Sensory-based conservation of seabirds: a review of management strategies and animal behaviours that facilitate success
ABSTRACT
Sensory-based conservation harnesses species' natural communication and signalling behaviours to mitigate threats to wild populations. To evaluate this emerging field, we assess how sensory-based manipulations, sensory mode, and target taxa affect success. To facilitate broader, cross-species application of successful techniques, we test which behavioural and life-history traits correlate with positive conservation outcomes. We focus on seabirds, one of the world's most rapidly declining groups, whose philopatry, activity patterns, foraging, mate choice, and parental care behaviours all involve reliance on, and therefore strong selection for, sophisticated sensory physiology and accurate assessment of intra- and inter-species signals and cues in several sensory modes. We review the use of auditory, olfactory, and visual methods, especially for attracting seabirds to newly restored habitat or deterring birds from fishing boats and equipment. We found that more sensory-based conservation has been attempted with Procellariiformes (tube-nosed seabirds) and Charadriiformes (e.g. terns and gulls) than other orders, and that successful outcomes are more likely for Procellariiformes. Evolutionary and behavioural traits are likely to facilitate sensory-based techniques, such as social attraction to suitable habitat, across seabird species. More broadly, successful application of sensory-based conservation to other at-risk animal groups is likely to be associated with these behavioural and life-history traits: coloniality, philopatry, nocturnal, migratory, long-distance foraging, parental care, and pair bonds/monogamy.
I. INTRODUCTION
Conservation biology traditionally focusses on reducing key threats such as habitat loss or overfishing (Soulé, 1985). More recently, conservation has begun to integrate evolutionary biology and the natural behaviours of target species (Crandall et al., 2000). Sensory ecology offers a powerful opportunity to influence animal behaviour for management purposes because acoustic, olfactory, and visual cues and signals are fundamental in many interactions with conspecifics, prey, predators, the environment, and human activities. However, sensory-based conservation efforts often produce variable results and can lack adequate controls. This popular and emerging sub-discipline of conservation biology merits rigorous review to improve success rates, and importantly, to facilitate application of effective techniques to a wider range of at-risk species by identifying key behavioural and life-history traits associated with successful conservation attempts.
Seabirds are excellent candidates for sensory-based conservation because they employ a range of sensory modes, display complex behaviours and cognition (Schreiber & Burger, 2001), and are often highly threatened (Croxall et al., 2012; IUCN, 2014; Paleczny et al., 2015). Seabirds have experienced steep recent population declines – 28% of the ∼347 seabird species are globally threatened and 5% are critically endangered (Croxall et al., 2012). Key orders include the Procellariiformes (tube-nosed seabirds), Sphenisciformes (penguins), Suliformes (gannets and boobies), some species of Charadriiformes (e.g. terns), Pelecaniformes (e.g. pelicans), and Phaethontiformes (tropicbirds). Of these orders, the Procellariiformes and Sphenisciformes are the most threatened, both on land and at sea (Croxall et al., 2012). On land, colonies succumb to residential and recreational development (Beatley, 1991), and even protected sites are nearly always threatened by physical disturbance or invasive predators (rats, cats, dogs, and mustelids; Burger & Gochfeld, 1994; Micol & Jouventin, 1995). At sea, one of the greatest threats is incidental bycatch by fishing vessels, especially when ensnared by hooked baits and longlines (Brothers, 1991; Murray et al., 1993; Tasker et al., 2000; Taylor, 2000; Bull, 2007; Richard, Abraham & Filippi, 2011). Estimates exceed 160000 seabirds killed per year across 68 fisheries (Anderson et al., 2011). These threats make seabird conservation a priority, ideally via practical, cost-effective techniques that can be applied generally across many species. Fortunately, seabirds' shared life-history traits and sophisticated sensory systems mean that sensory-based conservation techniques are a strategic option.
Traditionally, seabird conservation managers have focused on pest eradication, chick translocations, fisheries bycatch reductions, and social attraction to restored nesting sites (e.g. Parker et al., 2007; Jones & Kress, 2012). Although rarely explicitly recognized, sensory-based techniques are often used (Table 1) with variable success (see Section 4). Attracting birds to secure nesting habitat often involves broadcasting vocalizations or installing decoys to suggest a burgeoning colony (Kress, 1998; Gummer, 2003; Miskelly & Taylor, 2004; Table 1). Bycatch reduction techniques involve equipping fishing vessels with unpleasant sounds or vibrant visual banners, as well as changing the timing of line setting and hook weighting (Bull, 2007). Again, effectiveness varies (Table 1), especially based on species' responses to specific threats (for a review see Melvin et al., 1999). Despite the popularity of these sensory-based conservation efforts, there is little recognition that their success depends upon evolved behaviours and sensory ecology, and that measuring success requires comparison to unmanipulated controls.
