African trypanosomes and brain infection – the unsolved question
ABSTRACT
African trypanosomes induce sleeping sickness. The parasites are transmitted during the blood meal of a tsetse fly and appear primarily in blood and lymph vessels, before they enter the central nervous system. During the latter stage, trypanosomes induce a deregulation of sleep–wake cycles and some additional neurological disorders. Historically, it was assumed that trypanosomes cross the blood–brain barrier and settle somewhere between the brain cells. The brain, however, is a strictly controlled and immune-privileged area that is completely surrounded by a dense barrier that covers the blood vessels: this is the blood–brain barrier. It is known that some immune cells are able to cross this barrier, but this requires a sophisticated mechanism and highly specific cell–cell interactions that have not been observed for trypanosomes within the mammalian host. Interestingly, trypanosomes injected directly into the brain parenchyma did not induce an infection. Likewise, after an intraperitoneal infection of rats, Trypanosoma brucei brucei was not observed within the brain, but appeared readily within the cerebrospinal fluid (CSF) and the meninges. Therefore, the parasite did not cross the blood–brain barrier, but the blood–CSF barrier, which is formed by the choroid plexus, i.e. the part of the ventricles where CSF is produced from blood. While there is no question that trypanosomes are able to invade the brain to induce a deadly encephalopathy, controversy exists about the pathway involved. This review lists experimental results that support crossing of the blood–brain barrier and of the blood–CSF barrier and discuss the implications that either pathway would have on infection progress and on the survival strategy of the parasite. For reasons discussed below, we prefer the latter pathway and suggest the existence of an additional distinct meningeal stage, from which trypanosomes could invade the brain via the Virchow–Robin space thereby bypassing the blood–brain barrier. We also consider healthy carriers, i.e. people living symptomless with the disease for up to several decades, and discuss implications the proposed meningeal stage would have for new anti-trypanosomal drug development. Considering the re-infection of blood, a process called relapse, we discuss the likely involvement of the newly described glymphatic connection between the meningeal space and the lymphatic system, that seems also be important for other infectious diseases.
I. INTRODUCTION
African trypanosomes comprise a group of flagellated protozoans of medical and agricultural importance as they infect humans or farm animals. In this review we concentrate on Trypanosoma brucei, a group of subspecies which infect humans causing sleeping sickness (T. b. gambiense and T. b. rhodesiense), or infect farm and game animals causing nagana (T. b. brucei). Today, more than 90% of human African trypanosomiasis (HAT) cases are induced by T. b. gambiense (the Western form), which causes a more chronic disease with a usual survival time of several years. T. b. rhodesiense, primarily located in Eastern and Southern Africa, is responsible for approximately 10% of cases and has a more acute progression, with death occurring within several months. Untreated, both forms of HAT are fatal. Following the bite of an infected tse-tse fly, the transmitted metacyclic insect form differentiates spontanously into a blood form of slender morphology that is completely covered by a densely packed glycoprotein coat. This protein belongs to a library of several hundred distinctly encoded genes that can be sequentially expressed, thus allowing antigenic variation (Marcello & Barry, 2007). The variant surface glycoprotein (VSG) coat protects the parasite against the host's cellular immune system and complement reactions. Antibodies are formed against the N-terminal part of each VSG type, allowing the infecting parasite carrying this specific VSG to be opsonized by antibodies and destroyed. However, since variants expressing different VSGs appear independently of the host's immune reactions, continuous fluctuation of parasite density in the blood results. In addition, high parasite density leads to a differentiation process and the formation of so-called ‘stumpy parasites’ which are unable to proliferate but are involved in tse-tse fly infection (Hamm et al., 1990).
Sleeping sickness was well known among African populations since ancient times as a cause of periodic devastating epidemics (Slane, 1968; Atkins, 1978). Trypanosomes were recognized as causative agents of the disease in 1903 (Davies, 1962), after the parasite was first detected in the blood of infected humans (Dutton, 1902). The observable symptoms, notably the sleep disorder, led to the assumption that trypanosomes invade the brain. This was experimentally confirmed in 1903, when T. b. rhodesiense was for the first time observed within the cerebrospinal fluid (CSF) (Castellani, 1903; Calwell, 1937).
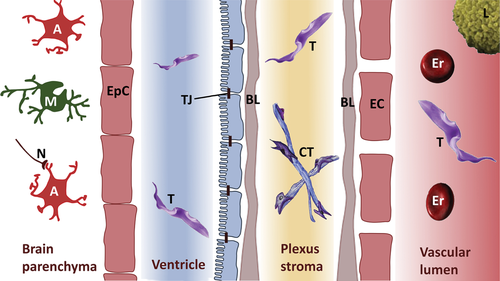
Calwell (1937) first discussed involvement of the choroid plexus (CP), which is responsible for the production of CSF, in trypanomosiasis. Thus, this parasite was thought to be able to cross the blood–CSF barrier (BCB) (Fig. 1). By contrast, imaging of cerebral trypanosomiasis revealed perivascular cuffing within the white matter (substantia alba) of the brain (Enanga et al., 2002), gliosis (Poltera, Owor & Cox, 1977), haemorrhages (Kennedy, 2004), and subcortical perivascular demyelination (Enanga et al., 2002). In some cases, hyperintense signals of the white matter were found in T2-weighted magnetic resonance images, which were interpreted as oedema or bleeding (Spinazzola et al., 1989; Braakman et al., 2006). Furthermore, lesions within the grey matter, the cerebellum and the brain stem were found (Kager et al., 2009). Together, these findings suggest that trypanosomes can cross the blood–brain barrier (BBB), although infiltration via the Virchow–Robin space (see Section IV.1) cannot be excluded (Fig. 2). All these studies were limited by the fact that human brain samples can only be analysed post-mortem. An autopsy would have to take place immediately after death in order to detect trypanosomes in situ. This is especially difficult in endemic regions. Therefore, the route of infection has never been systematically followed using patient material, and publications showing histological sections with trypanosomes in the human brain are extremely rare (Poltera et al., 1977). Much effort has been made to investigate trypanosomiasis in animal models, especially rodents and monkeys.
