (McNab, 113; Ellis & Gabrielsen, 46; White et al., 185)]. Both species-specific BM ratio and BMR were poor predictors of the variation in the Hg–body condition association. However, only a third of the species included in the analysis had known BMR information, and BMR also can vary depending on other factors, such as temperature and latitude (Ellis & Gabrielsen, 46; White et al., 185), or the presence of other environmental contaminants such as persistent organic pollutants (Blévin et al., 19). The influence of energy storage strategies and BMR on the association between Hg contamination and body condition needs further investigation, and likely works at the individual level, which cannot be accounted for by meta-analytical approaches.
Physiological factors could also confound the relationship between Hg concentration and body condition in birds. An example of this is the potential mismatch between the temporal integration of Hg into feathers and the timing of body condition measures (see also Section II.1). In addition, the Hg–body condition relationship could reflect mechanisms for dilution (or concentration) of Hg in tissues following body mass gain (or loss). However, this has been shown only in two studies on healthy individuals [Hg dilution in blood in growing juvenile birds (Ackerman, Eagles-Smith & Herzog, 1); Hg concentration in blood in fasting passerines during simulated migratory fasting (Seewagen et al., 153)], and in seabirds that died from starvation (Fort et al., 54). Further evidence from multiple avian species is necessary to confirm whether adaptive changes in body mass and body mass composition, which are necessary to sustain energy-demanding activities such as moulting, migrating and breeding (Bech, Langseth & Gabrielsen, 18), could drive variation in circulating Hg concentrations. To this end, we encourage the use of other non-invasive indices of body condition, such as pectoral muscle thickness (a proxy of lean mass; e.g. Sears, 148), or body composition assessed via quantitative magnetic resonance (Seewagen et al., 153; Ma et al., 106), and to account for sampling time of day (Adams et al., 7).
Another possible factor confounding the Hg–body condition association could be selenium (Se) status. Se can play a protective role against Hg toxicity at the biochemical level (Cuvin-Aralar & Furness, 36; Ralston, Blackwell & Raymond, 129; Scheuhammer et al., 143). The formation of apparently nontoxic Hg–Se granules observed in wildlife after MeHg demethylation is considered to be the primary detoxification mechanism of MeHg, and enables long-term storage of Hg (Manceau et al., 108). However, the mutual sequestration of Hg and Se can be detrimental. Specifically, Hg can inhibit Se-dependent enzymes (selenoenzymes), which are critical for brain health function, especially in early life (Ralston et al., 130; Ralston, Ralston & Raymond, 131). Sublethal effects of Hg and the Hg–body condition association could thus be influenced by the presence and bioavailability of Se in the diet, but this is still understudied with respect to the toxic effects of Hg in avian species. Quantifying the Se:Hg molar ratio (Scheuhammer et al., 143), and/or a risk assessment criterion that accounts for concurrent intake of MeHg and Se (Se health benefit value; Ralston et al., 131), could improve our understanding of the sublethal effects of Hg in birds.
V CONCLUSIONS
- Our meta-analysis indicates that body condition is an unreliable endpoint of the sublethal effects of Hg in wild birds.
- Associations of Hg with body condition appear to be clearer under controlled conditions and further investigations are needed.
- Wintering birds were more likely to show a negative association between Hg and body condition in the wild, but further studies should confirm this in additional taxa.
- We highlight a substantial knowledge gap on the metabolic effects of Hg in waders and raptors, birds in freshwater habitats and from tropical/subtropical regions, and especially in migrating and overwintering birds.
- Our results indicate the need for further studies in both the laboratory and the field on the effects of Hg on feeding rates, foraging efficiency, and energy storage and use in a larger sample of individuals and species.
VI ACKNOWLEDGEMENTS
The authors declare that there are no conflicts of interest. We thank Gholam Alaie, Françoise Amélineau, Michael J. Anteau, Yuri V. Albores-Barajas, Charles Clarkson, Silvia Espín, Kirsty E. B. Gurney, Anna L. Hargreaves, Megan E. Kobiela, Miriam Lerma, Manuel E. Ortiz-Santaliestra, Linnea M. Rowse, Manrico Sebastiano, Sabrina Tartu, Kjetil Sagerup, Jennifer F. Provencher, and Shari A. Weech, for providing additional information and raw data from their published work. We also thank Mark Mallory and two anonymous reviewers for providing constructive comments that greatly improved this review. This study is a contribution to the projects ARCTIC-STRESSORS (ANR-20-CE34-0006), ILETOP (ANR-16-CE34-0005), PolarTOP (ANR-10-CESA-0016), ORNITHO-ECO (IPEV-109), ADACLIM (IPEV-388), ORNITHO-ENDOCRINO (IPEV-330), The Research Council of Norway (AVITOX), Project MULTISTRESS (Region Nouvelle Aquitaine), PIG-CNRS (SENTINEL), to the international initiative ARCTOX (arctox.cnrs.fr) and to the Excellence Chair ECOMM funded by the Region Nouvelle Aquitaine (France). O.V. was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences and by the New National Excellence Programme of the Hungarian Ministry of Innovation and Technology. The IUF (Institut Universitaire de France) is acknowledged for its support to P.B. as a senior member. J.T.A. was funded by the U.S. Geological Survey, Ecosystems Mission Area, Environmental Health Program (Contaminant Biology and Toxic Substances Hydrology). G.Y. and O.G. are funded by the French Polar Institute-IPEV (Program ‘IVORY-1210’). We thank Guillaume Evanno, Alex Hartman, Mark Herzog, and Matt Toney. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Hg data from Tromelin and Ile du Lys (Indian Ocean) were measured on subsamples of blood collected within the SPILE program (Camille Lebarbenchon). We thank Henri Weimerskirch for managing the CLIMOM program, C. Lebarbenchon, Matthieu Le Corre, Karen McKoy, David Grémillet and Céline Toty for their help in the field, as well as the Terres Australes et Antarctiques Françaises (TAAF) personnel for ensuring field logistics. St. Lawrence Island sampling was funded by the North Pacific Research Board (NPRB #1612-6) and the Japan Society for the Promotion of Science (KAKENHI, grant number JP16H02705), and the Arctic Challenge for Sustainability (ArCS) program of the Japan Ministry of Education, Culture, Sports, Science and Technology (MEXT). We thank Akinori Takahashi, Jean-Baptiste Thiebot, Michael Toolie and Punguk Shoogukwruk. Fieldwork was conducted under the following permissions/permits: Native Village of Savoonga, Kukulget Land Crossing Permit, UAF IACUC protocol #470122, USFWS scientific collection permit #MB70337A, master banding permit #23350, and ADFG permits #19-140, 18-131, 17-104, 16-089.