| Goal | Family | Species | Management effort | Sensory mode targeted | Approach | Findings | Source |
|---|---|---|---|---|---|---|---|
| Attraction | Alcidae | Ancient murrelet, Synthliboramphus antiquus | Playback | Auditory | Experimental | Activity increased by 271% and 458 at two sites against standard activity | Major & Jones (2011) |
| Deterrent | Alcidae | Rhinocerous auklet, Cerorhinca monocerata | Acoustic pingers | Auditory | Experimental | No significant effect | |
| Visual nets | Visual | Experimental | Reduced bycatch 42% with deep visible deterrent | Melvin, Parrish & Conquest (1999) | |||
| Deterrent | Alcidae | Common murre, Uria aalge | Acoustic pingers | Auditory | Experimental | Reduced bycatch 50% | |
| Visual nets | Visual | Experimental | Reduced bycatch 40–45% with both deep and moderate visual cue | Melvin et al. (1999) | |||
| Attraction | Alcidae | Common murre, Uria aalge | Decoys, vocalizations and mirrors | Visual & auditory | Experimental | Colony from no breeding pairs to six in first year; more murres close to mirrors | Parker et al. (2007) |
| Deterrent | Diomedeidae | Albatross sp. | Streamer lines | Visual | Observational | Streamers reduced baits taken by 60.6% | Brothers (1991) |
| Attraction | Diomedeidae | Laysan albatross, Diomedea immutabili | Playback and visual stimuli | Visual and auditory | Experimental | Combination of visual and auditory stimuli more successful than visual alone | Podolsky (1990) |
| Attraction | Hydrobatidae | Leach's storm-petrel, Oceanodroma leucorhoa | Playbacks | Auditory | Experimental | 49× more caught in response to conspecific calls, 29× more caught in response to colony calls | |
| Scented material | Olfactory | Experimental | No significance in orienting to odour | Buxton & Jones (2012) | |||
| Attraction | Hydrobatidae | Fork-tailed storm-petrel, Oceanodroma furcata | Playbacks | Auditory | Experimental | 20× more caught in response to conspecific calls, 5× more to colony calls | |
| Scented material | Olfactory | Experimental | Significantly orient to odour 69% of time | Buxton & Jones (2012) | |||
| Attraction | Laridae | Audouin's gull, Ichthyaetus audouinii | Decoys and impaired gulls | Visual | Observational | Limited-success translocation with some visitation | Oro et al. (2011) |
| Attraction | Procellariidae | Fluttering shearwater, Puffinus gavia | Playback and translocated chicks | Auditory | Observational | Method used, but not focus of study | Bell, Bell & Bell (2005) |
| Deterrent | Procellariidae | Wedge-tailed shearwater, Puffinus pacificus | Blue-dyed bait as bycatch deterrent | Visual | Experimental | Dyed squid was taken 3–8% compared to 75–98% of control squid; dyed fish not as effective | Cocking et al. (2008) |
| Deterrent | Procellariidae | Northern fulmar, Fulmarus glacialis (primarily) | Scaring line and funnel that forces bait under the water surface | Visual | Experimental | More effectively deterred using the bird scaring line than the setting funnel | Løkkeborg (2003) |
| Deterrent | Procellariidae | Northern fulmar, Fulmarus glacialis | Scaring streamers, line shooters, or both | Visual | Experimental | Zero birds caught with the bird scaring line, and one caught with both scaring and line shooting | Løkkeborg & Robertson (2002) |
| Attraction | Procellariidae | Common diving petrel, Pelecanoides urinatrix | Playback and chick translocations | Auditory | Observational | 20 translocated chicks returned to the island and 51 unbanded birds | Miskelly & Taylor (2004) |
| Deterrent | Procellariidae | Black petrel, Procellaria parkinsoni | Shark liver oil | Olfactory | Experimental | Shark liver oil decreased interest, but not significant | Norden & Pierre (2007) |
| Deterrent | Procellariidae | Flesh-footed shearwater, Puffinus carneipes | Shark liver oil | Olfactory | Experimental | Shark liver oil caused significant decrease in interest | Norden & Pierre (2007) |
| Deterrent | Procellariidae | Albatross sp.; giant petrel, Macronectes giganteus, Cape petrel, Daption capense | Shark liver oil | Olfactory | Experimental | Odour not effective deterrent | Norden & Pierre (2007) |
| Deterrent | Procellariidae | Many (flesh-footed shearwater, Puffinus carneipes and black petrel, Procellaria parkinsoni) | Shark liver oil | Olfactory | Experimental | Reduced both seabirds at vessels and dives on baits | Pierre & Norden (2006) |
| Attraction | Procellariidae | Galápagos petrel, Pterodroma phaeopygia | Playback | Auditory | Experimental | Increased rate of capture to 0.83 petrels per hour from 0.38 | Podolsky & Kress (1992) |
| Attraction | Procellariidae | Fluttering shearwater, Puffinus gavia | Playback | Auditory | Observational | First bird recorded at site 11 months after playback commenced | Sawyer & Fogle (2010) |
| Attraction | Procellariidae | Grey-faced petrel, Pterodroma gouldi | Playback | Auditory | Observational | First bird recorded at site 7 months after playback commenced | Sawyer & Fogle (2010) |
| Attraction | Sternidae | Common tern, Sterna hirundo | Decoys | Visual | Experimental | Decoys + sound were effective attractants, but decoys alone were not effective | |
| Playback | Auditory | Experimental | Sound alone and decoys + sound were both effective attractants | Arnold, Nisbet & Veit (2011) | |||
| Attraction | Sternidae | New Zealand fairy tern, Sterna nereis davisae | Decoys | Visual | Experimental | Significant effect with 80% more landing to decoys, decoys + sound also effective | |
| Playback | Auditory | Experimental | No significant difference to plots with decoys + sound and decoys alone | Jeffries & Brunton (2001) | |||
| Attraction | Sternidae | Arctic tern, Sterna paradisaea, common tern, Sterna hirundo | Decoys and playback | Auditory & visual | Observational | Increased terns from 59% of observation days (1974–1977) to 96% (1978) | Kress (1983) |
| Attraction | Sternidae | Caspian tern, Hydroprogne caspia | Decoys and playback | Visual & auditory | Observation | Over 3 years, ∼ 8900 pairs shifted from previous colony to new site | Roby et al. (2002) |
| Attraction | Sternidae | Forster's tern, Sterna forsteri, least tern, Stemula antillarum | Decoys and playback | Visual & auditory | Observation | Successful at attracting birds but failed fledging due to nest predation | Ward et al. (2011) |
| Attraction | Sulidae | Australasian gannet, Morus serrator | Decoys and playback | Visual & auditory | Observation | First birds began roosting at the site ∼52 days after treatments commenced | Sawyer & Fogle (2013) |
| Deterrent | Many | Blue-dyed bait tested for catching fish and camouflaging from seabirds | Visual | Observation | Trial inconclusive on effectiveness | Lydon & Starr (2005) | |
| Deterrent | Many | Paired and single streamer lines | Visual | Observational | Paired streamers were 88–100% effective; single streamer line was 71–91% effective | Melvin (2004) | |
| Attraction | Grey-faced petrel, Pterodroma gouldi | Playback | Auditory | Experimental | 6× higher counts during playback | Buxton et al. (2015) | |
| Attraction | Procellariidae | Fluttering shearwater, Puffinus gavia | Playback | Auditory | Experimental | 4.9× higher counts during playback | Buxton et al. (2015) |
| Attraction | Procellariidae | Flesh-footed shearwater, Puffinus carneipes | Playback | Auditory | Experimental | No difference due to playback | Buxton et al. (2015) |
Seabirds have evolved a variety of unique physiological and sensory adaptions in response to the selective pressures imposed by their pelagic and colonial lifestyle. Seabirds live almost entirely at sea where foraging and navigation requires long-distance sensory modes that are less apparent in non-pelagic birds (Stager, 1967; Hunt & Schneider, 1987; Boyd & Croxall, 1996; Jarvis et al., 2014). For example, many seabird taxa can see fish under the ocean surface (e.g. albatross; Martin, 1998) or forage via chemical cues (e.g. Procellariiformes and Sphenisciformes; Nevitt, Veit & Kareiva, 1995; Cunningham, Strauss & Ryan, 2008; Pollonara et al., 2015). Natal philopatry and colonial breeding mean many seabird species must also navigate to, assess and recognize their breeding colonies, which are often on isolated islands (Schreiber & Burger, 2001). Some species use audition; playback experiments with recorded bird calls can emulate this (e.g. for Galápagos petrels Pterodroma phaeopygia; Podolsky & Kress, 1992). Seabirds may also use scents to locate colonies (Grubb, 1973; Bonadonna, Spaggiari & Weimerskirch, 2001). For example, when Cory's shearwaters (Calonectris borealis) were made anosmic, orientation and navigation were impaired and they could not return to their colony when displaced (Gagliardo et al., 2013).