Most animal studies using T. b. brucei (Masocha et al., 2004), T. b. rhodesiense (Bafort & Schmidt, 1983) or T. evansi (Tuntasuvan et al., 2000; Rodrigues et al., 2009) showed trypanosomes 21–63 days after infection within the brain of rats (Poltera et al., 1980; Schmidt & Bafort, 1985). To assist in the development of medications with minimal side effects, a crucial question is whether (and when) the parasite crosses the BCB or the BBB.
The focus of this review is on aspects essential for understanding the interaction of trypanosomes with the BBB and the BCB. We consider literature from a wide range of disciplines: areas from entomology to inflammation research, from developmental biology to public health, from pharmacology to protozoology, and from clinical neurology to sleep research.
II. SYMPTOMS OBSERVED IN SLEEPING SICKNESS
Sleeping sickness is transmitted by tse-tse flies, which belong to the genus Glossinae. Thus the endemic area is limited to the so-called tse-tse belt, i.e. sub-Saharan Africa. During the feeding process, blood-form trypanosomes are taken up by the fly and develop into a midgut form that enters the salivary gland, where it differentiates firstly into a cell-attached epimastigote form and finally to a free-swimming metacyclic form. During a subsequent blood meal, these trypanosomes enter the skin lesion with the release of anti-coagulatory saliva. After a brief period in the skin, the parasite may travel to blood and lymph vessels to begin the infection process (Caljon et al., 2016). A localized immune reaction at the site of the bite often leads to the formation of a chancre of about one inch in diameter that is associated with pruritus. Shortly after the bite, a typical first symptom of Western HAT is the Winterbottom's sign, i.e. a lymphadenopathy in the lateral cervical region of the neck.
Winterbottom's sign is usually absent in the haemolymphatic stage of Eastern HAT, which progresses more rapidly and often leads to pericardial effusion, pulmonary oedema and acute myocarditis (Fig. 3).
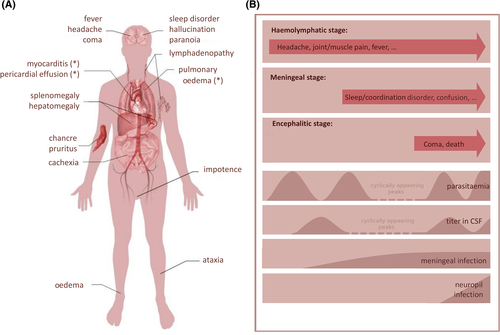
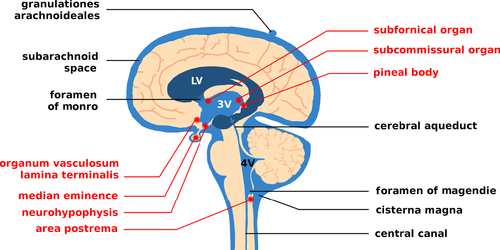
The second stage of infection, the so-called meningo-encephalitic stage, commences after a delay of weeks (Eastern HAT) or months (Western HAT). Since treatment and prognosis depend on the infection stage, the World Health Organization (WHO) recommends a lumbar puncture for diagnosis. Interestingly, trypanosomes do not survive long in CSF as it contains trypanocidal compounds (Pentreath, Owolabi & Doua, 1992; Wolburg et al., 2012). Thus, if parasites are found in the blood but not in the CSF, a cell count of more than five lymphocytes per microliter is considered positive for CSF infection. The appearance of trypanosomes in CSF is only indicative of parasites in the meningeal area and does not necessarily mean there is an infection of brain parenchyma (neuropil). The latency with which trypanosomes are detected in the central nervous system (CNS) varies considerably. A chronic stage with eventual exacerbation is now thought to exist after several case reports emerged describing patients who showed progression after more than a decade (reviewed in Sudarshi et al., 2014). From the CSF trypanosomes spread to susceptible tissues close to the ventricular system that do not have the BBB. This includes the circumventricular organs (CVOs), which are organized around the ventricles and possess fenestrated capillaries. Impairment of their physiological functions corresponds to commonly observed HAT symptoms. For example, the area postrema controls the emetic reflex and may become activated to cause nausea. Likewise, progressing somnolence and fragmented sleep patterns may be caused by deregulated melatonin secretion from the pineal gland. In addition, trypanosomes are able to produce and secrete prostaglandin D2 (PGD2) (Figarella et al., 2005, 2006; Duszenko et al., 2006), which may be at least partially responsible for elevated PGD2 levels recorded in late-stage HAT patients (Pentreath et al., 1990). PGD2 induces sleep and may also be involved in the deregulation of sleep–wake cycles (Urade & Hayaishi, 2011). Infection of the neurohypophysis, the vascular organ of the lamina terminalis and the subfornical organ affects regulation of the secretion of vasopressin and luteinizing hormone releasing hormone (LHRH). Eventually, HAT patients show oedema as well as impotence and infertility (Kennedy, 2012). However, other observed symptoms such as gait ataxia and facial nerve paralysis await explanation.