Seabird colonies may have thousands of birds (Schreiber & Burger, 2001), selecting for mechanisms to identify mates or nests within a crowd. Some seabirds can use vocalizations to find chicks after foraging bouts (e.g. Adélie and gentoo penguins, Pygoscelis adeliae and P. papua; Aubin & Jouventin, 2002). Visual discrimination is unlikely for nocturnal seabirds such as Procellariformes, which instead can use olfactory signals to identify species or individuals (Bonadonna & Nevitt, 2004; Bonadonna et al., 2007; Bonadonna & Mardon, 2010; Mardon et al., 2010). A classic test with captive nocturnal Leach's storm petrels (Oceanodroma leucorrhoa) found they were more likely to orient to the smell of conspecific nesting material than a control forest odour (Grubb, 1974), although the role of individual versus conspecific odours in this experiment is unclear. Chemosensory communication is not necessarily restricted to nocturnal species; diurnal black-legged kittiwakes (Rissa tridactyla; Laridae) have both individual and sex differences in odour (Leclaire et al., 2011).
Another seabird life-history trait selecting for sophisticated sensory behaviour is monogamy and the formation of long-term pair bonds, necessitating accurate assessment of conspecific quality during mate choice (Warham, 1990; Schreiber & Burger, 2001). Breeding birds often use vocal signals to differentiate between sexes (Brooke, 1978; James, 1984; Storey, 1984; Taoka & Okumura, 1990). Amongst closely related shearwater species, both sex- and species-specific vocalizations are common (Curé et al., 2012). For example, the exhalent, low part of the call is sufficient for species discrimination between Yelkouan (Puffinus yelkouan), Balearic (P. mauretanicus) and Cory's shearwaters, and is likely also important for sexual differentiation (Curé et al., 2012). By contrast, in the Australasian gannet (Morus serrator), male and female vocalizations do not appear to differ (Krull et al., 2012), but plumage colour does when modelled into a bird visual system (Ismar et al., 2014). Mate choice via assessment of visual signals is well documented, e.g. the brightly coloured legs of the blue-footed booby (Sula nebouxii) indicate individual quality, and affect mate choice, and therefore male breeding success (Velando, Beamonte-Barrientos & Torres, 2006). Similarly, male frigatebirds (Fregata spp.) use distinctive red gular pouches in sexual displays (Juola, McGraw & Dearborn, 2008). Evidence for mate choice via odour cues is rare for seabirds, however the major histocompatibility complex (MHC), which is associated with immune function and individual odours, influenced mate choice in the blue petrel (Halobaena caerulea; Strandh et al., 2012). In short, seabirds have evolved unique behaviours which in turn require sophisticated sensory mechanisms. These involve several signal modes, conspecific and heterospecific signals, and long- and short-distance signal reception – all of which could be harnessed for conservation and management.
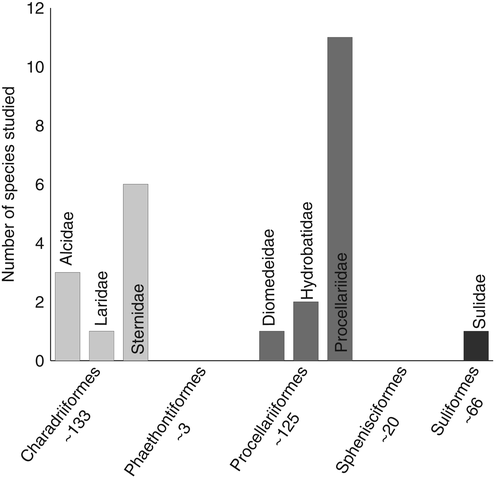
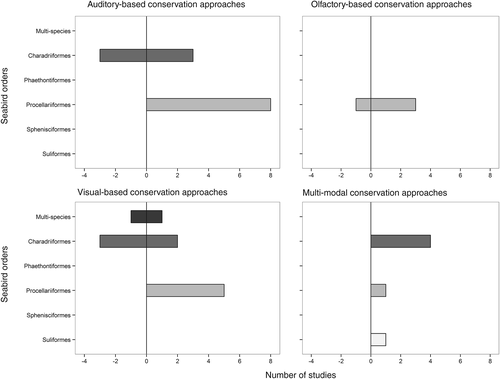
Although we focus on seabirds, our findings can be broadly applied to other animals with similar foraging, navigation, colonial, mate choice, and individual and breeding-site recognition behaviours. We analyse how sensory traits associated with these behaviours can be co-opted for conservation purposes, highlighting which approaches and taxa yield the greatest conservation benefits. We begin by reviewing the seabird literature to describe and quantify the range of sensory-based conservation techniques, factors associated with success, and the general potential for sensory-based conservation biology. We assess research effort according to seabird taxonomic groups (Section 2, Fig. 1), and the sensory mode manipulated (auditory, olfactory, visual, or combined; Fig. 2). Importantly, we provide a summary list of behaviours typically associated with successful sensory-based conservation efforts in seabirds, which can be broadly applied to other at-risk taxa. An understanding of the uses and ecological basis of sensory-based techniques is critical for improving conservation efforts, and heralds a new, more evolutionary approach to species management.