Neurological and psychological symptoms are common in second-stage HAT but can be misdiagnosed as symptoms of depressive disorders. Personality changes, hallucinations and paranoid delusions coincide with encephalitis. Soon after observation of these symptoms, patients become less responsive and fall below a score of 12 on the Glasgow Coma Scale (GCS) (MacLean et al., 2012). Death usually occurs by heart failure following pancarditis, opportunistic infection, malnourishment or encephalitis. Interestingly, in experimental monkey infections, cases of early deaths and late deaths could be differentiated. Early deaths occurred primarily by heart failure, meningitis or other organ damage, while the latter was exclusively induced by encephalitis (Schmidt, 1983).
There is still no complete biological explanation of HAT symptoms (Bruzzone et al., 2009). Neurological disorders might be due to factors secreted by the parasite, or by the host's immune system or by mutual modulation (Kristensson et al., 2010). Sleep–wake deregulation has also been observed in T. b. brucei-infected rats (Grassi-Zucconi et al., 1995; Darsaud et al., 2004; Seke Etet et al., 2012). This may be caused by the presence of trypanosomes within the pineal gland and the secretion of sleep inducers like PGD2 (Pentreath et al., 1990; Lundkvist, Kristensson & Bentivoglio, 2004; Figarella et al., 2006; Urade & Hayaishi, 2011) or tryptophol (Richards, 1965; Seed, Seed & Sechelski, 1978; Cornford et al., 1981). However, secondary effects on cytokine production also might lead to disruption of the circadian rhythm (Kristensson et al., 2010). African sleeping sickness causes changes to a number of hormones, including cortisol, prolactin and melatonin (Kristensson et al., 1998; Lundkvist et al., 2004). The photo-optic calibration of the sleep–wake rhythm takes place inside the suprachiasmatic nucleus. Neurotransmitters (especially glutamate) induce expression of the phosphoprotein c-fos. T. b. brucei-infected rats down-regulate glutamate receptors and thus have decreased levels of c-fos, maybe in response to an increase in levels of interferon gamma (IFNγ) (Lundkvist et al., 2004). In addition, changes to levels of tumor necrosis factor alpha (TNFα) also have an impact on the sleep–wake rhythm (Rodgers, 2010). Last but not least, cytokine-driven production of nitric oxide led not only to increased permeability of the BBB, but also to increased synthesis of prostaglandins (Enanga et al., 2002).
Other symptoms such as confusion, disturbed consciousness, coordination and sensory disorders could be the consequence of an increased autoimmune response (Galvao-Castro, Hochmann & Lambert, 1978). Antibodies against galactocerebrosides and neurofilaments circulate in CSF of HAT patients that may cause demyelination of nervous tissue (Enanga et al., 2002). However, all symptoms are reversible after eradication of the parasite by successful medical treatment. Thus it seems unlikely that trypanosomes have a direct cytotoxic effect on brain cells, as this would prevent full recovery. It has also been shown that trypanosomes do not have any impact on CNS cells during in vitro cultivation (Stoppini et al., 2000).
Attempts to link the appearance of different symptoms with progression of the disease have failed as published case reports are inconsistent (MacLean et al., 2010). Therefore, there is currently no clear symptom-based indication of the stage at which trypanosomes appear in CSF, when they move to the meninges and the CVOs and when they cross the BBB to invade the brain. We have attempted in Fig. 3 to link some prominent symptoms to different stages of the disease. It should be noted, that most symptoms are related to the function of the CVOs and meninges, while encephalopathy is clearly related to infiltration into the neuropil.
III. THE BRAIN STAGE IN SLEEPING SICKNESS
(1) Structure and function of the BBB
The human brain is pervaded by a network of capillaries that extend to a total length of about 600 km (Wolf, 1996; Fagerholm, 2007). The energy requirements of the brain constitute 20% of whole-body energy consumption, although the brain represents only 2% of body mass. A continuous supply of glucose and oxygen is essential as neurons do not have the ability to store nutrients and are obligatorily aerobic. To protect the brain from pathogens and toxins, the capillaries form a physical and physiological barrier (Sage, 1982; Brenner, 2006). Furthermore, the BBB (Fig. 2) prevents changes in pH or electrolyte concentrations in blood from directly affecting the membrane potential of neurons. It also ensures that neurotransmitters (glutamate, noradrenaline, etc.) circulating in the blood do not interfere with the nervous system. The BBB maintains homeostasis by ensuring the supply of nutrients and removal of metabolites using a number of selective transporters and ion channels (Ohtsuki, 2004; Abbott, Ronnback & Hansson, 2006), while water transport is via aquaporins (Nico et al., 2001; Amiry-Moghaddam & Ottersen, 2003). Although the volume of the extracellular space in the brain is typically about 20% (Sykova & Nicholson, 2008), the blood vessels are intimately integrated into the neuropil and are in close contact with the surrounding cells. The BBB extends to all brain areas except for the ventricles and CVOs (Duvernoy & Risold, 2007).
As shown in Fig. 2A, the BBB is composed of endothelial cells interconnected by tight junctions (TJs) which restrict paracellular flux (Reese & Karnovsky, 1967; Brightman & Reese, 1969; Wolburg & Lippoldt, 2002; Gunzel & Fromm, 2012). TJs are formed by many molecules, most belonging to occludin or different claudins (Cld-1,-3,-5,-12) (Bauer et al., 2014; Haseloff et al., 2015). The molecular design of BBB TJs is unique compared with other endothelial cell TJs.
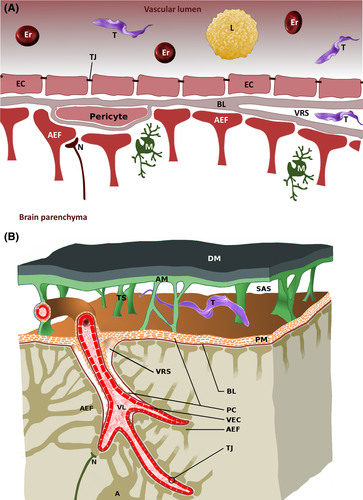
About 20% of the outer endothelial surface is coated by pericytes (Pardridge, 2005). They are completely surrounded by a basal lamina and possess contractile properties to regulate blood pressure. In addition, they fulfil similar functions to macrophages by phagocytozing pathogens and presenting their antigens. Finally, pericytes regulate proliferation and differentiation of endothelial cells and hence are important during angiogenesis. The coverage of pericytes on capillaries directly correlates with the barrier function of the BBB (Abbott, 1987). Endothelial cells have an underlying 50 nm thick basal lamina (Hawkins & Davis, 2005) (Fig. 2A) consisting mainly of type IV collagen, laminins, fibronectin, and heparan sulphate proteoglycans such as agrin. The basal lamina is easily visualized under electron microscopy. The blood capillaries, representing the barrier in the strictest sense, possess a basal lamina in common with that of pericytes and astrocytes. In the post-capillary venules and pre-capillary arterioles, the basal laminae are split into an endothelial and a pericytic/astrocytic lamina, leaving a perivascular space between. This space is continuous with the Virchow–Robin space that originates at blood vessels within the subarachnoid space when they enter the brain parenchyma (Fig. 2B).
Brain cells are divided into neurons, microglia and macroglia, with the latter a collective term for all brain cells which are neither neurons nor microglia. Astrocytes supply neurons with nutrients, regulate extracellular ion concentrations (Abbott, 2002), and send one process each to the intracerebral vessels forming so-called perivascular endfeet, covered by a basal lamina. The complex consisting of these endfeets and the basal lamina is named the glial-limiting membrane or membrana limitans gliae perivascularis. By contrast, astrocytic processes sending endfeet to the surface of the brain, form the membrana limitans gliae superficialis. Although perivascular astrocyte endfeet do not contribute directly to barrier function, they seem to be required for differentiation of endothelial cells and contribute to the formation of the BBB. By secreting cytokines, they are able to modulate permeability of the barrier within minutes (Abbott, 2002). It should be noted that blood vessels of the pia mater also show BBB properties without an association with astrocyte processes (Fig. 2B).
(2) Experimental evidence for trypanosomes crossing the BBB
Extravascular trypanosomes were found next to cerebral capillaries (Frevert et al., 2012), suggesting that they cross the BBB to invade the neuropil. This is especially the case for white, but also for grey matter areas of the brain (Schmidt & Bafort, 1987; Masocha et al., 2004). In rats that had been infected intravenously with a very high dose of fluorescence-labelled parasites, detection of perivascular trypanosomes was possible within hours (Frevert et al., 2012). The authors assumed that parasites had crossed the vascular endothelium of the BBB, but could not rule out the possibility that they had invaded the subarachnoid space and spread from there along the Virchow–Robin space (Frevert et al., 2012).
Since fluorescence-labelled trypanosomes have been detected in perivascular spaces, many attempts have been made to explain how they cross the BBB. Transmigration across the vascular endothelial cell layer would require the proteolytic opening of TJs and of the basal lamina. Trypanosomes do express enzymes that could facilitate tissue invasion, such as major surface metalloproteases (MSPs) (Grandgenett et al., 2007), oligopeptidases or cysteine proteases like brucipain (Lonsdale-Eccles & Grab, 2002). However, electron microscopic analysis of brain inflammation showed stable TJs during transcellular transmigration (Wolburg, Wolburg-Buchholz & Engelhardt, 2005). The permeability of basal laminae is influenced by the composition of laminins. It has been proposed that it may be easier for trypanosomes (and leucocytes) to overcome laminin α4 than laminin α5 (Masocha, Rottenberg & Kristensson, 2007). In addition, attachment to vascular endothelial cells could be mediated by increased expression of intercellular or vascular cell adhesion molecules (ICAM-1 and VCAM-1) during infection. For example, T. b. gambiense was shown to secrete soluble endothelium-activating factors (Grab & Kennedy, 2008). Moreover, cytokine-mediated permeabilization of the BBB may take place, involving an interaction between parasites, the host's immune system, and cellular or extracellular components of the BBB (Masocha et al., 2004; Grab et al., 2009).