II. METHODS
We searched for publications on seabird conservation and management efforts that explicitly involved the target species' auditory, visual, or olfactory senses using two databases (Google Scholar, Scopus) in English, with no limit on year. Database searches were made using a combination of the words: auditory, vocalization, playback, visual, decoy, olfactory, odour, sensory AND seabird conservation (N studies yielded in Google Scholar = 18500 of which first 3000 were searched based on relevance, N studies yielded in Scopus = 158; total N of studies included from both databases = 13 plus 2 reviews) OR seabird colony attraction (N studies yielded in Google Scholar = 7230 of which first 2000 were searched, N studies yielded in Scopus = 61; total N studies included from both databases not included in previous search = 4) OR seabird bycatch (N studies yielded in Google Scholar = 3890 of which first 1000 were searched, N studies yielded in Scopus = 26; total N studies included from both databases not included in previous search = 2) OR seabird translocation (N studies yielded in Google Scholar = 1820, N studies yielded in Scopus = 55; total N studies included from both databases not included in previous search = 4) OR seabird bycatch (N studies yielded in Google Scholar = 3890, N studies yielded in Scopus = 26; total N studies included from both databases not included in previous search = 2). Articles selected were read based on the title and abstract from the search, and then included in this review based on the content of the article. We excluded studies focused on censusing populations, sensory methods for deterring pest seabirds (e.g. gulls at landfill sites), or the sensory behaviour of other species interacting with seabirds (e.g. deterrents for invasive predators that eat birds). Further studies were included based on work reviewed by Jones & Kress (2012) and Melvin et al. (1999). We systematically searched the databases and aimed to incorporate all studies relevant for our study, but may have inadvertently excluded papers due to our specific search parameters.
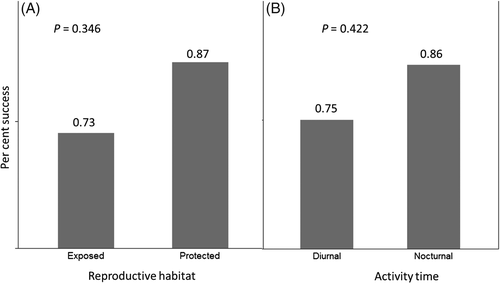
We then quantified the number of sensory-based conservation publications per seabird order (Fig. 1), and the sensory modes utilized per study (auditory, olfactory, visual, or multi-modal; Fig. 2). Based on our literature review (summarized in Table 1), we performed a logistic regression with chi-squared analysis of deviance comparing the sensory mode targeted in conservation efforts (acoustic, visual, olfactory, or multi-modal) based on the seabird order of targeted species (Procellariiformes or Charadriiformes; Suliformes were not included as only one study was reported). We hypothesized that species behaviours (reproductive habitat and activity times) influence the success of sensory-based colony attraction techniques. We performed one-tailed Fisher's Exact tests on the null hypothesis that success of sensory-based colony attraction was no different based on reproductive habitat (protected habitat – cliffs and burrows, and exposed habitat – ground), and activity period on colonies (nocturnal, diurnal).
III. SENSORY MODES, TAXONOMY, BEHAVIOUR AND SUCCESSFUL CONSERVATION OUTCOMES
Sensory-based conservation techniques have not been trialled equally across seabird orders or families. Procellariiformes are the most studied (N = 14 species out of ∼125 species in the order) with most of these focusing on the family Procellariidae (N = 11). Charadriiformes are the second most common order represented in the literature for sensory-based conservation (N = 10 out of ∼133 species). There have been no sensory conservation studies on Phaethontiformes or Sphenisciformes, and only one study on a suliform species (Fig. 1). Sensory techniques could and should be attempted across a broader taxonomic range, but this is impeded by the lack of data about sensory ecology in the neglected groups. The Sphenisciformes in particular should be a high priority for sensory research and sensory-based conservation because species in this order are some of the most critically threatened seabirds (Croxall et al., 2012).
Although there was no correlation between the sensory mode targeted and the likelihood of a successful conservation outcome, taxonomy did correlate with success (Table 2). Sensory-based conservation efforts are more likely to succeed with Procellariiformes than Charadriiformes (Table 2; Fig. 2). This is likely due to differences in the typical activity patterns and nesting sites for these two orders – Procellariiformes are typically nocturnal and burrow-nesting whereas Charadriiformes are generally surface-nesting. We found some possible trends between nocturnal activity time and protected (burrow and cliffs) versus exposed (surface/ground) breeding, but results were not significant (Fig. 3). These results are likely biased by the under-reporting of failed conservation attempts (Fanelli, 2011).
| d.f. | Deviance residual | P | |
|---|---|---|---|
| Sensory mode | 3 | 3.84 | 0.28 |
| Order | 1 | 4.59 | 0.032** |
| Interaction: Sensory mode × order | 2 | 0.20 | 0.90 |
- ** , significant at P < 0.05.
IV. USE AND EFFECTIVENESS OF SENSORY MODES IN CONSERVATION
(1) Auditory signals
Our survey identified 24 out of a possible ∼347 seabird species that have received sensory-based conservation management, and auditory cues were the most common sensory mode used (Fig. 2). This is not surprising as the importance of sound for birds is well established, particularly for songbirds, whose intricate vocalizations have been studied in detail at many stages, including ontogenetic learning and physiological responses (e.g. Nottebohm, 1970; McCasland, 1987; Slabbekoorn & Smith, 2002). Many seabird species use calls, perhaps because they often nest in high-density, mixed-species colonies necessitating rapid and non-contact identification of partners and nests (Bretagnolle, Genevois & Mougeot, 1998). Vocalizations may be especially important for species that are nocturnally active on breeding sites, a common seabird trait. For example, Manx shearwaters (Puffinus puffinus), are more attracted to burrows emitting playbacks than silent burrows (James, 1985). Calls in dense colonies are used for individual (Jouventin & Aubin, 2002; Tibbetts & Dale, 2007) or sexual recognition (Taoka et al., 1989a), to display aggression or threats (Taoka, Won & Okumura, 1989b; Marler, 2004), and to synchronize breeding behaviours (Hunt, 1980; Bretagnolle, 1989). Additionally, vocalizations are important for courtship, and mate recognition and assessment (Bretagnolle, 1989; Jouventin & Aubin, 2002; McKown, 2008; Juola & Searcy, 2011). Reflecting this broad understanding of the importance of acoustic cues and signals for seabirds, acoustic stimuli such as playback recordings have been used relatively extensively in conservation and management (Table 1).
(a) Sound as an attractant
The use of acoustic signals to attract seabirds to new colonies or restored habitats is one of the most common examples of sensory-based conservation. This ‘acoustic anchoring’ has been trialled in at least 15 studies (successful in 14, unsuccessful in 1, including multi-modal approaches involving an acoustic mode; Table 1). Given the highly colonial behaviour of seabirds, and inter-generational site fidelity, the use of vocalizations that emulate colony sounds has been one of the most effective approaches for seabird attraction. Successful examples involve Galápagos petrel (Podolsky & Kress, 1992), Australasian gannet (Sawyer & Fogle, 2013) and many other species (Table 1).