As a hypothetical mechanism for transmigration it was suggested that secretion of brucipain could activate a protease-sensitive G-protein-coupled endothelial receptor (Grab et al., 2009). Signal transduction would then lead to activation of phospholipase C, release of inositol triphosphate and subsequently to increased cytosolic calcium concentration. As a result, proteinkinase C could activate myosin light chain kinase, which in turn would alter the cytoskeleton to open TJs. In addition, the role of GTPases like Ras homolog gene family member A (RhoA) and its effect on actin filaments has been discussed (Grab et al., 2009).
IFNγ is released in response to trypanosome infection, and may be involved in the opening of the BBB. For example, IFNγ is known to induce the production of chemokine ligands (i.e. chemokine C-X-C motif ligand 10, CXCL-10) in perivascular astrocytes, facilitating the passage of lymphocytes and possibly trypanosomes into the brain parenchyma. CXCL-10-deficient mice show reduced brain infections (Kristensson et al., 2010). In IFNγ receptor-deficient mice, brain parenchymal infection was absent (Masocha et al., 2004). Thus, on the one hand IFNγ is essential as an activator of the host's immune defences while on the other hand it could facilitate penetration of the BBB for trypanosomes. Increased concentrations of kinins have also been suggested to correlate with increased permeability of the endothelium (Richards, 1965; Boreham, 1970; Kreier, 1977).
Brain microendothelial cells have been cultured on collagen-coated Transwell filter units to simulate the BBB in vitro. T. b. gambiense could penetrate these artificial model systems much faster than T. b. brucei as measured by a decrease in electric impedance. By contrast, the trypanosome procyclic insect form was not able to penetrate the barrier, or attach to vascular endothelial cells (Grab et al., 2004; Grab & Kennedy, 2008). Further studies have shown that minocycline (a drug which inhibits the passage of leukocytes across the BBB) could also prevent trypanosomes from crossing the vessel endothelium, suggesting that leukocytes could in some way facilitate trypanosome infection (Grab & Kennedy, 2008).
However, note that while there is experimental evidence that trypanosomes are able to cross the BBB, they cannot survive inside the brain parenchyma. Whenever trypanosomes were injected directly into the brain of suckling and weanling mice (Bafort, Schmidt & Molyneux, 1987) or adult rats (Wolburg et al., 2012; Mogk et al., 2014b), the parasites degenerated within days. The same was shown for blood-infected mice, whose BBB was mechanically damaged to allow trypanosomes to enter the brain (Schmidt & Bafort, 1985).
IV. THE MENINGEAL STAGE OF SLEEPING SICKNESS
(1) Structure and function of the BCB and the circumventricular organs
The mammalian brain contains four ventricles, i.e. spaces filled with CSF and separated from the brain parenchyma by a layer of ependymal cells. Each ventricle contains a choroid plexus (CP) that invades the ventricle and is responsible for CSF formation. For this purpose, the blood vessels of the CP have fenestrated endothelial cells which allow diffusion of serum from the blood vessel into the plexus stroma (Fig. 1). The outer layer of the CP is formed by epithelial cells, which are interconnected by TJs. On its way from stroma to ventricle space, CSF is formed from serum and is rather similar in terms of its osmolarity, water content and pH, while levels of glucose, amino acids, cations and anions are reduced by a factor of 1.5–2. Most strikingly, the protein concentration drops by a factor of about 200 (Lentner, 1981; Ernst, Palacios & Siegel, 1986; Lindsey et al., 1990). Thus, CSF is not a simple ultra-filtrate but is specifically modified, including the addition of neuropeptides.
The CP epithelial cells are sometimes referred to as ependymal cells since they are in continuity with the ependyma of the periventricular lining, but they are not identical. The main differences between ependymal and epithelial cells are: (i) the presence of a basal lamina in the CP, but not in the ependyma, and (ii) that the CP epithelium has barrier properties (continuous TJs), while the ependyma contains only adherens junctions and fragmented discontinuous TJs (Fig. 1).
The ependymal and epithelial cell layers meet at the neck of the plexus. Due to their special topology controlled fluid release is possible across the ependymal border, while cells cannot pass (Linß & Fanghänel, 1998). Thus, the plexus epithelial cells with its TJs form the BCB. In contrast to the endothelial BBB, the epithelial CP is characterized by high expression of at least claudin-1, -2 and -11, resulting in TJs formed by parallel arrangements of strands (Wolburg et al., 2001).
Transmigration of pathogenic bacteria across the plexus epithelium of the BCB has been found to be a para- or transcellular process (Wewer et al., 2011; Schwerk et al., 2012). Based on a daily CSF production of 500–700 ml in humans, the total volume of 120–200 ml of CSF is renewed 3–4 times a day. CSF formed in both lateral ventricles combines and flows via the intraventricular foramen of Monro to the third ventricle to join with CSF formed here. It then reaches the fourth ventricle via the aqueduct of Sylvius. The fourth ventricle in turn is connected via the foramen of Magendie with the cisterna magna (Fig. 4). From here CSF flushes into the subarachnoid space, i.e. the open space between the arachnoid mater and pia mater (Fig. 2B), which also extends into the vertebral canal. Below the skull, CSF is reabsorbed by arachnoid granulations. These are vessel-free protrusions of the arachnoid that reach superficial brain veins through gaps in the dura mater or the dura-encapsulated cranial sinus. Working like a valve, arachnoid granulations maintain a unidirectional flow whenever the intracranial pressure exceeds a defined threshold (Siegel, Agranoff & Albers, 1999). In addition, a direct connection between CSF and lymphatic vessels was recently discovered and is named the glymphatic system (Jessen et al., 2015).