The degree of natal and breeding philopatry (the tendency to nest at the site of one's own hatching or previous breeding) varies among seabirds and is likely to be critical for the success of acoustic attractants in establishing new colonies. Terns are less philopatric to either breeding or natal locations than many other seabird species, and have a tendency to change colony location in the presence of predators or during storms (Gummer, 2003). These transient behaviours impact the success of relocation management. Attempts to mediate this transience have involved both acoustic and visual attractants. For example, the largest colony of Caspian tern (Hydroprogne caspia) in North America, located on Rice Island in the basin of the Columbia River, was successfully re-located to East Sand Island to mediate the tern colony's impact on local juvenile salmonids, which are protected as evolutionary significant units (ESUs) under the Endangered Species Act of 1973 (16 U.S.C. Sec. 1531; Roby et al., 1998; Collis et al., 2002). This Caspian tern relocation began in 1999, was accomplished over 3 years, and eventually achieved 8900 pairs breeding on East Sand Island. In addition to habitat landscaping, gull control, and other sensory techniques (see Section 4.3), an audio system broadcast vocalizations recorded from the Rice Island Caspian terns (Roby et al., 2002). However, the use of sensory cues in tern species has not always been effective. Several projects in the United Kingdom have been unsuccessful at relocating little terns (Sterna albifrons) via playback of non-aggressive calls and visual attractants (Gummer, 2003). The lack of responsiveness in these tern species could be a result of traits related to their specific reproductive habitat (surface breeding), which might cause them to be more ephemeral and highly selective when choosing breeding locations.
When species have high philopatry to natal colony locations, this creates the challenge of how to incentivize individuals to nest at new or newly restored locations (Podolsky & Kress, 1992; Miskelly & Taylor, 2004; Sawyer & Fogle, 2010). Successful attraction to new nesting sites may rely on the tendency of non-breeders to be more responsive to acoustic playback. Playback experiments with thin-billed prions (Pachyptila belchei) found that non-breeders were more likely to respond to calls from both sexes than individuals that already had a mate (Bretagnolle et al., 1998). Additionally, some studies have shown a higher degree of genetic flow between colonies of conspecifics, indicating a greater level of movement between philopatric species than previously thought (Lawrence, Lyver & Gleeson, 2014).
Many seabird species are likely to have sophisticated abilities to differentiate between conspecific calls. In translocation efforts, playbacks were successfully used to increase the capture rate of Galápagos petrels near potential nest sites in the Galápagos Islands (Podolsky & Kress, 1992). In addition to the attraction of seabirds to conspecific vocal cues, there is evidence that some species are also highly attracted to other types of noise. For example, black-winged petrels (Pterodroma nigripennis) increased nesting activity near a generator that produced a loud noise (Ismar et al., 2010). Additionally, many seabirds are attracted to a human produced ‘wah-wah’ sound that does not seemingly reflect birds' natural calls (Tennyson & Taylor, 1990).
(b) Sound as a deterrent
Noxious sounds have been trialled for deterring seabirds and other marine animals such as sea turtles from fishing vessels, with varying success (Southwood et al., 2008). Acoustic pingers in coastal gillnet fisheries reduced bycatch common murres (Uria aalge) by 50%, and did not impair the number of salmon caught in gill nets (Melvin et al., 1999). However, the effect that such artificial noises may have on other nearby organisms such as cetaceans should be investigated further as noise pollution is considered a threat to some species (Erbe, 2002). As with any regularly used external stimuli, habituation and the association of the sound with a food source could become concerns (see Section 24).
(c) Sound in conservation for other taxa
Acoustic-based conservation has been attempted in a range of species, providing examples of useful approaches, and important issues relevant to seabirds and other taxa of high conservation priority. Amongst songbirds, conspecific vocalizations have been effective attractants for translocation and anchoring (Ward & Schlossberg, 2004). A notable study on translocated New Zealand robins (Petroica australis), involved control sites and clearly demonstrated that more individuals returned to locations with conspecific playback (Bradley et al., 2011). Marine animals have similar sensory constraints as seabirds and have also been studied from a sensory-based conservation approach using auditory stimuli. For example, acoustic pingers have been successful for deterring cetaceans from driftnets (Kraus et al., 1997; Trippel et al., 1999; Barlow & Cameron, 2003). Studies on loggerhead turtles (Caretta caretta) with acoustic deterrents highlight some of the limitations of acoustic techniques in natural settings such as hearing damage or habituation leading to decreased responsiveness (O'Hara & Wilcox, 1990; Southwood et al., 2008). These lessons from marine mammals should be considered for seabird acoustic sensory conservation and the broader application of such approaches to other taxa.
(2) Chemosensory signals
Scent is the least-explored mode of sensory-based conservation in seabirds (N = 4; Table 1). Despite this lack of application in conservation programs, many seabirds, especially the tube-nosed Procellariiformes, are renowned for their excellent sense of smell, making them ideal candidates for chemosensory-based conservation techniques (e.g. Bang, 1966; Bang & Wenzel, 1985; Warham, 1996), but outcomes are highly variable.
(a) Scent as an attractant
Scent has been successfully used in one out of two species on Procellariiformes relocation (Table 1). For example, chick translocations of the Chatham Island taiko (Pterodroma magentae), a critically endangered New Zealand seabird (IUCN, 2014), included taking scented nesting material from the original nest and placing it in a new nest site to provide chicks with familiar odours. This was intended to prevent wandering, and encourage birds to return to the new location as adults but success has not been formally assessed (G. A. Taylor, unpublished data). There has only been one quantitative and controlled experimental study testing seabird attraction to the odours of nesting material. In a T-maze test, fork-tailed storm petrels (Oceanodroma furcata) were significantly more attracted to the smell of conspecifics than a control, but this preference was not shown by Leach's storm petrels (Buxton & Jones, 2012). Together with the growing number of case studies demonstrating the existence and sensitivity of seabird olfaction (Hagelin, Jones & Rasmussen, 2003; Mardon et al., 2010; Leclaire et al., 2011), these early trials suggest adding natural scents to artificial nest boxes may be a simple means to enhance seabird visits and/or permanent use of new sites. More experimental trials, especially those involving controls, are essential.
Scents could also be useful for familiarizing young birds with natural species-specific odour cues during hand-rearing. Pre-fledged chicks of some petrel species can distinguish odours (Mínguez, 1997; De León, Mínguez & Belliure, 2003), and early exposure to some odours could influence behaviours such as foraging or mate choice. Furthermore, to our knowledge, the effect of human or artificial scents on chicks has not been investigated. This may be an important consideration for hand-rearing seabirds or other endangered and highly olfactory species such as kakapo parrots (Strigops habroptila; Sibley, 1994).
(b) Scent as deterrent
Odour cues have been successful deterrents in two studies involving several species including flesh-footed shearwaters (Puffinus carneipes) and black petrels (Procellaria parkinsoni; Pierre & Norden, 2006; Norden & Pierre, 2007; Table 1). These studies address a bycatch avoidance method used by some commercial fishing vessels where shark liver oil is released into the water (BirdLife International, 2004; Hansford, 2004; Pierre & Norden, 2006; Norden & Pierre, 2007). The deterrent effects reported for shark liver oil are somewhat surprising because releasing other fish oils and burley from boats attracts birds, as when chum is deployed to attract seabirds to boats during pelagic birdwatching tours. However, releasing shark liver oil won an incentive-driven competition from Sociedad Española de Ornitología (SEO) and BirdLife International, where anglers submitted new and practical methods of reducing seabird bycatch (BirdLife International, 2004). Experimental tests with shark liver oil in New Zealand waters show a significant reduction in the number of seabirds attracted to boats, and fewer birds diving to baits (focusing particularly on flesh-footed shearwaters; Pierre & Norden, 2006; Norden & Pierre, 2007). However, shark harvesting and releasing oil from boats creates other conservation and political issues. For example, there are legislative controls on the release of all oil from shipping vessels in New Zealand waters (via International Maritime Organisation, Jan 2013). Analysis of shark liver oil chemical components and synthetic production of any chemical deterrents is the next step for assessing this controversial method.
Other options for the chemical deterrence of seabirds include chilli and pepper concentrates. Preliminary research in which mackerel (Scomber scombrus) were treated with capsaicin (crushed chilies, Capsicum frutescens) or a capsaicin and piperine mixture (using tobacco and black pepper) indicated this may be effective in deterring white-chinned petrels (Procellaria aequinoctialis) from swallowing baits (Gasco & Pierre, 2007). More broadly, bird eggs treated with capsaicin discourage mammalian predators (Baylis, Cassey & Hauber, 2012).
(c) Scent in the conservation of other taxa
Chemical signals are rarely used in the conservation of non-seabird birds. Repellents have little or variable success in deterring passerine birds from areas of human conflict (Stevens, Clark & Weber, 1998). Laboratory studies with methyl anthranilate (MA), a grape-flavoured chemical disliked by birds, suggest some deterrent qualities for passerines (Askham, 1992; Avery et al., 1996), but field trials are inconsistent (Avery, 1992). The repellent use of MA has also been trialled in Canada geese (Branta canadensis) with varying success (Cummings et al., 1995; Belant et al., 1996). Increasing awareness of the importance of olfaction for birds (especially seabirds; Bonadonna & Bretagnolle, 2002; Bonadonna & Nevitt, 2004; Bonadonna et al., 2007; Leclaire et al., 2011; Pollonara et al., 2015) and the usage of chemosensory manipulations for conservation in other animal groups suggests that this area has applications to a range of animals, but requires more focus.
(3) Visual signals
The success of vision-based conservation efforts reflects the importance of vision in birds' conspecific interactions and foraging. Visual stimuli successfully deter birds from boats, allowing longer fishing periods and larger catches (Løkkeborg, 2003). Visual cues are considered a cost-effective alternative to direct translocations of chicks when attempting to establish a new colony (Jones & Kress, 2012).
(a) Visual attractants
The success of using visual cues in seabird conservation (seven successful trials, two unsuccessful) is primarily based on the role of vision in mate choice, and is a less costly technique for establishing new colonies than the physical translocation of birds to a new site (Jones & Kress, 2012). Decoys, such as models of birds, can work well for colonial breeding species that are attracted to nesting locations by the presence of conspecifics (Evans & Cash, 1985; Podolsky, 1990). Coloniality reflects not only the social behaviour of many species, but also influences habitat choice in black-legged kittiwakes based on apparent success of conspecifics in the location (Danchin, Boulinier & Massot, 1998).
Sight cues, such as decoys, were used as attractants and deterrents in the relocation of nearly 9000 pairs of Caspian terns to East Sand Island (see Section 4.1a; Roby et al., 2002). At the target colony, 380 model Caspian terns were used to lure conspecifics while streamers and eagle decoys were used as deterrents at the old nest site (Roby et al., 2002). As for many sensory-based conservation efforts, controlled experimental assessments are important to demonstrate causality. Decoys with and without a playback stimulus attracted 80% more endangered New Zealand fairy terns (Sterna nereis davisae) than the control treatment (Jeffries & Brunton, 2001); an example of effective use of experimental controls for sensory-based conservation. Another study was able to determine that sites with decoys were no different from control non-breeding sites at attracting nesting or loafing brown pelicans (Pelecanus occidentalis; Walter et al., 2013). Although the use of visual decoys has not been explored for seabird groups that are nocturnal on breeding colonies, trials are in progress to attract petrel species active at night using decoys (S. Sawyer, personal communication). Despite the importance of plumage colour for mate choice in birds (Hill, 1993; Hunt et al., 1999) no studies have tested the effectiveness of decoys of varying colouration, or tested whether decoys accurately represent bird colour when perceived by birds. Similarly, data regarding target species' sensitivity to ultraviolet (UV) and the application of an appropriate amount of UV reflectance could increase the effectiveness of decoys.
(b) Visual deterrents
Most seabird bycatch fatalities occur within 100 m of the fishing vessel (e.g. albatross in the Southern Ocean; Brothers, 1991). Within this distance, visual deterrents can be effectively used to scare seabirds away from boats, or make fishing bait less visible. Of the visual-deterrent methods trialled in eight species, seven were successful and one had an undetermined outcome (Table 1). A successful visual deterrent for auklet species involved mesh-panelled streamers extending ∼90 m from the back of the boat, sometimes with brightly coloured tubing and UV protection to improve longevity (Melvin, 2004). Streamers reduced bycatch by 40–45%, depending on mesh size (Melvin et al., 1999). In other trials, streamers were used with or without a lineshooter to repel Northern fulmars (Fulmarus glacialis) from North Atlantic longline fisheries. During at-sea trials, no birds were caught with streamers alone and only one bird was caught when streamers were paired with line shooters, compared to 32 birds caught during the control period (1270 hooks set; Løkkeborg & Robertson, 2002). Further trials with streamers, lineshooters, and underwater setting of nets showed that all three methods reduced the number of Northern fulmars caught in hooks. Scaring lines, where moving streamers scare seabirds from foraging on hooked baits, were the most successful. Only two birds were caught in 185000 hooks, compared to 205 birds on similar control lines (Løkkeborg, 2003, 2011).