Keeping the brain floating in CSF is necessary for several reasons: it serves to protect the tissues from suddenly arising acceleration or deceleration forces which could lead to severe trauma. Minimal volume changes (e.g. inflammatory swellings) of the brain can create life-threatening intracranial pressure increases. This is partially mitigated by controlled CSF reabsorption. In addition, CSF contributes to nutrient supply and clearance of metabolites by crossing the ependymal cell border. In this way, secreted substances can be easily transported. A role in signal transduction has also been discussed (Siegel et al., 1999; Jessen et al., 2015).
The CVOs are unpaired and located medial of the ventricle walls (Fig. 4). Except for the subcommissural organ, they do not possess a BBB, but are organized similarly to the BCB (Duvernoy & Risold, 2007). This is essential, as they secrete hormones or fulfil sensory tasks. The pineal gland, for example, releases the melatonin involved in defining the circadian rhythm. The eminentia mediana secretes neuropeptides, and the posterior pituitary produces antidiuretic hormone and oxytocin. Other CVOs have to react to substances circulating in the blood. The organum vasculosum of the lamina terminalis is involved in the regulation of thirst, hunger and fever. The sub-fornical organ reacts to angiotensin II in order to regulate electrolyte and water balance, and the vomiting centre (area postrema) needs to detect potential toxins. To meet these requirements, the CVOs have fenestrated capillaries, like those within the CP. The barrier to the adjacent brain parenchyma is formed by specialized ependymal cells, so-called tanycytes, which are interconnected by TJs that block cellular crossings.
(2) Experimental evidence for trypanosomes crossing the BCB
The passage of trypanosomes across the BCB (Ormerod & Venkatesan, 1970; Poltera et al., 1980; Kristensson et al., 2010) and the barrier of the CVOs (Mhlanga, Bentivoglio & Kristensson, 1997) has been reported several times. The first observation of trypanosomes in CSF was in 1903 (Castellani, 1903). Vervet monkeys (Cercopithecus aethiops) infected with T. b. rhodesiense showed CSF infection and developed inflammatory infiltrates in proximity to the ventricular system (Schmidt, 1983). During the course of infection, parasites appeared inside the subarachnoid space and the associated Virchow–Robin spaces. In experiments with sheep, hyperaemia, oedema and lymphocyte accumulation was found in the CP (Moulton, 1986). The infection path could be clearly followed by electron microscopy in T. b. brucei-infected rats for about 30 days (Wolburg et al., 2012). Following the appearance of parasites during an artificial laboratory infection of rats, trypanosomes were localized within the stroma of the plexus, the ventricle, the subarachnoid space and the pia mater, but not within the neuropil.
There is general consensus that T-cells, monocytes and parasites first overcome the fenestrated capillaries within the CP to enter the stroma and then the CP epithelial cells with their TJ-based barrier to enter the ventricle. From there, the cells can access the external CSF to reach the subarachnoid space and the Virchow–Robin space. Reaching this area could of course also occur by egression of parasites from pial blood vessels, but this is highly restricted by the BBB properties of these non-fenestrated vessels.
Most of these experimental results suggest an infection mechanism where trypanosomes initially cross the BCB (Schultzberg et al., 1988). Following these initial steps, inflammatory responses may allow the parasite to invade CSF and to enter ventricles and CVOs (Kristensson et al., 2010). It was shown that trypanosome lymphocyte-triggering factor induces an immune response and thus the production of IFNγ (Bakhiet et al., 2002). Macrophages were activated and released interleukins, TNFα and nitric oxide. In fact, enhanced concentrations of interleukin-6, interleukin-10 and TNFα were measured in CSF of vervet monkeys suffering from trypanosomiasis (Nyawira Maranga et al., 2013). It should be noted, however, that the appearance of soluble factors (especially IFNγ) has also been used to explain modulation of the BBB (see Section III.2).
V. IS THERE A CHRONIC MENINGEAL STAGE IN SLEEPING SICKNESS?
In HAT, detection of trypanosomes in CSF is a diagnostic tool for second-stage infection. Usually, finding trypanosomes in CSF is considered equivalent to the appearance of the parasite within the brain, hence the term ‘meningo-encephalitic’ stage. In principle, however, parasites crossing the BCB have no access to brain parenchyma, but spread throughout the subarachnoid space. Likewise, trypanosomes passing the equivalent of the BCB, i.e. the fenestrated blood vessels of CVOs, stay within these CVOs but cannot proceed to the brain parenchyma because tanycytes act as a physical barrier. As in CSF, trypanosomes within the CP seem unable to seed permanently within this organ as they lose their VSG coat and degrade (Mogk et al., 2014b). Instead, with each newly appearing population peak in the blood, trypanosomes recross the BCB and a new infection episode occurs, detectable by cyclic infection of CSF (Mogk et al., 2014b). In contrast to the fluctuating population in the plexus, the ventricles and the subarachnoid space, parasites are found permanently within the pia mater (Wolburg et al., 2012). Here they must be safe from any trypanocidal activity, most likely from neuropeptides (Gonzalez-Rey, Chorny & Delgado, 2006; Delgado et al., 2009), that is present in CSF. It is also likely that this acts as a source for trypanosomes reappearing in the blood. So-called ‘relapse’ is observed following trypanosome eradication from the blood by drugs such as suramin, which do not cross the BBB. By contrast, it is difficult to imagine how trypanosomes could reappear in blood starting from the neuropil.
There is no doubt that African trypanosomes are able both to cross the BCB and to appear within the neuropil; both facts have been well documented (see Sections III and IV). There is controversy, however, regarding when during an ongoing infection the parasite escapes from the haemolymphatic system. Generalized statements claim that it takes several months for T. b. gambiense and several weeks for T. b. rhodesiense to appear in the brain. This proposed delayed crossing of the BBB or BCB, however, has not been systematically established in natural human infections. If true, latency of CNS infection would certainly require a suitable explanation, but so far this is purely speculative. A plausible argument would be that antigen–antibody complexes are needed (Poltera et al., 1980; Lambert, Berney & Kazyumba, 1981), but an experimental approach in a rodent model could not confirm this (Wolburg et al., 2012). Instead, in rat infections periodic appearance of parasites in CSF, 1 day following a parasitic population peak in the blood, was found (Fig. 3B). In this case an additional mechanism to open the BCB is not necessary, as opening may depend on the appearance of short stumpy parasites, which express a surface-bound metalloprotease, major metalloprotease B (MSP-B; Grandgenett et al., 2007; de Sousa, Atouguia & Silva, 2010) to open the basal lamina surrounding the plexus blood vessels (Kleiner & Stetler-Stevenson, 1999). Artificial placement of bloodform trypanosomes into brain parenchyma never induced a brain infection; these parasites did not grow and died within a few days (Mogk et al., 2014b).
As mentioned above, several experimental approaches led to the observation that trypanosomes can cross the BBB, appearing in perivascular locations within the brain parenchyma (Masocha et al., 2004; Frevert et al., 2012). Interestingly, using a rather high parasite number injected intravenously into mice, trypanosomes appeared in perivascular locations just hours following injection (Frevert et al., 2012). Unfortunately, the fate of these parasites was not analysed over subsequent days and their ability to undergo cell division was not assessed. The question thus remains whether trypanosomes are able to survive and grow within the brain parenchyma for the several years of a natural T. b. gambiense infection. This is especially so for the ‘healthy carrier of trypanosomiasis’, i.e. asymptomatic humans infected with HAT that have been followed up for up to 15 years (Wurapa et al., 1984; Franco et al., 2014a), or in the case of an African who developed sleeping sickness symptoms almost 30 years after leaving Africa (Sudarshi et al., 2014). It is hard to believe that a symptomless brain infection was present for 30 years without causing neurological disabilities. Another argument against brain infection is that virtually no neurological symptoms remain after successful treatment. In this context, experiments performed on vervet monkeys are interesting. Following infection, two populations could be clearly distinguished: one group died rather early, usually because of heart failure, while the other group died later from encephalitis (Schmidt, 1983). This result suggests that trypanosomes may enter the brain only in a late phase of infection and that this encephalitic stage is inevitably fatal.
During the meningeal stage, a cell density-control mechanism must be in place to keep the parasite population within the available space, i.e. up to six pial cell layers. Since CSF has trypanocidal activity, parasites pushed out of the pia mater will be killed unless they can reach a blood vessel to induce a relapse. It is thus interesting to note that evidence exists suggesting a direct connection of CSF with the lymphatic system in the nasal pharynx (Zakharov, Papaiconomou & Johnston, 2004; Pollay, 2012). It is tempting to speculate that this glymphatic pathway is the route that trypanosomes use from the meninges to the haemolymphatic system during a relapse. This pathway seems also be important for other pathogens that invade the meninges and the brain, such as protozoan (e.g. Toxoplasma gondii causing toxoplasmosis), bacterial (e.g. Treponema pallidum causing syphilis) or viral (e.g. tick-borne encephalitis virus) infections. An increasing population density of trypanosomes induces differentiation from the slender to the stumpy morphology (Reuner et al., 1997; Seed & Black, 1997). Stumpy parasites produce PGD2 (Figarella et al., 2005), which by a paracrine or autocrine mechanism leads to caspase-free apoptosis of stumpy cells (Duszenko et al., 2006). Since slender trypanosomes do not undergo apoptosis, persisting infection is ensured (Duszenko et al., 2006). This view is also supported by the observation that PGD2 is markedly elevated in CSF of late-stage HAT patients (Pentreath et al., 1990). PGD2 is also well known as a sleep inducer (Urade & Hayaishi, 2011) and this prostanoid of parasitic origin may thus contribute to the name-giving symptom of sleeping sickness.
VI. SYNOPSIS AND FUTURE PERSPECTIVES
It is surprising that after a period of more than 100 years of intense research on sleeping sickness and African trypanosomes, the exact details of the meningo-encephalitic stage are still elusive. Herein we discuss representative studies in an attempt to present a clear and comprehensive picture about what is known and what is still unknown regarding this stage.