Seabird bycatch can also be reduced by camouflaging bait in longline fisheries (Gilman, Brothers & Kobayashi, 2005). This was tested by modelling how the colour/spectral reflectance of blue-dyed bait squid and fish would be seen by wedge-tailed shearwaters (Puffinus pacificus; Cocking et al., 2008). While blue-dyed squid was much less attractive than natural-coloured squid (with only 3–8% of dyed squid taken compared to 75–98% of control squid), blue-dyed and control fish were similarly attractive due to more difficulty in achieving a cryptic colour in fish (Cocking et al., 2008). This method exemplifies novel thinking in fisheries where squid baits are used. Facilitating a broad-scale usage of this technique might be problematic, as it requires access to blue-dyed baits (that likely have been thawed). Implementation of these types of techniques may require further investigation.
(c) Visual cues in conservation of other taxa
Despite the importance of visual signals for many taxa, there are few published reports of the use of visual stimuli for conservation of non-seabird species. Visual deterrents have been used in other taxa in attempts to minimize human–animal conflict. For example, flying raptor decoys have been deployed over areas where other birds, like doves (Columbidae) and crows (Corvidae) come into conflict with vehicles like planes. Although initially effective, the birds tend not to be permanently deterred (Sugimoto, 1999).
Visual lures are an important aspect of pest management (Foster & Harris, 1997). Visual lures have been used to attract brushtail possums (Trichosurus vulpecula) in New Zealand where they are a pest species, for management practices. These visual attractants were more effective when they included a luminescent strip as opposed to plain or UV reflective strips (Short, Turner & Risbey, 2003; Ogilvie et al., 2006). Decoys are widely used as attractants for hunting, strongly suggesting wider applicability in conservation efforts for any colonial or aggregating species. For example, duck hunting decoys may effectively attract endangered duck species to new suitable habitat. Testing the effectiveness of visual lures should prove a highly productive area of research.
(4) Multi-modal signals
Many taxa communicate and detect cues in multiple sensory modes, i.e. simultaneous acoustic, olfactory and visual signals (Candolin, 2003; Hebets & Papaj, 2005). This can assist in conservation management as multi-modal stimuli may provoke a stronger behavioural response than a single sensory mode. For example, in the diurnal Laysan albatross (Diomedea immutabilis), more individuals landed at sites where both vocalizations and visual stimuli were utilized than sites using visual stimuli alone (Podolsky, 1990). Fork-tailed storm petrels were attracted in trials with both acoustic and olfactory cues (Buxton & Jones, 2012). Predator-avoidance training with visual and species-specific auditory cues (playbacks and stuffed decoys) in the endangered New Zealand robin, provoked a stronger anti-predator response than single-sensory mode training. However, responsiveness was still greatest after experiences with wild, live predators (Maloney & McLean, 1995). As for all sensory modalities, more controlled experiments, broader taxonomic coverage and more sophisticated consideration of sensory perception capacities are required. This will improve conservation outcomes and provide fundamental data on the role of the different sensory modalities in the life history of any taxa, including seabirds.
V. BEHAVIOURS FACILITATING SENSORY-BASED CONSERVATION
Since sensory approaches to conservation rely on natural interactions between target animals and their conspecifics, heterospecifics and the environment rather than any specific individual species traits, lessons from seabird efforts can be applied to a broad range of at-risk animal taxa. Sensory-based conservation takes advantage of species' natural behaviours. Animals clearly differ in their sensory ecologies and amenability to sensory manipulation for management outcomes. However, our survey has revealed several life-history traits that characterize a species as particularly suitable for sensory-based conservation.
(1) Coloniality/sociality
Animals that breed in colonial groups often use sensory cues to choose or identify mates in aggregations (e.g. vocalizations in king penguins, Aptenodytes patagonicus; Lengagne et al., 2004), transfer information about food locations (e.g. honey bees, Apis mellifera; Seeley, 1994), in defence from predators (e.g. vervet monkeys, Chlorocebus pygerythrus; Seyfarth, Cheney & Marler, 1980), and as a way of identifying suitable breeding habitat (Danchin et al., 1998). Some of the most successful examples of sensory-based conservation efforts are those that use visual or auditory stimuli typically produced by individuals or groups of conspecifics to attract new colonizers (Table 1). Although this approach has usually targeted birds, other mobile species that use aggregation cues are likely candidates.
(2) Long-distance mate attraction
Species that use long-distance communication modes such as acoustic or chemical signals in mate attraction and mate choice are excellent candidates for sensory-based communication. This technique is commonly used in pest insect control (Foster & Harris, 1997). However, this approach will typically influence male rather than female behaviour so further stimuli may be required if behavioural manipulation is required for both sexes, e.g. stimuli associated with female behaviours such as nesting or oviposition.
(3) Philopatry
Philopatric traits that are often found in seabirds are present in other animals including salmon (Dittman & Quinn, 1996), sharks (Hueter et al., 2005), and bats (Kurta & Murray, 2002). In addition, many species are attracted to locations where a critical number of conspecifics are already present, as for seabirds. Allee effects can be critical for small populations and pose serious issues for conservation managers (Courchamp, Clutton-Brock & Grenfell, 1999). Coloniality and philopatry can make species more vulnerable to Allee effects by geographically concentrating breeding species, making them more susceptible to intense human modification (e.g. alterations to wetland environments and the effects on breeding and migratory shorebirds; Piersma, 2007). In seabirds, using sensory cues such as recorded vocalizations to imitate an existing population can curtail the drive of philopatry and reduce Allee effects by creating the auditory illusion of a larger colony (Podolsky & Kress, 1992). As such, sensory-based attraction methods could be successful in other taxa that share this challenging behaviour.
(4) Territoriality
Species that use sensory markers to advertise their home ranges, e.g. wolves and coyotes (Canidae), which use odours to define territories, could be excellent candidates for sensory-based management of human–wildlife interactions (Peters & Mech, 1975). Male red-winged blackbirds (Agelaius phoeniceus) use both vocal and colour displays of epaulet feathers to defend and maintain the nesting territory (Peek, 1972). Sensory cues that warn of negative consequences like those used in territorial displays or by predators have potential to be applied as deterrents for species from risky sites such as roads.
(5) Nocturnality
Animals that are active after dark have often evolved specialized senses. For example, nautilus (Nautilus pompilius), use odour cues to locate food in dark oceans (Basil et al., 2000), while barn owls (Tyto alba) use a combination of audio-visual cues for hunting at night (Takahashi, 2010). Other nocturnal animals have evolved specialized visual systems (e.g. nocturnal colour vision in helmet geckos, Tarentola chazaliae; Roth & Kelber, 2004), or tactile sensory traits (vibration detection in scorpions, Paruroctonus mesaensis; Brownell & Farley, 1979). When one sensory mode is made more difficult by environmental conditions, or has evolved specializations, sensory-based conservation approaches are likely to be even more targetable and effective.
VI. COGNITIVE CHALLENGES FOR SENSORY-BASED CONSERVATION
A nuanced sensory-based approach to conservation must consider ways in which an animals' cognition may limit sensory management efforts. The tendency of animals to habituate to sensory treatments is especially important because deterrents, unlike true threats, do not impose negative consequences on the receiver. Playing different combinations or cycles of stimuli might prolong deterrent effects and minimize habituation, as well as decrease interference with other non-target species. In tests with harbor porpoises (Phocoena phocoena), some habituation to sounds occurs with sound cycling, but intermittent pings were still relatively successful (Carlström, Berggren & Tregenza, 2009). Use of high-frequency noise to deter tammar wallabies (Macropus eugenii) from areas of human conflict were shown to have no effect, regardless of the presence of food. This might be because the sounds were artificial and not part of the animals' natural behaviour (Muirhead et al., 2006; also shown in kangaroos Macropus spp.; Bender, 2003). This indicates the importance of investigating the natural sensory ecology and responses of target conservation species to different stimuli.
Anthropogenic sensory traps are human activities that lure animals from natural sensory cues and are associated with urbanization (e.g. artificial light; Schlaepfer, Runge & Sherman, 2002). Anthropogenic sensory traps can cause significant individual- and population-level effects, e.g. artificial light and fires disorient newly hatched sea turtles (Madliger, 2012). A well-studied example is the consumption of plastic particles in the ocean by albatrosses and other seabirds (Harper, 1979; Robards, Piatt & Wohl, 1995; Auman et al., 1997; Schlaepfer et al., 2002), effectively reducing food consumption (Ryan, 1988). A second major topic for recent research is how urban sounds interfere with bird songs, negatively impacting communication in many species worldwide (Slabbekoorn & Ripmeester, 2008). Determining when an animal is subject to ecological traps and applying appropriate mitigation measures is becoming a more widely recognized aspect of sensory-based conservation applicable across various taxa (Madliger, 2012). For example, anthropogenic noises, such as traffic sounds that interfere with predators' ability to detect prey could be modified by reducing the speed limit (Madliger, 2012). By considering the sensory perceptions of target species, conservation biologists can predict where such traps may exist and use their knowledge of the animals' sensory modes to alleviate the impact of traps.
Another risk of sensory-based conservation efforts is negative effects on unintended receivers or eavesdroppers. For example, acoustic pingers to deter seals from aquaculture pens also deter non-target harbor porpoises (Johnston, 2002). Another risk of eavesdroppers involves predators and parasites, which find prey via multiple sensory modes, including chemical (Stowe et al., 1995) and auditory cues (Deecke, Ford & Slater, 2005). The risk of attracting predators and parasites may be intensified if management efforts broadcast sensory stimuli, such as playing vocalizations for translocation. While the effect of eavesdroppers has not been thoroughly examined in sensory-based conservation examples, we recommend that predator and parasite activity be carefully monitored before and during sensory-based conservation activities.
VII. CONCLUSIONS
(1) Sensory-based conservation incorporates behavioural and sensory ecology by considering how signalling and responses evolve under selection from conspecifics, heterospecifics and the environment. Conservation approaches informed by evolutionary principles are likely to have several advantages over more-interventionist methods such as captive breeding and catch limits as they are transferable among taxa, and can be applied simultaneously to several species with similar behaviours (e.g. bycatch species).
(2) Sensory-based approaches may prove to be more cost-effective than interventionist approaches, especially if they can be automated (e.g. acoustic anchoring versus manual translocations).
(3) Sensory conservation methods have been successfully applied in a number of seabird taxa (e.g. Sternidae, Alcidae, Procellariidae; Table 1). While there are probably examples of sensory-based conservation in seabirds not encompassed by our search parameters, we found examples of attraction attempts that had limited success (e.g. Audouin's gulls, Ichthyaetus audouinii; Table 1), and other groups whose responsiveness to such techniques remains seemingly unstudied (e.g. booby and gannet species, frigatebirds, and tropicbirds; Jones & Kress, 2012; Fig. 1).
(4) This review identifies several conservation contexts where sensory-based techniques have considerable potential to improve outcomes such as establishing new colonies and deterrence from anthropogenic traps. We recommend:
(a) Life-history and behavioural traits that select for sensory signalling to inform the best candidate species for sensory-based conservation. These traits are: long-distance travel, navigation in featureless environments (e.g. seas, deserts), pair-bonding and mate choice, high parental care by both sexes, conspecific and heterospecific recognition (prey and predators, and mates, nests and chicks), coloniality, philopatry.
(b) Using multiple sensory modes to promote stronger behavioural responses from the target species. For example, combining sound and visual lures could be more effective than either lure on its own when attempting to counter effects of strong philopatry.
(c) Evaluating the potential risks of unintended consequences from using sensory-based methods. Predator cues should be investigated to avoid luring seabirds, or other prey species, to ecological traps, or attracting predators to the target species.
(d) Incorporating an experimental control to improve the ability to evaluate conservation outcomes and quantifying success of management efforts. For example, studies on attracting seabirds to new locations rarely observe other suitable nearby habitat without experimental stimuli to ensure that the movement of birds to the new location is a result of sensory method and not just a coincidental outcome of animal movement.
(e) Undertaking additional research on sensory-based approaches to conservation of seabirds and other taxa to optimize conservation outcomes. In seabirds, olfaction is underutilized. Further work (and controlled experimentation) on visual attractants is required to understand why some attempts are successful and others are not. A high priority is to understand the ethological response of seabirds to different vocalizations, such as identifying which calls are most effective in enticing seabirds to colony locations.
(5) Systematic investigations into sensory ecology could profoundly improve the success of conservation strategies. This type of sensory management is broadly applicable to many systems, and may be more cost-effective than more interventionist activities.
VIII. ACKNOWLEDGEMENTS
Our thanks to G. Taylor, C. Gaskin, and S. Sawyer for providing information for this review and to J. Rudolph, W. Lee, C. Painting, H. Schultz, C. Simpkins, J. Zhang, R. Sagar and E. Kennedy for comments. We acknowledge V. Ward for creative assistance with figures. M.R.F. was supported by the George Mason Scholarship Fund, NZ Forest and Bird (JS Watson), the Centre for Biodiversity and Biosecurity (CBB), and the University of Auckland.