There is consensus that trypanosomes are present within the meninges and within the brain parenchyma at a certain phases of infection. However, there is no conclusive evidence as to exactly when trypanosomes cross the BBB or the BCB. Since a defined mechanism explaining how trypanosomes cross either of these two barriers has not been provided, initiation of the second stage remains speculative. Statements that it takes weeks to months in rhodesiense infections and months to years in gambiense infections obviously derive either from speculations about the infection period based on when an individual had been in an endemic area, or from observations correlating appearance of a chancre with the first detection of typical HAT symptoms. However, this is not necessarily identical with the first appearance of parasites outside the haemolymphatic system. In fact, in a rodent model, it takes some 20 days for T. b. brucei to be detectable in brain after intraperitoneal infection (Poltera et al., 1980; Schmidt & Bafort, 1985); CSF was not examined in these experiments. In another study, trypanosomes in a rat infection appeared in CSF just 1 day after the first blood population peak and then periodically, linked to waves in blood infection (Mogk et al., 2014b). This result suggests that trypanosomes may appear in CSF at a very early point of infection to seed within the pia mater and also that crossing of the BCB depends on population density and thus, as already discussed, possibly on the formation of stumpy parasites.
Taking all the experimental evidence and medical observations into consideration, we propose that infection in sleeping sickness may include three phases. The first haemolymphatic stage starts shortly after the tsetse fly's bite, as the parasite has access to the blood and lymphatic systems. The second meningeal stage starts after the first sufficiently high blood population density peak and may depend on the appearance of short stumpy parasites to achieve entry across the BCB. The third encephalitic stage can be characterized by a breakdown of the brain's protective borders thus allowing mass invasion of trypanosomes to induce a fatal encephalopathy (Fig. 3B). This view is consistent with observations by Schmidt (1983) on artificially infected vervet monkeys. This model could explain several observations in natural infections: (i) trypanosomes cross the fenestrated endothelium as no additional mechanism is necessary besides high cell density allowing formation of stumpy parasites. (ii) Meningeal infection includes infection of CVOs and thus explains the onset of many symptoms observed during sleeping sickness. (iii) Because of controlled cell-density regulation of parasites within the pia mater by PGD2-induced apoptosis, inflammation processes are prevented and a chronic phase may last for years or even decades. (iv) Relapses, regularly observed after clearance of parasites from blood, can be explained by reabsorption of CSF into the venous system by CSF–lymphatic bridges, while retrograde transfer across the BBB is difficult to imagine. (v) Full recovery after successful treatment of HAT is possible if parasites reside in the meninges only, since no brain structures would have been damaged. (vi) A non-life-threatening meningeal stage would suit transmission of the parasite, as it allows permanent blood infection even if parasitaemia is controlled by a successful immune response.
The model does not explain infection of the brain parenchyma, as observed in experimental and natural infections. Consistent with the above-described model, a meningeal infection could overwhelm control mechanisms and parasites infiltrate via the Virchow–Robin space to induce lethal encephalopathy (Fig. 3). This could happen during an acute attack, perhaps due to a weakened constitution of a receptive individual. Another scenario would include permanent crossing of the BBB by some trypanosomes which are then removed by e.g. microglia (H. Wolburg & M. Duszenko, unpublished observations), as observed in experimental intracerebral infection studies (Mogk et al., 2014b). In this case, lethal encephalopathy would occur after successful brain defences collapse either due to ongoing permanent infiltration of parasites or a generally weakened constitution. In contrast to these models, we consider a permanent and long-lasting infection of the brain parenchyma as rather unlikely, although we cannot exclude this possibility.
Despite common assumptions that the brain stage of HAT is well understood, questions still remain. The most important question is what molecular mechanism allows the trypanosome to move beyond the haemolymphatic system to infect the meninges and the CNS. Solving this will provide information on the length of time after an infected tse-tse fly bite that the parasite resides exclusively within the haemolymphytic system, and whether or not an explicit meningeal stage exists. Currently available drugs for second-stage HAT all have severe side effects, because they have been selected for their ability to cross the BBB. By flooding the brain, these drugs also affect delicate neural structures. If, however, a prolonged meningeal stage does exist, drugs should be selected for their ability to distribute within the meninges instead of the brain parenchyma. Such drugs would be in direct contact with the parasite and side effects thus may be minimized.
It is hoped that sleeping sickness will be eradicated or become manageable within the next decade, as suggested by the WHO (Franco et al., 2014b). However, with central questions about the infection mechanism unsolved, the disease may reappear at cyclic intervals as chronically infected individuals or animal reservoirs become infectious again.
VII. CONCLUSIONS
- Trypanosomes do not survive within the neuropil during the early stage of HAT.
- Cerebrospinal fluid is cyclically infected depending on parasite density in the blood.
- Infections of the circumventricular organs and the pia mater account for most of the observable symptoms in HAT.
- Transition from the meningeal to the cerebral stage may occur after long time periods.
- Massive brain infiltration of parasites lead to encephalitis as a proposed final stage in HAT.
VIII. ACKNOWLEDGEMENTS
This was supported by a grant (DU136) of the Deutsche Forschungsgemeinschaft (DFG), Bonn, Germany.




